Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors
Abstract
:1. Introduction
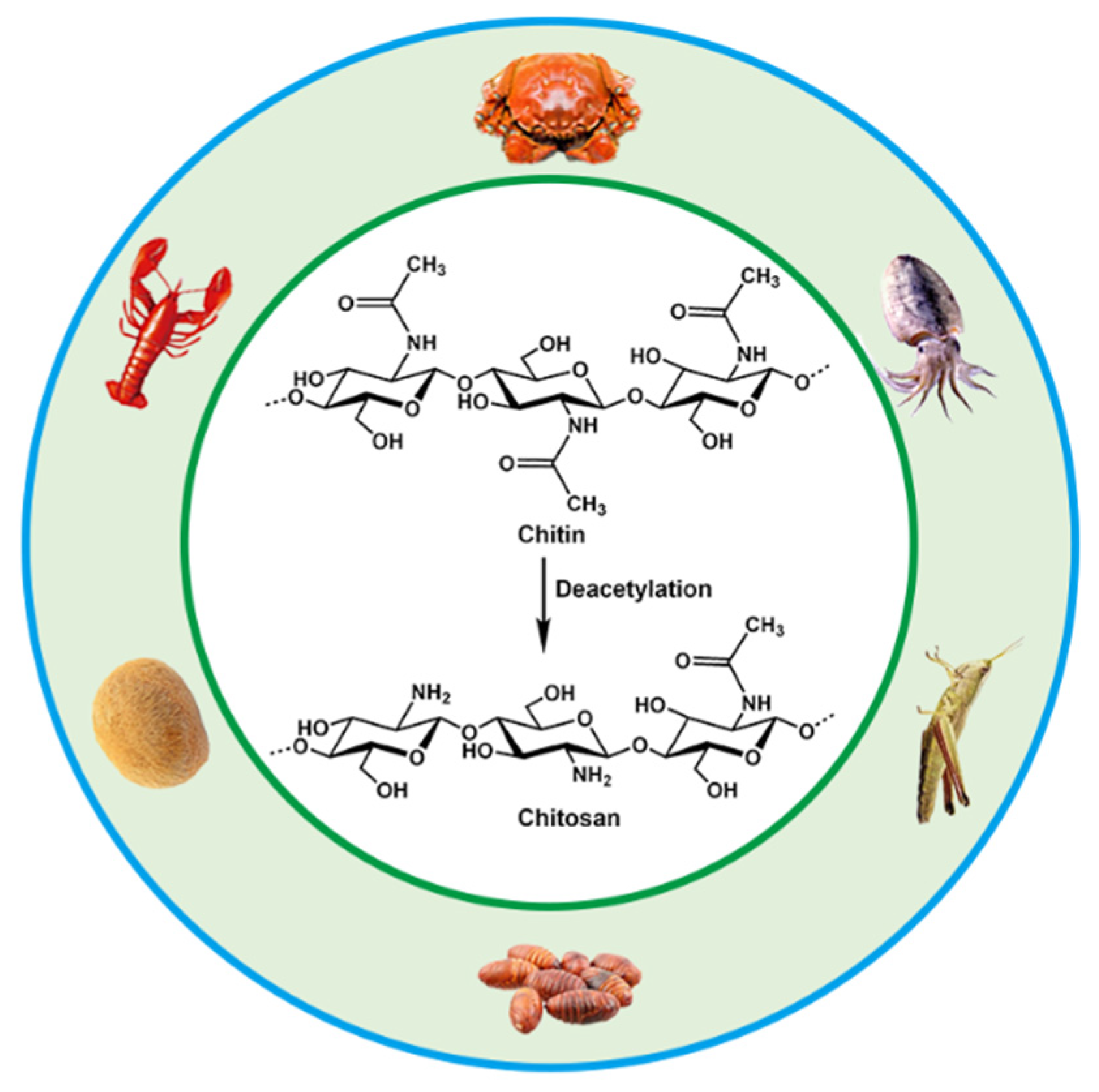
2. Structure and Properties of CS
3. Design and Preparation of CS Hydrogels
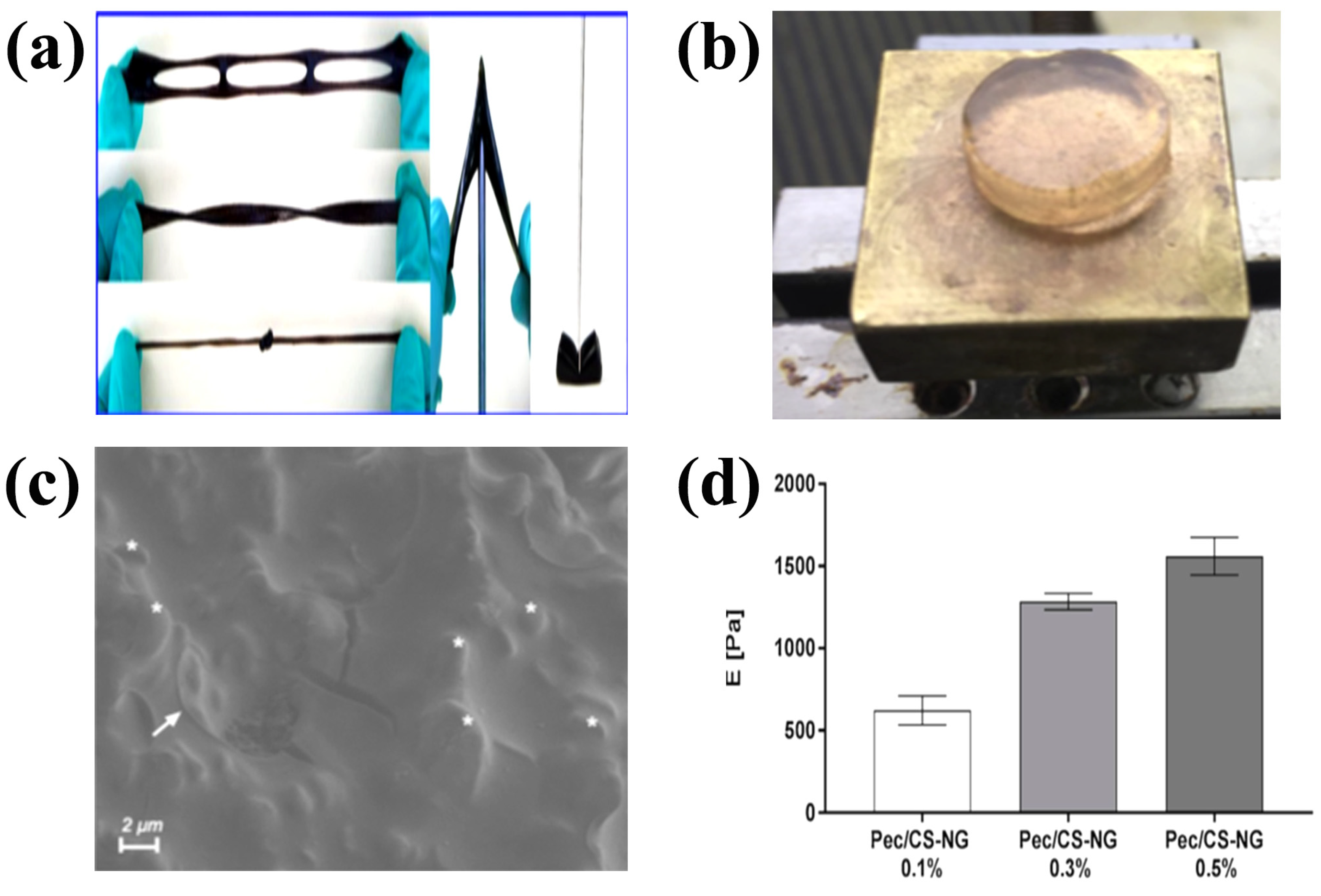
3.1. Physically Crosslinked CS Hydrogels
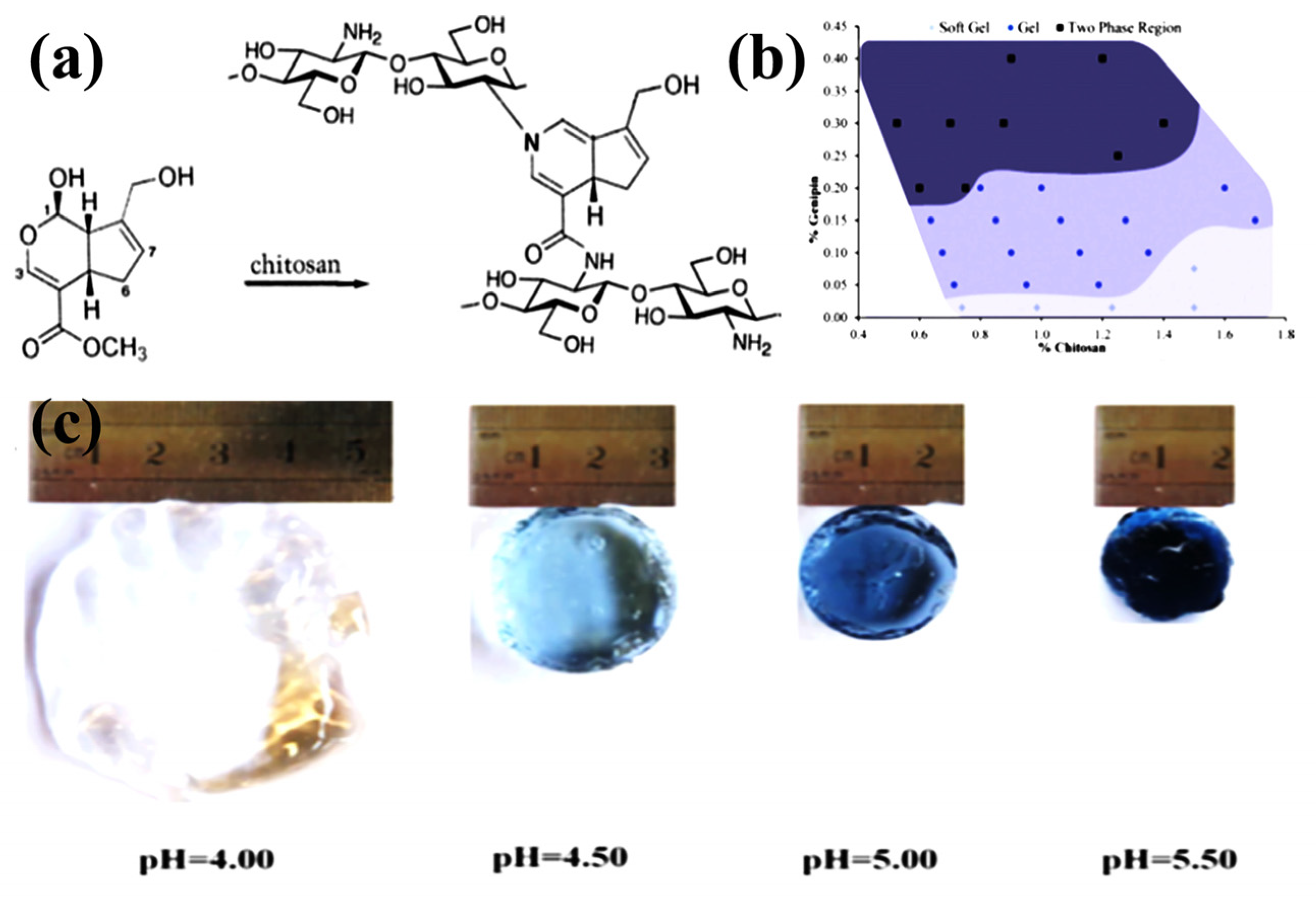
3.2. Chemically Crosslinked CS Hydrogels
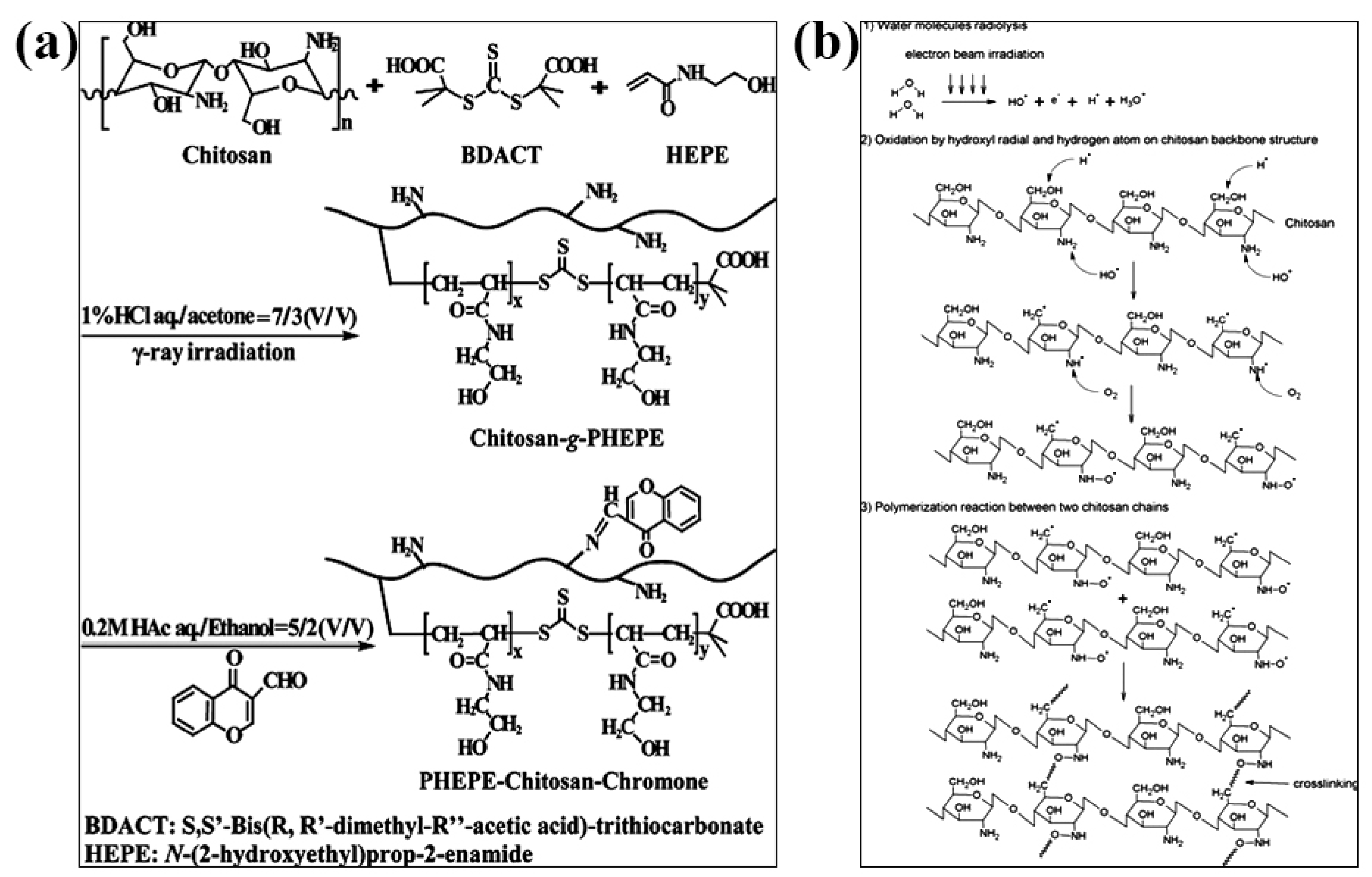
3.3. Irradiation-Crosslinked CS Hydrogels
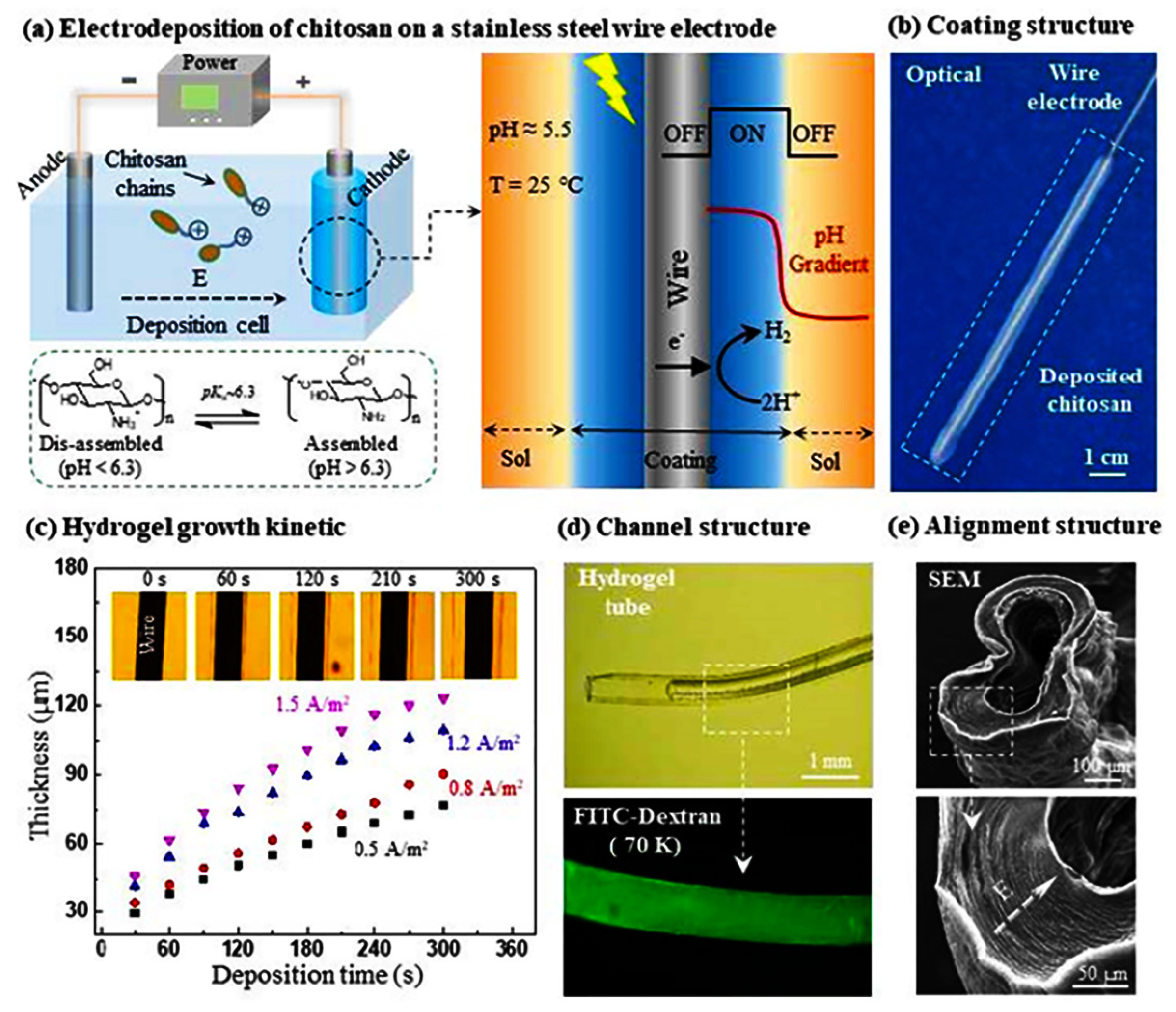
3.4. Electrodeposited CS Hydrogels
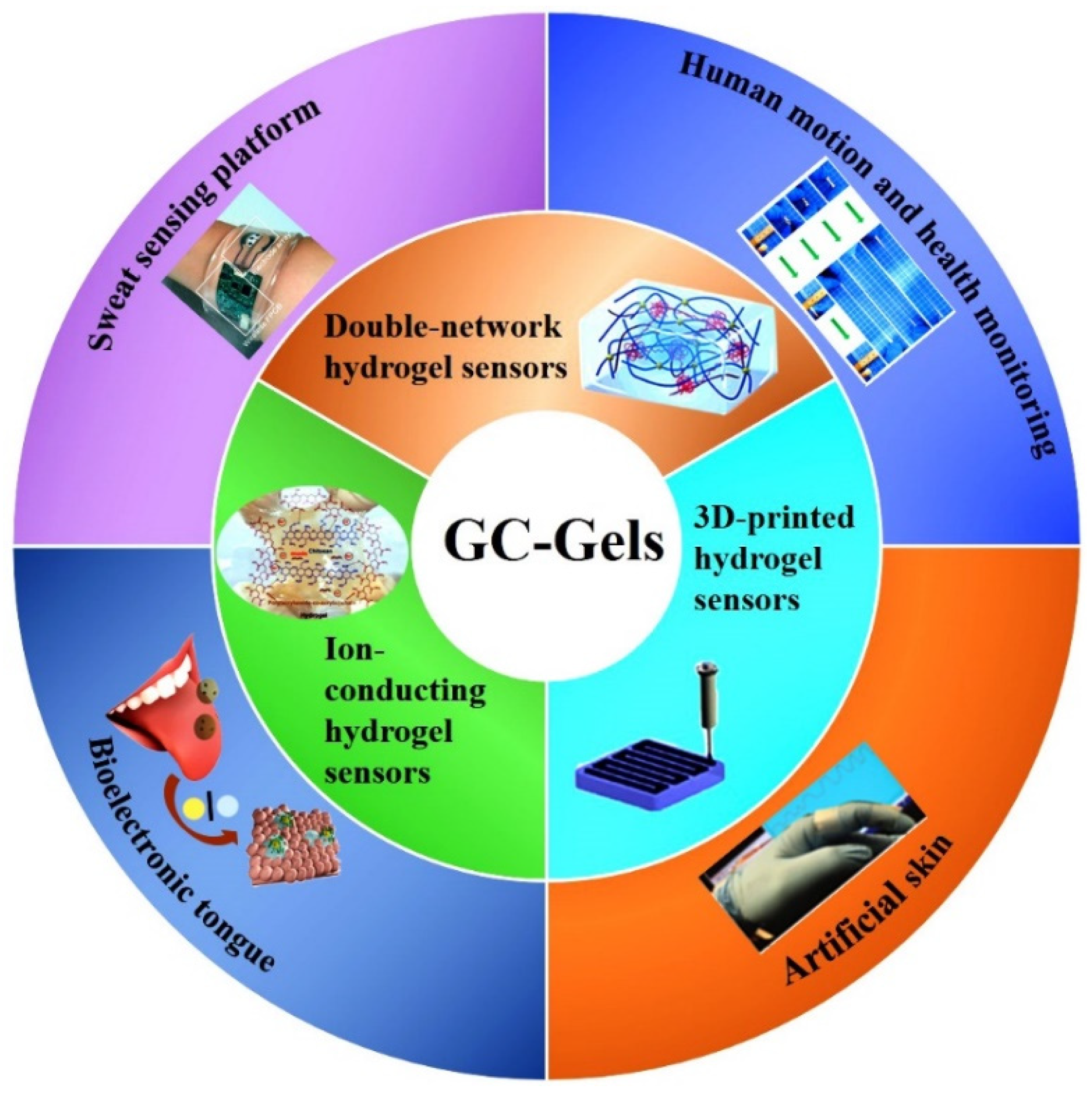
4. Synthesis and Modification of CS-Based Hydrogels for Flexile Wearable Sensors
4.1. CS/Carbon-Based Nanomaterial Composite Hydrogel Sensors
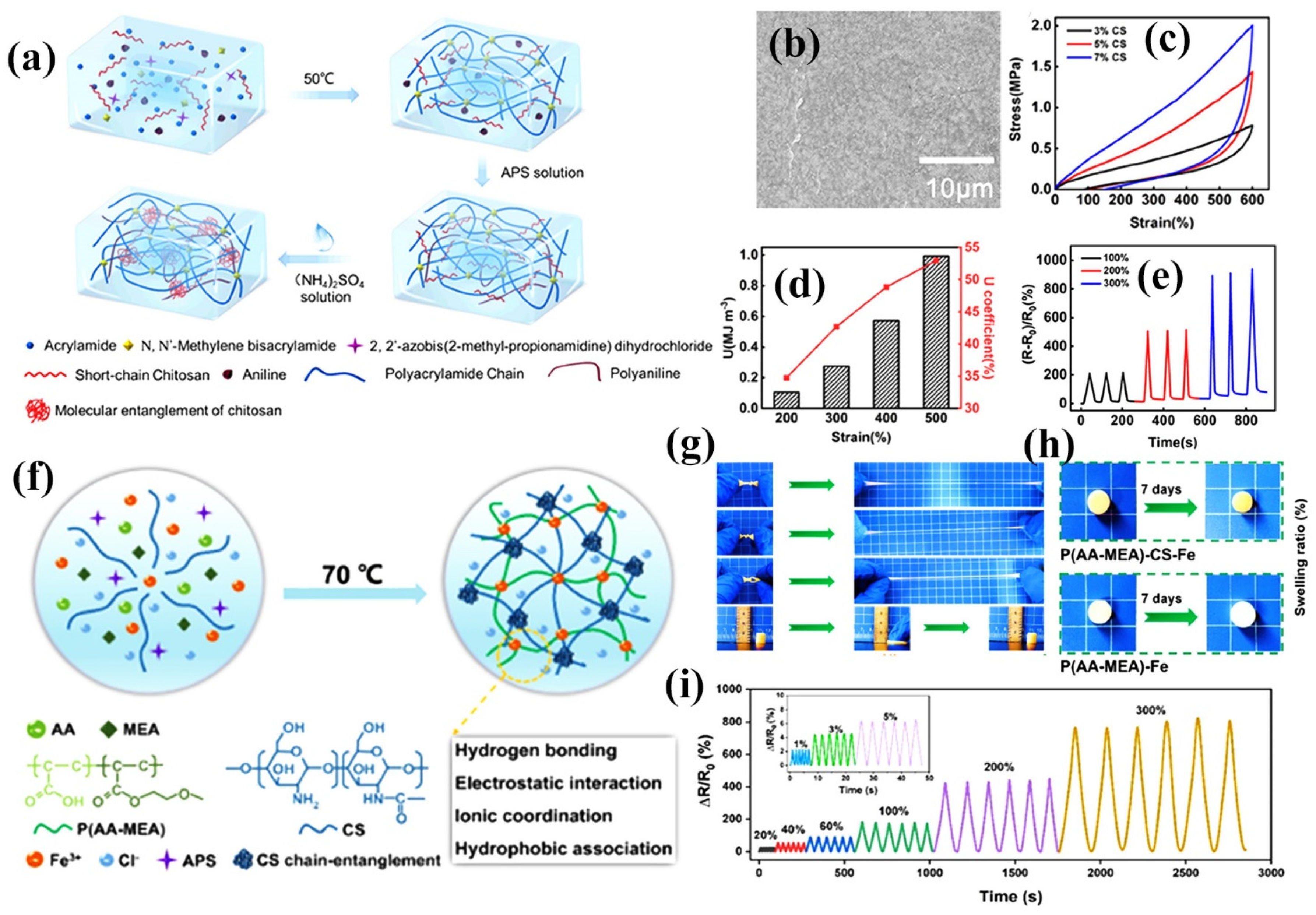
4.2. CS-Based Double-Network Hydrogel Sensors
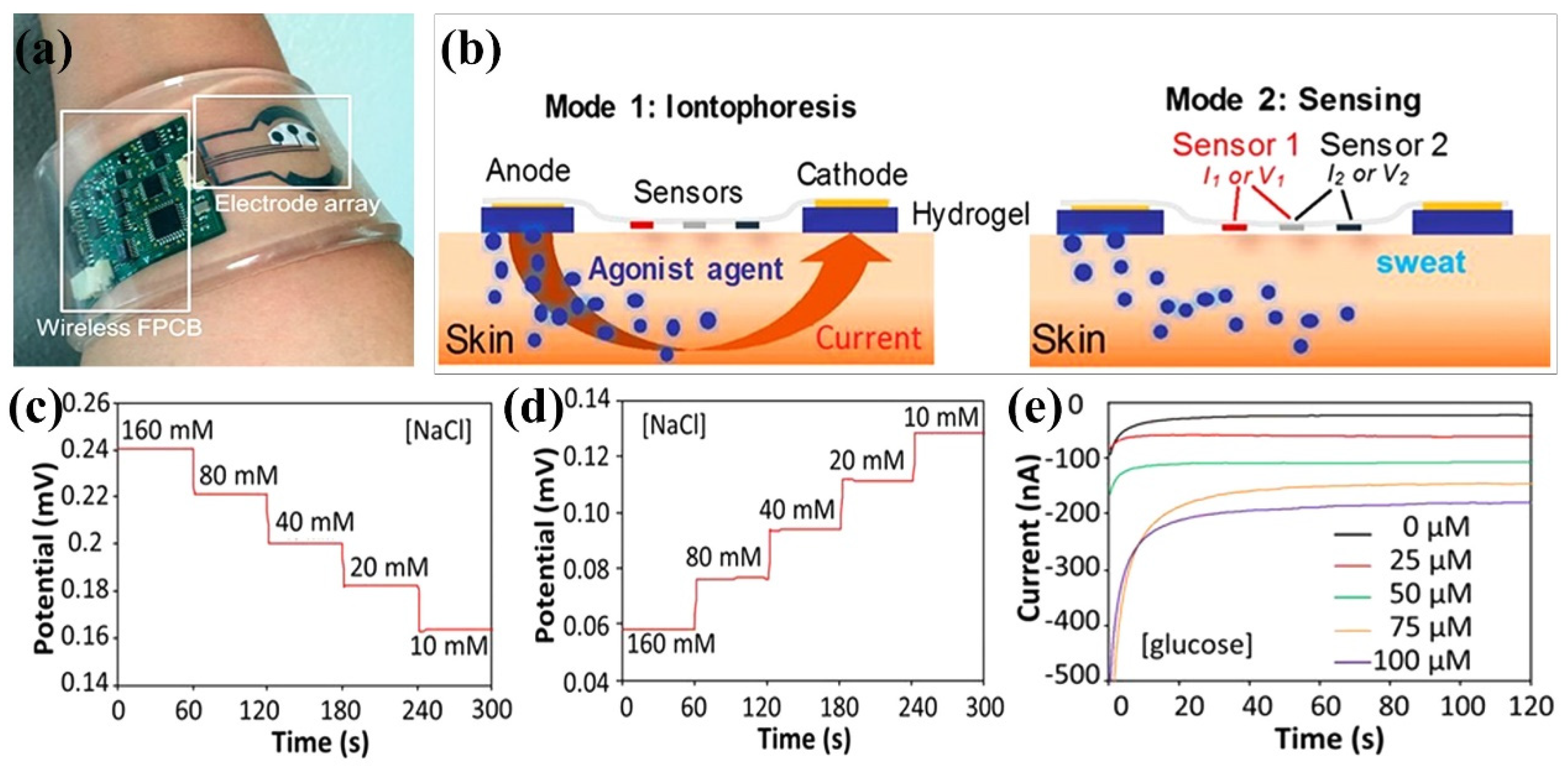
4.3. CS-Based Ion-Conducting Hydrogel Sensors
4.4. Three-Dimensional Printed CS Hydrogel Sensors
5. Problems and Challenges
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Xu, T.; Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent progress in natural biopolymers conductive hydrogels for flexible wearable sensors and energy devices: Materials, structures, and performance. ACS Appl. Bio Mater. 2020, 4, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, J.; He, S.; Liu, A.; Zhang, J.; Xu, H.; Shao, W. Flexible wearable sensors based on lignin doped organohydrogels with multi-functionalities. Chem. Eng. J. 2022, 430, 132653. [Google Scholar] [CrossRef]
- Anwer, A.H.; Khan, N.; Ansari, M.Z.; Baek, S.-S.; Yi, H.; Kim, S.; Noh, S.M.; Jeong, C. Recent advances in touch sensors for flexible wearable devices. Sensors 2022, 22, 4460. [Google Scholar] [CrossRef]
- del Bosque, A.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Flexible wearable sensors based in carbon nanotubes reinforced poly (Ethylene Glycol) Diglycidyl ether (PEGDGE): Analysis of strain sensitivity and proof of concept. Chemosensors 2021, 9, 158. [Google Scholar] [CrossRef]
- Fu, X.; Wang, L.; Zhao, L.; Yuan, Z.; Zhang, Y.; Wang, D.; Wang, D.; Li, J.; Li, D.; Shulga, V. Controlled Assembly of MXene Nanosheets as an Electrode and Active Layer for High-Performance Electronic Skin. Adv. Funct. Mater. 2021, 31, 2010533. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Mohamed, M.E.B.; Attia, N.F.; Elashery, S.E. Greener and facile synthesis of hybrid nanocomposite for ultrasensitive iron (II) detection using carbon sensor. Microporous Mesoporous Mater. 2021, 313, 110832. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D.; Morin-Crini, N. Dye removal by biosorption using cross-linked chitosan-based hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.-V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Cui, C.; Shao, C.; Meng, L.; Yang, J. High-strength, self-adhesive, and strain-sensitive chitosan/poly (acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Xu, J.; Duan, L.; Gao, G. Chitosan-driven skin-attachable hydrogel sensors toward human motion and physiological signal monitoring. Carbohydr. Polym. 2021, 268, 118240. [Google Scholar] [CrossRef]
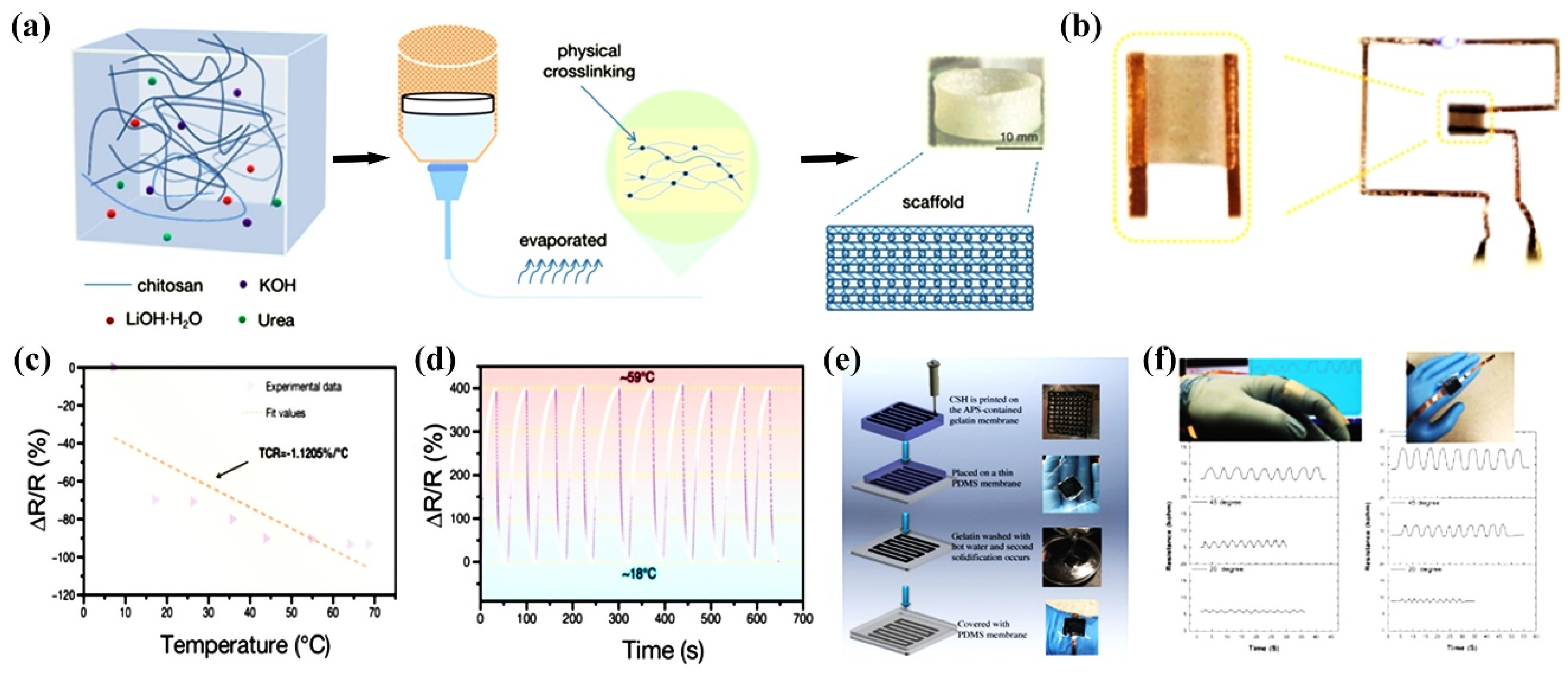
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W. Polyacrylamide/chitosan-based conductive double network hydrogels with outstanding electrical and mechanical performance at low temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saheed, I.O.; Da Oh, W.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.; Maia, P.C.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent advancement of molecular structure and biomaterial function of chitosan from marine organisms for pharmaceutical and nutraceutical application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Franca, E.F.; Freitas, L.C.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and chitosan polymer-based mucoadhesive liquid crystalline systems intended for buccal drug delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Win, P.; Lin, C.-G.; Long, Y.; Chen, W.; Chen, G.; Song, Y.-F. Covalently cross-linked layered double hydroxide nanocomposite hydrogels with ultrahigh water content and excellent mechanical properties. Chem. Eng. J. 2018, 335, 409–415. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Feksa, L.R.; Troian, E.A.; Muller, C.D.; Viegas, F.; Machado, A.B.; Rech, V.C. Hydrogels for biomedical applications. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 403–438. [Google Scholar]
- Li, Y.; Yang, J.; Yu, X.; Sun, X.; Chen, F.; Tang, Z.; Zhu, L.; Qin, G.; Chen, Q. Controlled shape deformation of bilayer films with tough adhesion between nanocomposite hydrogels and polymer substrates. J. Mater. Chem. B 2018, 6, 6629–6636. [Google Scholar] [CrossRef]
- Wang, B.; Hua, J.; You, R.; Yan, K.; Ma, L. Electrochemically deposition of catechol-chitosan hydrogel coating on coronary stent with robust copper ions immobilization capability and improved interfacial biological activity. Int. J. Biol. Macromol. 2021, 181, 435–443. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemical deposition to construct a nature inspired multilayer chitosan/layered double hydroxides hybrid gel for stimuli responsive release of protein. J. Mater. Chem. B 2015, 3, 7577–7584. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Wang, J.; Minev, I.R. Electro-assisted printing of soft hydrogels via controlled electrochemical reactions. Nat. Commun. 2022, 13, 1353. [Google Scholar] [CrossRef]
- Helú, M.A.B.; Liu, L. Rational shaping of hydrogel by electrodeposition under fluid mechanics for electrochemical writing on complex shaped surfaces at microscale. Chem. Eng. J. 2021, 416, 129029. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Deng, X.; Wang, T.; Yang, Z.; Zhou, W.; Wang, C. Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 2016, 49, 5660–5668. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yu, X.; Sun, X.; Zhu, L.; Qin, G.; Dai, Y.; Chen, Q. Tough and conductive dual physically cross-linked hydrogels for wearable sensors. Ind. Eng. Chem. Res. 2019, 58, 17001–17009. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, X.; Yang, B.; Lai, L.; Chen, N.; Hu, J.; Lu, Q. Dual physically cross-linked hydrogels incorporating hydrophobic interactions with promising repairability and ultrahigh elongation. Adv. Funct. Mater. 2021, 31, 2008187. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Zhang, Y.; Zheng, J.; Wu, H.; Chen, Y.; Xu, S.; Yang, J.; Liu, C.; Zhang, Y. Dual cross-linked ion-based temperature-responsive conductive hydrogels with multiple sensors and steady electrocardiogram monitoring. Chem. Mater. 2020, 32, 7670–7678. [Google Scholar] [CrossRef]
- Yang, J.; Kang, Q.; Zhang, B.; Fang, X.; Liu, S.; Qin, G.; Chen, Q. Strong, tough, anti-freezing, non-drying and sensitive ionic sensor based on fully physical cross-linked double network hydrogel. Mater. Sci. Eng. C 2021, 130, 112452. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Chiao, M.; Mahmoud, K.A.; Wu, L.; Wang, B. Preparation and characterization of PVA/PVP conductive hydrogels formed by freeze–thaw processes as a promising material for sensor applications. J. Mater. Sci. 2022, 57, 8029–8038. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Hyun, J. Free-form three-dimensional nanocellulose structure reinforced with poly (vinyl alcohol) using freeze-thaw process. Carbohydr. Polym. 2022, 298, 120055. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; He, S.; Ma, S.; Zeng, A.; Chen, G.; Tang, X.; Sun, Y.; Xu, F.; Zeng, X.; Lin, L. Catechol-based all-wood hydrogels with anisotropic, tough, and flexible properties for highly sensitive pressure sensing. Chem. Eng. J. 2022, 427, 131896. [Google Scholar] [CrossRef]
- Du, C.; Zhang, X.N.; Sun, T.L.; Du, M.; Zheng, Q.; Wu, Z.L. Hydrogen-bond association-mediated dynamics and viscoelastic properties of tough supramolecular hydrogels. Macromolecules 2021, 54, 4313–4325. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly (vinyl alcohol)/poly (acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, injectable, and self-healing conductive hydrogel enabled by multiple hydrogen bonding toward wearable electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Li, X.; Liu, W.; Yu, X.; Yan, F.; Sun, J. Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly (vinyl alcohol)-based hydrogels with excellent self-healing ability. ACS Mater. Lett. 2020, 2, 764–770. [Google Scholar] [CrossRef]
- Qi, C.; Dong, Z.; Huang, Y.; Xu, J.; Lei, C. Tough, Anti-Swelling Supramolecular Hydrogels Mediated by Surfactant–Polymer Interactions for Underwater Sensors. ACS Appl. Mater. Interfaces 2022, 14, 30385–30397. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, S.; Li, Y.; Gao, G. Highly sensitive and wearable gel-based sensors with a dynamic physically cross-linked structure for strain-stimulus detection over a wide temperature range. J. Mater. Chem. C 2019, 7, 11303–11314. [Google Scholar] [CrossRef]
- Shitrit, Y.; Davidovich-Pinhas, M.; Bianco-Peled, H. Shear thinning pectin hydrogels physically cross-linked with chitosan nanogels. Carbohydr. Polym. 2019, 225, 115249. [Google Scholar] [CrossRef]
- Khapre, M.A.; Pandey, S.; Jugade, R.M. Glutaraldehyde-cross-linked chitosan–alginate composite for organic dyes removal from aqueous solutions. Int. J. Biol. Macromol. 2021, 190, 862–875. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.-B.; Kim, K.-W.; Lee, Y. Enhanced Gold (III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef]
- Marrakchi, F.; Hameed, B.; Hummadi, E. Mesoporous biohybrid epichlorohydrin crosslinked chitosan/carbon–clay adsorbent for effective cationic and anionic dyes adsorption. Int. J. Biol. Macromol. 2020, 163, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Xiao, N.; Ma, X.; Liu, C.; Zhong, L.; Xiao, G. Preparation and properties of epichlorohydrin-cross-linked chitosan/hydroxyethyl cellulose based CuO nanocomposite films. Cellulose 2022, 29, 4413–4426. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Isaacs-Páez, E.D.; Rodríguez-Méndez, I.; González-García, R.; Labrada-Delgado, G.J.; Aragón-Piña, A.; García-Arreola, M.E. Formaldehyde and tripolyphosphate crosslinked chitosan hydrogels: Synthesis, characterization and modeling. Int. J. Biol. Macromol. 2021, 183, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Atangana, E. Adsorption of Zn (II) and Pb (II) ions from aqueous solution using chitosan cross-linked formaldehyde adsorbent to protect the environment. J. Polym. Environ. 2019, 27, 2281–2291. [Google Scholar] [CrossRef]
- Tavares, L.; Flores, E.E.E.; Rodrigues, R.C.; Hertz, P.F.; Noreña, C.P.Z. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Crica, L.E.; Pandele, A.M.; Ionita, M. Graphene oxide reinforcing genipin crosslinked chitosan-gelatin blend films. Coatings 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, R.; Li, M.; Yu, F.; He, C. Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohydr. Polym. 2019, 220, 141–148. [Google Scholar] [CrossRef]
- Kang, M.L.; Ko, J.-Y.; Kim, J.E.; Im, G.-I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Zhang, H.; Luo, J.; Gao, X. Green Fabrication of Chitin/Chitosan Composite Hydrogels and Their Potential Applications. Macromol. Biosci. 2021, 21, 2000389. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.K.; Shahi, V.K. Selective adsorption of Pb (II) from aqueous medium by cross-linked chitosan-functionalized graphene oxide adsorbent. ACS Sustain. Chem. Eng. 2018, 7, 1427–1436. [Google Scholar] [CrossRef]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, M.; Morsali, A.; Beyramabadi, S.A. An applied quantum-chemical model for genipin-crosslinked chitosan (GCS) nanocarrier. Int. J. Biol. Macromol. 2020, 165, 1229–1240. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Swelling behaviour and glass transition in genipin-crosslinked chitosan systems. Int. J. Biol. Macromol. 2020, 164, 3075–3083. [Google Scholar] [CrossRef]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Zhang, S.; Huang, L.; Hua, D.; Zhu, X. A facile approach for controlled modification of chitosan under γ-ray irradiation for drug delivery. Macromolecules 2013, 46, 814–818. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Chan, M.Y.; Koay, S.C. Biodegradation and thermal properties of crosslinked chitosan/corn cob biocomposite films by electron beam irradiation. Polym. Eng. Sci. 2019, 59, E59–E68. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, B.H.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.W.; Shen, J. Chitosan to connect biology to electronics: Fabricating the bio-device interface and communicating across this interface. Polymers 2014, 7, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Nawrotek, K.; Tylman, M.; Adamus-Włodarczyk, A.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M.; Wach, R. Influence of chitosan average molecular weight on degradation and stability of electrodeposited conduits. Carbohydr. Polym. 2020, 244, 116484. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Ino, K.; Ida, H.; Nashimoto, Y.; Shiku, H. Electrodeposition-based rapid bioprinting of 3D-designed hydrogels with a pin art device. Biofabrication 2019, 11, 035018. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Nawrotek, K.; Grams, J. Understanding electrodeposition of chitosan–hydroxyapatite structures for regeneration of tubular-shaped tissues and organs. Materials 2021, 14, 1288. [Google Scholar] [CrossRef]
- Yan, K.; Yang, C.; Zhong, W.; Lu, Z.; Li, X.; Shi, X.; Wang, D. Wire templated electrodeposition of vessel-like structured chitosan hydrogel by using a pulsed electrical signal. Soft Matter 2020, 16, 9471–9478. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Wang, W.; Liu, H.; Deng, H.; Du, Y.; Shi, X. Electrodeposition induced covalent cross-linking of chitosan for electrofabrication of hydrogel contact lenses. Carbohydr. Polym. 2022, 292, 119678. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Luo, Y.; Wu, T.; Chen, X.; Wang, Y.; Xie, J. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Technol. 2021, 110, 822–832. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical application. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef]
- Han, F.; Wang, T.; Liu, G.; Liu, H.; Xie, X.; Wei, Z.; Li, J.; Jiang, C.; He, Y.; Xu, F. Materials with Tunable Optical Properties for Wearable Epidermal Sensing in Health Monitoring. Adv. Mater. 2022, 34, 2109055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, B.; Zhang, Y.; Xu, L.; Huang, Q.; He, Y.; Li, X.; Wu, J.; Yang, J.; Chen, Q. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mater. Chem. B 2020, 8, 3171–3191. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, J.; Duan, L.; Gao, G. A highly conductive hydrogel driven by phytic acid towards a wearable sensor with freezing and dehydration resistance. J. Mater. Chem. A 2021, 9, 22615–22625. [Google Scholar] [CrossRef]
- Kim, J.-N.; Lee, J.; Lee, H.; Oh, I.-K. Stretchable and self-healable catechol-chitosan-diatom hydrogel for triboelectric generator and self-powered tremor sensor targeting at Parkinson disease. Nano Energy 2021, 82, 105705. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Shen, B.; Wang, Y.; Peng, P.; Tang, F.; Feng, J. Highly transparent, self-healing, injectable and self-adhesive chitosan/polyzwitterion-based double network hydrogel for potential 3D printing wearable strain sensor. Mater. Sci. Eng. C 2020, 117, 111298. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Zhou, Z.; Chen, Q.; Hong, M.; Yang, S.; Fu, H. Highly elastic anti-fatigue and anti-freezing conductive double network hydrogel for human body sensors. Ind. Eng. Chem. Res. 2021, 60, 6162–6172. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Jiang, Z.; Ni, M.; Xu, M. A self-healing and self-adhesive chitosan based ion-conducting hydrogel sensor by ultrafast polymerization. Int. J. Biol. Macromol. 2022, 209, 1975–1984. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Y.; Sun, X.; Liang, H. Preparation of stretchable and self-healable dual ionically cross-linked hydrogel based on chitosan/polyacrylic acid with anti-freezing property for multi-model flexible sensing and detection. Int. J. Biol. Macromol. 2021, 193, 629–637. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- Pirahmadi, P.; Kokabi, M.; Alamdarnejad, G. Polyvinyl alcohol/chitosan/carbon nanotubes electroactive shape memory nanocomposite hydrogels. J. Appl. Polym. Sci. 2021, 138, 49995. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, M.; Han, D.; Deng, Z.; Cao, X.; Tian, J.; Ye, Q. A high-performance GelMA–GelMA homogeneous double-network hydrogel assisted by 3D printing. J. Mater. Chem. B 2022, 10, 3906–3915. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, X.; Cao, L.; Li, J.; Wei, Y. Chitosan-enhanced nonswelling hydrogel with stable mechanical properties for long-lasting underwater sensing. Int. J. Biol. Macromol. 2022, 212, 123–133. [Google Scholar] [CrossRef]
- Sahoo, S.D.; Vasudha, T.; Muthuvijayan, V.; Prasad, E. Chitosan-Based Self-Healable and Adhesive Hydrogels for Flexible Strain Sensor Application. ACS Appl. Polym. Mater. 2022, 4, 9176–9185. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-stimuli-responsive and cellulose conductive ionic hydrogel for smart wearable devices and thermal actuators. ACS Appl. Mater. Interfaces 2020, 13, 1353–1366. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J. Muscle-inspired highly anisotropic, strong, ion-conductive hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Zhang, B.; Wan, K.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Hydrogen-bonded network enables semi-interpenetrating ionic conductive hydrogels with high stretchability and excellent fatigue resistance for capacitive/resistive bimodal sensors. Chem. Eng. J. 2021, 411, 128506. [Google Scholar] [CrossRef]
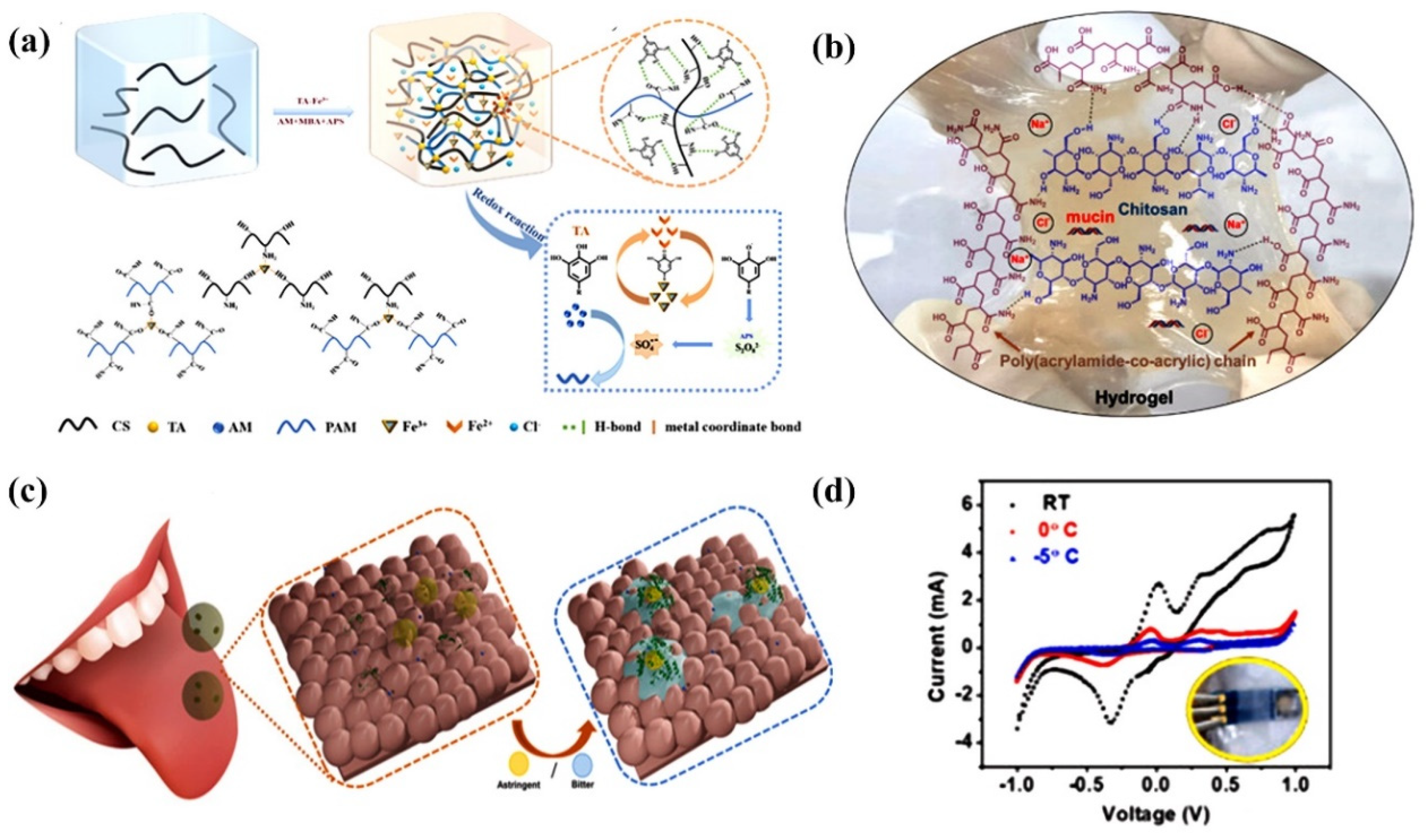
- Khan, A.; Ahmed, S.; Sun, B.-Y.; Chen, Y.-C.; Chuang, W.-T.; Chan, Y.-H.; Gupta, D.; Wu, P.-W.; Lin, H.-C. Self-healable and anti-freezing ion conducting hydrogel-based artificial bioelectronic tongue sensing toward astringent and bitter tastes. Biosens. Bioelectron. 2022, 198, 113811. [Google Scholar] [CrossRef] [PubMed]
- Emon, M.O.F.; Alkadi, F.; Philip, D.G.; Kim, D.-H.; Lee, K.-C.; Choi, J.-W. Multi-material 3D printing of a soft pressure sensor. Addit. Manuf. 2019, 28, 629–638. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, X.; Huang, H.; Wang, H.; Lin, W.; Peng, Z. Full 3D printing of stretchable piezoresistive sensor with hierarchical porosity and multimodulus architecture. Adv. Funct. Mater. 2019, 29, 1807569. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Yap, Y.L.; Goh, G.D.; Yu, H.; Yeong, W.Y.; Tran, T. Development of bendable strain sensor with embedded microchannels using 3D printing. Sens. Actuators A Phys. 2017, 263, 593–599. [Google Scholar] [CrossRef]
- Ragones, H.; Schreiber, D.; Inberg, A.; Berkh, O.; Kósa, G.; Freeman, A.; Shacham-Diamand, Y. Disposable electrochemical sensor prepared using 3D printing for cell and tissue diagnostics. Sens. Actuators B Chem. 2015, 216, 434–442. [Google Scholar] [CrossRef]
- Hao, F.; Maimaitiyiming, X. Fast 3D Printing with Chitosan/Polyvinyl alcohol/Graphene Oxide Multifunctional Hydrogel Ink that has UltraStretch Properity. ChemistrySelect 2022, 7, e202200201. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Wei, X.; Ma, K.; Cheng, Y.; Sun, L.; Chen, D.; Zhao, X.; Lu, H.; Song, B.; Yang, K.; Jia, P. Adhesive, conductive, self-healing, and antibacterial hydrogel based on chitosan–polyoxometalate complexes for wearable strain sensor. ACS Appl. Polym. Mater. 2020, 2, 2541–2549. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Guo, M.; Sun, Z.; Chen, Y.; Wu, Y.; Li, Y.; Xiang, D.; Li, H.; Li, Z. Double-network hydrogel-based stretchable, adhesive, and conductive e-skin sensor coupled human skin-like biocompatible and protective properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129803. [Google Scholar] [CrossRef]
- Duan, J.; Liu, F.; Kong, Y.; Hao, M.; He, J.; Wang, J.; Wang, S.; Liu, H.; Sang, Y. Homogeneous chitosan/graphene oxide nanocomposite hydrogel-based actuator driven by efficient photothermally induced water gradients. ACS Appl. Nano Mater. 2020, 3, 1002–1009. [Google Scholar] [CrossRef]
- Heijmans, K.; Nab, S.; Holkenborg, B.K.; Pathak, A.D.; Gaastra-Nedea, S.; Smeulders, D. Development of a reactive force field for CaCl2· nH2O, and the application to thermochemical energy storage. Comput. Mater. Sci. 2021, 197, 110595. [Google Scholar] [CrossRef]
| CS-Based Hydrogel Materials | Role/Function of CS | Sensing Types | Conductivity | Stretchability | Applications | Ref. |
|---|---|---|---|---|---|---|
| CS/AMPS/PAAm a | Electrostatic interaction; improved the mechanical properties | Strain sensor | 0.0848 S/cm | 2839% | Human motion and physiological signal monitoring | [17] |
| CS/PA/PAAm b | Polymerization; anti-freezing and moisturizing properties | Strain sensor | 0.041 S/cm | 1266% | Electrocardiography (ECG) signal detection | [96] |
| CCDHG c | Hydrogen bonding and reversible dynamic bonding; bio-availability and applicability | Triboelectric nanogenerator | ─ | 185% | Monitoring the health condition of Parkinson’s disease (PD) patients | [97] |
| P(SBMA-co-AAc)/CS-Cit d | Electrostatic interaction and hydrogen bonding; self-healing and self-adhesive | Strain sensor | 0.11 S/cm | 800% | Human motion detection and health monitoring | [98] |
| CS/P(AA-co-SS)/NaCl e | Hydrogen bonds, electrostatic interactions, and hydrophobic domains; high elasticity | Strain sensor | 4.5 S/m | 620% | Detect various movements of the human body | [99] |
| PAAm @CS | Ion coordination; self-healing and self-adhesive | Strain sensor | 1.7 mS/cm | 636% | Electronic skin and human activity monitoring | [100] |
| CS/PAA f | Ionic interaction; high deformability, self-healing and anti-freezing | Self-healable and strain sensor | 4.58–5.76 S/m | 1190–1550% | Detect the conventional motion signals of human body | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Xu, C.; Zhao, Y.; Shi, W.; Li, H.; Cai, J.; Ding, F.; Qu, P. Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors. Chemosensors 2023, 11, 39. https://doi.org/10.3390/chemosensors11010039
Wu S, Xu C, Zhao Y, Shi W, Li H, Cai J, Ding F, Qu P. Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors. Chemosensors. 2023; 11(1):39. https://doi.org/10.3390/chemosensors11010039
Chicago/Turabian StyleWu, Shuping, Chao Xu, Yiran Zhao, Weijian Shi, Hao Li, Jiawei Cai, Fuyuan Ding, and Ping Qu. 2023. "Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors" Chemosensors 11, no. 1: 39. https://doi.org/10.3390/chemosensors11010039






