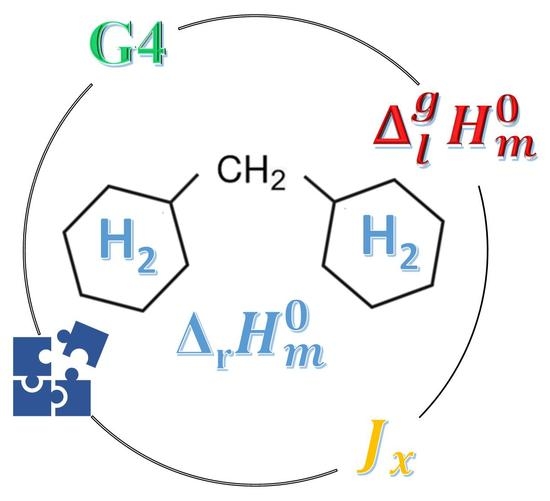
Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs
Abstract
:1. Introduction
2. Methodology
2.1. Basics of the Group-Additivity Concept (“Centerpiece” Approach)
2.2. Quantum Chemistry: From Doubts to Enthusiasm
2.3. Empirical Correlations: Vaporisation Enthalpies vs. Kovats Retention Indices
2.4. Empirical Correlations: Vaporisation Enthalpies vs. Normal Boiling Temperatures
3. Results and Discussion
3.1. Quantum Chemistry: Theoretical Enthalpies of Formation
3.2. Empirical Correlations to Assess Vaporisation Enthalpies of Alkyl-Cyclohexanes
3.3. Liquid-Phase Enthalpies of Formation and Thermodynamic Analysis of the Dehydrogenation/Hydrogenation of the Alkyl-Cyclohexane-Based LOHC Systems
3.4. Absolute Vapour Pressures of Alkyl-Cyclohexane Based LOHC Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef]
- Müller, K.; Aslam, R.; Fikrt, A.; Krieger, C.; Arlt, W. Water Removal from LOHC Systems. Hydrogen 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Siewert, R.; Pimerzin, A.A. Energetics of LOHC: Structure-Property Relationships from Network of Thermochemical Experiments and in Silico Methods. Hydrogen 2021, 2, 101–121. [Google Scholar] [CrossRef]
- Cao, T.; Lee, W.; Huang, R.; Gorte, R.J.; Vohs, J.M. Liquid-Organic hydrogen carriers as endothermic fuels. Fuel 2022, 313, 123063. [Google Scholar] [CrossRef]
- Konnova, M.E.; Li, S.; Bösmann, A.; Müller, K.; Wasserscheid, P.; Andreeva, I.V.; Turovtzev, V.V.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. Thermochemical Properties and Dehydrogenation Thermodynamics of Indole Derivates. Ind. Eng. Chem. Res. 2020, 59, 20539–20550. [Google Scholar] [CrossRef]
- Kondratev, S.O.; Zaitsau, D.H.; Vostrikov, S.V.; Li, S.; Bösmann, A.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Thermochemical properties of 6,7-benzindole and its perhydrogenated derivative: A model component for liquid organic hydrogen carriers. Fuel 2022, 324, 124410. [Google Scholar] [CrossRef]
- Safronov, S.P.; Vostrikov, S.V.; Samarov, A.A.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Comprehensive thermodynamic study of substituted indoles/perhydro indoles as potential liquid organic hydrogen carrier system. Fuel 2023, 331, 125764. [Google Scholar] [CrossRef]
- Müller, K.; Skeledzic, T.; Wasserscheid, P. Strategies for Low-Temperature Liquid Organic Hydrogen Carrier Dehydrogenation. Energy Fuels 2021, 35, 10929–10936. [Google Scholar] [CrossRef]
- Pedley, J.B.; Naylor, R.D.; Kirby, S.P. Thermochemical Data of Organic Compounds; Hapman and Hall: New York, NY, USA, 1986; pp. 1–792. [Google Scholar]
- Jang, M.; Jo, Y.S.; Lee, W.J.; Shin, B.S.; Sohn, H.; Jeong, H.; Jang, S.C.; Kwak, S.K.; Kang, J.W.; Yoon, C.W. A High-Capacity, Reversible Liquid Organic Hydrogen Carrier: H2-Release Properties and an Application to a Fuel Cell. ACS Sustain. Chem. Eng. 2019, 7, 1185–1194. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Emel’yanenko, V.N.; Pimerzin, A.A.; Verevkin, S.P. Benchmark properties of biphenyl as a liquid organic hydrogen carrier: Evaluation of thermochemical data with complementary experimental and computational methods. J. Chem. Thermodyn. 2018, 122, 1–12. [Google Scholar] [CrossRef]
- Konnova, M.E.; Vostrikov, S.V.; Pimerzin, A.A.; Verevkin, S.P. Thermodynamic analysis of hydrogen storage: Biphenyl as affordable liquid organic hydrogen carrier (LOHC). J. Chem. Thermodyn. 2021, 159, 106455. [Google Scholar] [CrossRef]
- Steele, W.; Chirico, R.D.; Smith, N.K. The standard enthalpies of formation of 2-methylbiphenyl and diphenylmethane. J. Chem. Thermodyn. 1995, 27, 671–678. [Google Scholar] [CrossRef]
- Chirico, R.D.; Steele, W.V. Thermodynamic Properties of Diphenylmethane. J. Chem. Eng. Data 2005, 50, 1052–1059. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Vostrikov, S.V.; Leinweber, A.; Bösmann, A.; Wasserscheid, P.; Müller, K. Thermochemical properties of benzyl toluenes and their hydrogenated counter-parts as potential LOHC. Fuel 2023, 335, 126618. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/Chemistry/ (accessed on 5 December 2022).
- Serijan, K.T.; Wise, P.H. Dicyclic Hydrocarbons. III. Diphenyl- and Dicyclohexylalkanes through C15. J. Am. Chem. Soc. 1951, 73, 4766–4769. [Google Scholar] [CrossRef]
- Wise, C.H.; Serijan, K.T.; Goodman, I.A. NACA Technical Report 1003. NACA Tech. Rep. 1951, 1003, 1–10. [Google Scholar]
- Gollis, M.H.; Belenyessy, L.I.; Gudzinowicz, B.J.; Koch, S.D.; Smith, J.O.; Wineman, R.J. Evaluation of Pure Hydrocarbons as Jet Fuels. J. Chem. Eng. Data 1962, 7, 311–316. [Google Scholar] [CrossRef]
- Verevkin, S.P. Gibbs Energy and Helmholtz Energy. In Gibbs Energy and Helmholtz Energy: Liquids, Solutions and Vapours; Wilhelm, E., Letcher, T.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; ISBN 978-1-83916-201-5. [Google Scholar]
- Verevkin, S.P.; Zaitsau, D.H.; Schick, C.; Heym, F. Development of Direct and Indirect Methods for the Determination of Vaporization Enthalpies of Extremely Low-Volatile Compounds. In Handbook of Thermal Analysis and Calorimetry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 1–46. [Google Scholar]
- Verevkin, S.P.; Emel’Yanenko, V.N.; Diky, V.; Muzny, C.D.; Chirico, R.D.; Frenkel, M. New Group-Contribution Approach to Thermochemical Properties of Organic Compounds: Hydrocarbons and Oxygen-Containing Compounds. J. Phys. Chem. Ref. Data 2013, 42, 033102. [Google Scholar] [CrossRef] [Green Version]
- Benson, S.W. Thermochemical Kinetics, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1976; pp. 1–320. [Google Scholar]
- Ducros, M.; Gruson, J.F.; Sannier, H. Estimation des enthalpies de vaporisation des composes organiques liquides. Partie 1. Applications aux alcanes, cycloalcanes, alcenes, hydrocarbures benzeniques, alcools, alcanes thiols, chloro et bromoalcanes, nitriles, esters, acides et al.dehydes. Thermochim. Acta 1980, 36, 39–65. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emelyanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-Toluene Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Turovtsev, V.V.; Andreeva, I.V.; Orlov, Y.D.; Pimerzin, A.A. Webbing a network of reliable thermochemistry around lignin building blocks: Tri-methoxy-benzenes. RSC Adv. 2021, 11, 10727–10737. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Andreeva, I.V.; Zherikova, K.V.; Pimerzin, A.A. Prediction of thermodynamic properties: Centerpiece approach—How do we avoid confusion and get reliable results? J. Therm. Anal. Calorim. 2022, 147, 8525–8534. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Heintz, A.; Stark, K.; Arlt, W. Liquid Organic Hydrogen Carriers: An Upcoming Alternative to Conventional Technologies. Thermochemical Studies. Ind. Eng. Chem. Res. 2012, 51, 12150–12153. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N.; Schleyer, P.v.R.; Allen, W.D. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef] [Green Version]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Pacáková, V.; Felti, L. Chromatographic Retention Indices: An Aid to Identification of Organic Compounds. In Ellis Horwood Series in Analytical Chemistry; Ellis Horwood Ltd.: Chichester, UK, 1992. [Google Scholar]
- The Pherobase: Database of Pheromones and Semiochemicals. Available online: https://www.pherobase.com/Database/Kovats/ (accessed on 5 December 2022).
- Verevkin, S.P. Vapour pressures and enthalpies of vaporization of a series of the linear n-alkyl-benzenes. J. Chem. Thermodyn. 2006, 38, 1111–1123. [Google Scholar] [CrossRef]
- Benson, S.W. New Methods for Estimating the Heats of Formation, Heat Capacities, and Entropies of Liquids and Gases. J. Phys. Chem. A 1999, 103, 11481–11485. [Google Scholar] [CrossRef]
- SciFinder—Chemical Abstracts Service. Available online: http://scifinder.cas.org/ (accessed on 5 December 2022).
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K.; Rassolov, V.; Pople, J.A. Gaussian-3 theory using reduced Mo/ller-plesset order. J. Chem. Phys. 1999, 110, 4703–4709. [Google Scholar] [CrossRef] [Green Version]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Verevkin, S.P.; Emel’yanenko, V.N.; Pimerzin, A.A.; Vishnevskaya, E.E. Thermodynamic Analysis of Strain in the Five-Membered Oxygen and Nitrogen Heterocyclic Compounds. J. Phys. Chem. A 2011, 115, 1992–2004. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Kozlova, S.A.; Emel’yanenko, V.N.; Goodrich, P.; Hardacre, C. Thermochemistry of Ionic Liquid-Catalyzed Reactions. Experimental and Theoretical Study of Chemical Equilibria of Isomerization and Transalkylation of Tert -Butylbenzenes. J. Phys. Chem. A 2008, 112, 11273–11282. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemical investigation on α-methyl-styrene and parent phenyl substituted alkenes. Thermochim. Acta 1999, 326, 17–25. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- Fuchs, R.; Peacock, L.A. Heats of vaporization of monoalkylcyclohexanes by the gas chromatography—Calorimetry method. Can. J. Chem. 1978, 56, 2493–2498. [Google Scholar] [CrossRef]
- Reid, R.C.; Wilhort, R.C. Handbook on Vapor Pressure and Heats of Vaporization of Hydrocarbons and Related Compounds; Wilhort, R.C., R.C., Zwolinski, B.J., Eds.; Texas A&M Research Foundation: College Station, TX, USA, 1972; Volume 18, p. 1278. [Google Scholar] [CrossRef]
- Antheaume, J.; Guiochon, G. Application de La Chromatographie En Phase Gazeuse à l’étude de La Composition Des Fractions Moyennes d’un Brut Pétrolier. Bull. Soc. Chim. Fr. 1965, 2, 298–307. [Google Scholar]
- Rang, S.; Orav, A.; Kunigas, K.; Eisen, O. Capillary gas chromatography of monosubstituted cyclopentenes and cyclohexenes. Chromatographia 1977, 10, 115–122. [Google Scholar] [CrossRef]
- Beens, J.; Tijssen, R.; Blomberg, J. Prediction of comprehensive two-dimensional gas chromatographic separations: A theoretical and practical exercise. J. Chromatogr. A 1998, 822, 233–251. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 5 December 2022).
- Guidechem Chemical Network. Available online: https://www.guidechem.com (accessed on 5 December 2022).
- Månsson, M.; Sellers, P.; Stridh, G.; Sunner, S. Enthalpies of vaporization of some 1-substituted n-alkanes. J. Chem. Thermodyn. 1977, 9, 91–97. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hesse, D.G.; Hosseini, S.; Liebman, J.F.; David Mendenhall, G.; Verevkin, S.P.; Rakus, K.; Beckhaus, H.-D.; Rüchardt, C. Enthalpies of vaporization of some highly branched hydrocarbons. J. Chem. Thermodyn. 1995, 27, 693–705. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hyman, A.S.; Ladon, L.H.; Liebman, J.F. Measurement and estimation of the heats of vaporization of hydrocarbons. J. Org. Chem. 1981, 46, 4294–4296. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemical properties of branched alkylsubstituted benzenes. J. Chem. Thermodyn. 1998, 30, 1029–1040. [Google Scholar] [CrossRef]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Determination of Vapor Pressures and Vaporization Enthalpies of the Aliphatic Branched C5 and C6 Alcohols. J. Chem. Eng. Data 2001, 46, 1593–1600. [Google Scholar] [CrossRef]
- Willingham, C.B.; Taylor, W.J.; Pignocco, J.M.; Rossini, F.D. Vapor pressures and boiling points of some paraffin, alkylcyclopentane, alkylcyclohexane, and alkylbenzene hydrocarbons. J. Res. Natl. Bur. Stand. 1945, 35, 219. [Google Scholar] [CrossRef]
- QS Study. Available online: https://qsstudy.com/vapour-pressure-of-diesel-fuel/ (accessed on 5 December 2022).
- Stephenson, R.M.; Malanowski, S. Handbook of the Thermodynamics of Organic Compounds; Springer: Dordrecht, The Netherlands, 1987; ISBN 978-94-010-7923-5. [Google Scholar]
- Osborn, A.G.; Douslin, D.R. Vapor-Pressure Relations for 15 Hydrocarbons. J. Chem. Eng. Data 1974, 19, 114–117. [Google Scholar] [CrossRef]
- Lainez, A.; Rodrigo, M.M.; Wilhelm, E.; Grolier, J.P.E. Excess Volumes and Excess Heat Capacities of Some Mixtures with Trans,Trans,Cis-1,5,9-Cyclododecatriene at 298.15 K. J. Chem. Eng. Data 1989, 34, 332–335. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Verevkin, S.P. Vapor Pressure of Pure Methyl Oleate—the Main Componene of Biodiesel. Russ. J. Gen. Chem. 2021, 91, 2061–2068. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat Capacity Corrections to a Standard State: A Comparison of New and Some Literature Methods for Organic Liquids and Solids. Struct. Chem. 1993, 4, 271–278. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2015. Part 1. C 1−C 10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Verevkin, S.P.; Sazonova, A.Y. Vapor Pressures and Vaporization Enthalpies of 5-Nonanone, Linalool and 6-Methyl-5-Hepten-2-One. Data Evaluation. Fluid Phase Equilib. 2015, 386, 140–148. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu-Wu, A.; Chickos, J.S.; Leitão, M.L.P.; Roux, M.V.; Torres, L.A. Reference Materials for Calorimetry and Differential Thermal Analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Ambrose, D.; Lawrenson, I.J.; Angus, S.; Paule, R.C. Recommended Reference Materials for Realization of Physicochemical Properties; Herington, E.F.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; ISBN 978-0-08-022340-7. [Google Scholar]
- Marsh, K.N. Recommended Reference Materials for the Realization of Physicochemical Properties; Blackwell Scientific Publications: Oxford, UK; Boston, MA, USA, 1987; ISBN 063201718X 9780632017188. [Google Scholar]
- Clarke, E.C.W.; Glew, D.N. Evaluation of Thermodynamic Functions from Equilibrium Constants. Trans. Faraday Soc. 1966, 62, 539. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Santos, L.M.N.B.F. The Design, Construction, and Testing of a New Knudsen Effusion Apparatus. J. Chem. Thermodyn. 2006, 38, 778–787. [Google Scholar] [CrossRef]
- Malaspina, L.; Gigli, R.; Bardi, G. Microcalorimetric Determination of the Enthalpy of Sublimation of Benzoic Acid and Anthracene. J. Chem. Phys. 1973, 59, 387–394. [Google Scholar] [CrossRef]
- Davies, M.; Jones, J.I. The Sublimation Pressures and Heats of Sublimation of Some Carboxylic Acids. Trans. Faraday Soc. 1954, 50, 1042–1047. [Google Scholar] [CrossRef]
- Ribeiro Da Silva, M.A.V.; Cabral, J.I.T.A.; Gomes, P.; Gomes, J.R.B. Combined Experimental and Computational Study of the Thermochemistry of Methylpiperidines. J. Org. Chem. 2006, 71, 3677–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Site, A.D. The Vapor Pressure of Environmentally Significant Organic Chemicals: A Review of Methods and Data at Ambient Temperature. J. Phys. Chem. Ref. Data 1997, 26, 157–193. [Google Scholar] [CrossRef] [Green Version]
- Shiu, W.-Y.; Ma, K.-C. Temperature Dependence of Physica-Chemical Properties of Selected Chemicals of Environmental Interest. I. Mononuclear and Polynuclear Aromatic Hydrocarbons. J. Phys. Chem. Ref. Data 2000, 29, 41–130. [Google Scholar] [CrossRef]
- Růžička, K.; Fulem, M.; Růžička, V. Recommended Vapor Pressure of Solid Naphthalene. J. Chem. Eng. Data 2005, 50, 1956–1970. [Google Scholar] [CrossRef]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Enthalpies of Vaporization of a Series of Aliphatic Alcohols. Fluid Phase Equilib. 2001, 192, 187–207. [Google Scholar] [CrossRef]






| (liq) Dicyclohexyl-Methane | (liq) Diphenyl-Methane | (liq) a | (liq)/H2b |
|---|---|---|---|
| −824.0 [17] | 96.6 ± 0.8 [13,14,15] | 920.6 | 153.4 |
| −295.0 [18] | 96.6 ± 0.8 [13,14,15] | 391.6 | 65.3 |
| −375.1 [19] | 96.6 ± 0.8 [13,14,15] | 471.7 | 78.6 |
| −307.1 ± 2.0 c | 96.6 ± 0.8 [13,14,15] | 403.7 | 67.3 |
| Compound | (g, AT)G3MP2a | (g)expb | (g)theorc | Δ d |
|---|---|---|---|---|
| cyclohexane | −122.6 | −123.4 ± 0.8 | −122.1 | −1.3 |
| Me-cyclohexane | −154.7 | −154.7 ± 1.0 | −153.3 | −1.4 |
| trans-1,2-di-Me-cyclohexane | −182.8 | −179.9 ± 1.9 | −180.5 | 0.6 |
| cis-1,2-di-Me-cyclohexane | −175.9 | −172.1 ± 1.8 | −173.8 | 1.7 |
| trans-1,3-di-Me-cyclohexane | −179.3 | −176.5 ± 1.7 | −177.1 | 0.6 |
| cis-1,3-di-Me-cyclohexane | −186.7 | −184.6 ± 1.8 | −184.3 | −0.3 |
| trans-1,4-di-Me-cyclohexane | −186.6 | −184.5 ± 1.8 | −184.2 | −0.3 |
| cis-1,4-di-Me-cyclohexane | −179.4 | −176.6 ± 1.8 | −177.2 | 0.6 |
| Et-cyclohexane | −173.8 | −171.7 ± 1.6 | −171.8 | 0.1 |
| n-Pr-cyclohexane | −195.3 | −192.5 ± 1.0 | −192.6 | 0.1 |
| n-Bu-cyclohexane | −216.8 | −213.3 ± 1.3 | −213.5 | 0.2 |
| n-Dec-cyclohexane | −344.9 | −339.5 ± 2.5 | −337.8 | −1.7 |
| n-Dodec-cyclohexane | −387.6 | −378.7 ± 3.7 | −379.2 | 0.5 |
| trans-1,4-di-tBu-cyclohexane | −330.5 | −323.2 ± 2.4 | −323.8 | 0.6 |
| 1,3,5-tri-Me-cyclohexane | −218.8 | (−212.1 ± 3.2) | −215.4 | |
| n-Heptyl-cyclohexane | −280.9 | (−289.2 ± 2.4) | −275.7 | |
| trans-4-Me-iPr-cyclohexane | −229.7 | (−230.7 ± 3.2) | −226.0 | |
| 1,2,3-trimethyl-cyclohexane | −208.7 | −205.6 | ||
| dicyclohexyl-methane | −246.0 | −241.8 | ||
| perhydro-dibenzyl-toluene | −388.1 [25] | −387.4 ± 7.7 [25] | −379.7 |
| Compound | (g, AT)G3MP2a | (g)expb | (g)theorc | Δ d |
|---|---|---|---|---|
| toluene | 42.7 | 50.4 ± 0.6 | 51.1 | −0.7 |
| ethylbenzene | 23.2 | 29.9 ± 1.1 | 31.6 | −1.7 |
| n-propylbenzene | 0.5 | 7.9 ± 0.8 | 8.9 | −1.0 |
| iso-propylbenzene | −3.9 | 4.0 ± 1.0 | 4.5 | −0.5 |
| n-butylbenzene | −20.9 | −13.1 ± 1.1 | −12.5 | −0.6 |
| iso-butylbenzene | −30.7 | −21.5 ± 1.3 | −22.3 | 0.8 |
| tert-butylbenzene | −31.9 | −23.7 ± 0.6 [41] | −23.5 | −0.2 |
| sec-butylbenzene | −28.4 | −17.4 ± 1.2 | −20.0 | 2.6 |
| α-methylstyrene | 109.2 | 119.0 ± 0.9 [42] | 117.6 | 1.4 |
| Trans-Alkyl-Cyclohexane | (g)CPb | (g) c | Δ d |
|---|---|---|---|
| 1,4-diMe-cyclohexane | −186.0 ± 2.0 | −184.5 ± 1.8 [9] | 1.5 |
| 1,3,5-tri-Me-cyclohexane | −217.3 ± 2.0 | −215.4 ± 2.0 | 1.9 |
| 3-Me-1-Et-cyclohexane | −203.0 ± 2.0 | −203.2 ± 1.7 [9] | −0.2 |
| 1,3-di-iso-propyl-cyclohexane | −264.6 ± 2.0 | −268.8 ± 2.0 | −4.2 |
| 1,4-di-iso-propyl-cyclohexane | −264.6 ± 2.0 | −267.8 ± 2.0 | −3.2 |
| 1,3,5-tri-iso-propyl-cyclohexane | −335.2 ± 2.0 | −338.5 ± 2.0 | −3.3 |
| 1,3-di-tert-butyl-cyclohexane | −321.8 ± 2.0 | −324.4 ± 2.0 | −2.6 |
| 1,4-di-tert-butyl-cyclohexane | −321.8 ± 2.0 | −323.8 ± 2.0 | −2.0 |
| Trans-Alkyl-Cyclohexane | (CP) b | (exp) | Δ c |
|---|---|---|---|
| trans-1,4-di-Me-cyclohexane | 37.7 ± 1.5 | 38.0 ± 0.2 | −0.3 |
| trans-4-Me-Et-cyclohexane | 42.9 ± 1.5 | 44.3 ± 1.1 | −1.4 |
| 1,4-di-Et-cyclohexane | 48.1 ± 1.5 | 50.7 ± 1.1 | −2.6 |
| 1,3,5-tri-Me-cyclohexane | 41.5 ± 1.5 | 43.5 ± 1.5 | −2.0 |
| trans-4-Me-iPr-cyclohexane | 46.3 ± 1.5 | 46.8 ± 1.5 | −0.5 |
| 1,4-di-iPr-cyclohexane | 54.9 ± 1.5 | 54.9 ± 1.5 | 0.0 |
| 1,3,5-tri-iPr-cyclohexane | 65.8 ± 1.5 | 64.3 ± 1.5 | 1.5 |
| trans-4-Me-tBu-cyclohexane | 49.3 ± 1.5 | 51.3 ± 1.5 | −2.0 |
| trans-1,4-di-tBu-cyclohexane | 60.9 ± 1.5 | 61.3 ± 1.5 | −0.4 |
| 1,3,5-tri-tBu-cyclohexane | 74.8 ± 1.5 | 71.9 ± 1.5 | 2.9 |
| (exp) | (Jx) a | (Tb) b | (Average) c | |
|---|---|---|---|---|
| kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| cyclohexane | 33.1 ± 0.2 [43] | 32.6 | - | |
| Me-cyclohexane | 35.4 ± 0.2 [43] | 34.9 | 34.5 | |
| trans-1,2-di-Me-cyclohexane | 38.4 ± 0.2 [43] | 38.7 | 38.8 | |
| cis-1,2-di-Me-cyclohexane | 39.7 ± 0.2 [43] | 40.3 | 40.1 | |
| trans-1,3-di-Me-cyclohexane | 39.2 ± 0.2 [43] | 39.0 | 39.0 | |
| cis-1,3-di-Me-cyclohexane | 37.8 | 38.2 | 38.0 ± 1.1 | |
| trans-1,4-di-Me-cyclohexane | 38.0 ± 0.2 [43] | 37.7 | 38.1 | |
| cis-1,4-di-Me-cyclohexane | 39.1 ± 0.2 [43] | 38.9 | 39.0 | |
| trans-2-Me-Et-cyclohexane | 44.2 | 44.3 | 44.3 ± 1.1 | |
| cis-2-Me-Et-cyclohexane | 44.6 | 44.9 | 44.8 ± 1.1 | |
| trans-3-Me-Et-cyclohexane | 44.0 | 43.7 | 43.9 ± 1.1 | |
| cis-3-Me-Et-cyclohexane | 44.7 ± 0.9 [Table S5] | 43.2 | 43.5 | |
| trans-4-Me-Et-cyclohexane | 43.4 | 43.8 | 43.6 ± 1.1 | |
| cis-4-Me-Et-cyclohexane | 44.2 | 44.5 | 44.4 ± 1.1 | |
| Et-cyclohexane | 40.6 ± 0.2 [43] | 39.9 | 40.4 | |
| 1,2-di-Et-cyclohexane | 49.5 | 49.1 | 49.3 ± 1.1 | |
| 1,3-di-Et-cyclohexane | 48.4 | 48.3 | 48.4 ± 1.1 | |
| 1,4-di-Et-cyclohexane | 50.5 | 50.8 | 50.7 ± 1.1 | |
| i-Bu-cyclohexane | 47.6 ± 0.2 [43] | 47.8 | 48.0 | |
| n-Pr-cyclohexane | 45.1 ± 0.2 [43] | 44.9 | 45.2 | |
| 1,3,5-tri-Me-cyclohexane | 43.5 ± 1.5 [Table S5] | 41.3 | 42.1 | |
| 1,2,4,5-tetra-Me-cyclohexane | 49.3 | 48.4 | 48.9 ± 1.1 | |
| penta-Me-cyclohexane | 51.1 | - | 51.1 ± 1.5 | |
| hexa-Me-cyclohexane | 56.9 | - | 56.9 ± 1.5 | |
| n-Bu-cyclohexane | 49.4 ± 0.2 [43] | 50.6 | 49.9 | |
| n-Pe-cyclohexane | 53.9 ± 0.2 [43] | 54.2 | 54.3 | |
| n-Hex-cyclohexane | 59.0 ± 0.5 [44] | 59.0 | 58.3 | |
| n-Hep-cyclohexane | 63.7 ± 0.5 [44] | 64.4 | 62.3 | |
| n-Oct-cyclohexane | 69.8 ± 1.0 [45] | 69.4 | - | |
| n-Dec-cyclohexane | 78.8 ± 0.8 [44] | 78.5 | - | |
| n-Dodec-cyclohexane | 88.9 ± 0.8 [44] | 87.5 | - | |
| n-tetra-Dec-cyclohexane | 99.4 ± 1.0 [45] | 99.9 | - | |
| iso-Pr-cyclohexane | 44.0 ± 0.2 [43] | 44.4 | 44.9 | |
| trans-2-Me-iPr-cyclohexane | 48.0 | 48.0 ± 1.5 | ||
| cis-2-Me-iPr-cyclohexane | 48.0 | 48.0 ± 1.5 | ||
| trans-3-Me-iPr-cyclohexane | 48.0 | 47.4 | 47.7 ± 1.1 | |
| cis-3-Me-iPr-cyclohexane | 49.4 | 47.6 | 48.5 ± 1.1 | |
| trans-4-Me-iPr-cyclohexane | 46.8 ± 1.5 [Table S5] | 48.4 | 47.9 | |
| cis-4-Me-iPr-cyclohexane | 48.3 | 48.3 ± 1.5 | ||
| 1,2,3-tri-methyl-cyclohexane | 42.3 | 43.1 | 42.7 ± 1.1 | |
| 1,2-di-iPr-cyclohexane | 54.9 | 54.9 ± 1.5 | ||
| 1,3-di-iPr-cyclohexane | 54.9 | 54.9 ± 1.5 | ||
| 1,4-di-iPr-cyclohexane | 54.9 | 54.9 ± 1.5 | ||
| 1,3,5-tri-iPr-cyclohexane | 64.3 | 64.3 ± 1.5 | ||
| 1,2,4,5-tetra-iPr-cyclohexane | 72.8 | 72.8 ± 1.5 | ||
| t-Bu-cyclohexane | 47.0 ± 0.2 [43] | 47.8 | 48.1 | |
| 2-Me-tBu-cyclohexane | 50.5 | 50.5 ± 1.5 | ||
| 3-Me-t-Bu-cyclohexane | 50.5 | 50.5 ± 1.5 | ||
| trans-4-Me-tBu-cyclohexane | 51.5 | 51.0 | 51.3 ± 1.1 | |
| cis-4-Me-tBu-cyclohexane | 52.1 | 51.4 | 51.8 ± 1.1 | |
| 1,2-di-tBu-cyclohexane | 60.4 | 60.4 ± 1.5 | ||
| 1,3-di-tBu-cyclohexane | 63.3 ± 2.6 [Table S6] | 64.6 | 62.2 | |
| trans-1,4-di-tBu-cyclohexane | 61.3 | 61.3 ± 1.5 | ||
| cis-1,4-di-tBu-cyclohexane | 61.8 | 61.8 ± 1.5 | ||
| 1,3,5-tri-tBu-cyclohexane | 71.9 | 71.9 ± 1.5 | ||
| dicyclohexyl-methane | 64.5 ± 3.5 [Table S6] | 63.8 | 63.9 ± 1.4 c |
| Compound | (liq)HRb | (liq)HLc | (liq) d | /H2e |
|---|---|---|---|---|
| cyclohexane | −156.4 ± 0.8 | 49.0 ± 0.6 | −205.4 ± 1.0 | 68.5 |
| Me-cyclohexane | −190.1 ± 1.0 | 12.4 ± 0.6 | −202.5 ± 1.2 | 67.5 |
| trans-1,2-di-Me-cyclohexane | −218.2 ± 1.9 | −24.4 ± 1.1 | −193.8 ± 2.2 | 64.6 |
| cis-1,2-di-Me-cyclohexane | −211.8 ± 1.8 | −24.4 ± 1.1 | −187.4 ± 2.1 | 62.5 |
| trans-1,3-di-Me-cyclohexane | −215.7 ± 1.7 | −25.4 ± 0.8 | −190.3 ± 1.9 | 63.4 |
| cis-1,3-di-Me-cyclohexane | −222.9 ± 1.8 | −25.4 ± 0.8 | −197.5 ± 2.0 | 65.8 |
| trans-1,4-di-Me-cyclohexane | −222.4 ± 1.8 | −24.4 ± 1.0 | −198.0 ± 2.1 | 66.0 |
| cis-1,4-di-Me-cyclohexane | −215.6 ± 1.8 | −24.4 ± 1.0 | −191.2 ± 2.1 | 63.7 |
| trans-2-Me-Et-cyclohexane | −240.2 ± 0.9 | −46.4 ± 1.0 | −193.8 ± 1.3 | 64.6 |
| cis-2-Me-Et-cyclohexane | −236.2 ± 1.0 | −46.4 ± 1.0 | −189.8 ± 1.4 | 63.3 |
| trans-3-Me-Et-cyclohexane | −247.1 ± 1.2 | −48.7 ± 1.1 | −198.4 ± 1.6 | 66.1 |
| cis-3-Me-Et-cyclohexane | −246.4 ± 0.9 | −49.8 ± 1.3 | −196.6 ± 1.6 | 65.5 |
| trans-4-Me-Et-cyclohexane | −238.9 ± 1.1 | −49.8 ± 1.3 | −189.1 ± 1.7 | 63.0 |
| Et-cyclohexane | −211.9 ± 1.6 | −12.3 ± 0.9 | −199.6 ± 1.8 | 66.5 |
| n-Pr-cyclohexane | −237.4 ± 1.0 | −38.3 ± 0.8 | −199.2 ± 1.3 | 66.4 |
| 1,3,5-tri-Me-cyclohexane | −258.9 ± 2.3 f | −63.4 ± 1.3 | −195.5 ± 2.6 | 65.2 |
| n-Bu-cyclohexane | −263.1 ± 1.3 | −63.2 ± 1.1 | −199.9 ± 1.7 | 66.6 |
| n-Heptyl-cyclohexane | −339.4 ± 2.3 f | −140.9 ± 1.5 g | −198.5 ± 2.7 | 66.2 |
| n-Decyl-cyclohexane | −418.2 ± 2.4 | −218.3 ± 2.3 | −199.9 ± 3.3 | 66.6 |
| n-Dodecyl-cyclohexane | −467.6 ± 3.6 | −269.7 ± 1.5 g | −197.9 ± 3.9 | 66.0 |
| trans-1,4-di-tBu-cyclohexane | −384.5 ± 2.8 | −190.2 ± 2.0 [54] | −194.3 ± 3.5 | 64.8 |
| Compound | p/Pa at 293 K | p/Pa at 323 K | p/Pa at 373 K |
|---|---|---|---|
| cyclohexane [56] | 10,224 | 36,159 | 170,862 |
| 3-Me-ethyl-cyclohexane [Table S5] | 956 | 4874 | 35,685 |
| 4-Me-iso-Pr-cyclohexane [Table S5] | 582 | 3112 | 24,136 |
| 1,4-di-tBu-cyclohexane [Table S6] | 8.3 | 89 | 1578 |
| dicyclohexyl-methane [Table S6] | 4.4 | 48.9 | 960 |
| perhydro-dibenzyltoluene [24] | 0.0024 | 0.08 | 3.8 |
| soy methyl ester (SME) a | 0.007 | 0.17 | 9.5 |
| raps methyl ester (RME) a | 0.001 | 0.04 | 4.8 |
| biodiesel reference sample JRC a | 0.05 | 0.76 | 20.6 |
| fossil-derived diesel [57] | 53 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verevkin, S.P.; Samarov, A.A.; Vostrikov, S.V.; Wasserscheid, P.; Müller, K. Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs. Hydrogen 2023, 4, 42-59. https://doi.org/10.3390/hydrogen4010004
Verevkin SP, Samarov AA, Vostrikov SV, Wasserscheid P, Müller K. Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs. Hydrogen. 2023; 4(1):42-59. https://doi.org/10.3390/hydrogen4010004
Chicago/Turabian StyleVerevkin, Sergey P., Artemiy A. Samarov, Sergey V. Vostrikov, Peter Wasserscheid, and Karsten Müller. 2023. "Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs" Hydrogen 4, no. 1: 42-59. https://doi.org/10.3390/hydrogen4010004
APA StyleVerevkin, S. P., Samarov, A. A., Vostrikov, S. V., Wasserscheid, P., & Müller, K. (2023). Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs. Hydrogen, 4(1), 42-59. https://doi.org/10.3390/hydrogen4010004