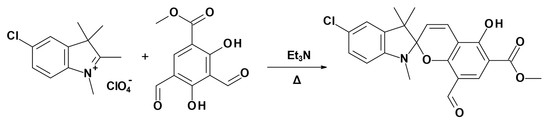
Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feringa, B.L.; Browne, W.R. Molecular Switches; Wiley-VCH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Berman, E.; Fox, R.E.; Thomson, F.D. Photochromic Spiropyrans. I. The Effect of Substituents on the Rate of Ring Closure. J. Am. Chem. Soc. 1959, 81, 5605–5608. [Google Scholar] [CrossRef]
- Rad, J.K.; Balzade, Z.; Mahdavian, A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C 2022, 51, 100487. [Google Scholar]
- Towns, A. Spiropyran dyes. Phys. Sci. Rev. 2021, 6, 20200197. [Google Scholar] [CrossRef]
- Kozlenko, A.S.; Pugachev, A.D.; Ozhogin, I.V.; El-Sewify, I.M.; Lukyanov, B.S. Spiropyrans: Molecules in motion. Chem. Heterocycl. Compd. 2021, 57, 984–989. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugachev, A.D.; Ozhogin, I.V.; Makarova, N.I.; Rostovtseva, I.A.; Lukyanova, M.B.; Kozlenko, A.S.; Borodkin, G.S.; Tkachev, V.V.; El-Sewify, I.M.; Dorogan, I.V.; et al. Novel polychromogenic fluorine-substituted spiropyrans demonstrating either uni- or bidirectional photochromism as multipurpose molecular switches. Dye. Pigm. 2022, 199, 110043. [Google Scholar] [CrossRef]
- Gao, M.; Lian, C.; Xing, R.; Wang, J.; Wang, X.; Tian, Z. The photo-/thermo-chromism of spiropyran in alkanes as a temperature abuse indicator in the cold chain of vaccines. New J. Chem. 2020, 44, 15350–15353. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Mukhanov, E.L.; Ozhogin, I.V.; Kozlenko, A.S.; Metelitsa, A.V.; Lukyanov, B.S. Isomerization and changes of the properties of spiropyrans by mechanical stress: Advances and outlook. Chem. Heterocycl. Compd. 2021, 57, 122–130. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, H.; Zhang, G.; Zhou, Y.; Fan, L.; Shi, L.; Zhang, C.; Shuang, S.; Dong, C. Substituent effect on the acid-induced isomerization of spiropyran compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 13–17. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and biosensing applications of spiropyran and its derivatives—A review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Kato, R.; Yamamoto, H.M. Light-induced superconductivity using a photoactive electric double layer. Science 2015, 347, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Minode, K.; Numai, Y.; Ohto, T.; Yamada, R.; Masai, H.; Tada, H.; Terao, J. Mechanical switching of current–voltage characteristics in spiropyran single-molecule junctions. Nanoscale 2020, 12, 7527–7531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sahoo, P.R.; Kumar, S. A MC-spiropyran for smartphone assisted reversible, selective and nanomolar level detection of formic acid in water and gas phase. J. Mol. Struct. 2021, 1223, 129249. [Google Scholar] [CrossRef]
- Ding, G.; Gai, F.; Gou, Z.; Zuo, Y. Multistimuli-responsive fluorescent probes based on spiropyrans for the visualization of lysosomal autophagy and anticounterfeiting. J. Mater. Chem. B 2022, 10, 4999–5007. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, X.; Zhu, S.; Liu, L.; Li, L. Spiropyran-Functionalized Gold Nanoclusters with Photochromic Ability for Light-Controlled Fluorescence Bioimaging. ACS Appl. Bio Mater. 2021, 4, 2790−2797. [Google Scholar] [CrossRef]
- Cardano, F.; Del Canto, E.; Giordani, S. Spiropyran for light-controlled drug delivery. Dalton Trans. 2019, 48, 15537–15544. [Google Scholar] [CrossRef]
- Barman, S.; Das, J.; Biswas, S.; Maitib, T.K.; Pradeep Singh, N.D. A spiropyran–coumarin platform: An environment sensitive photoresponsive drug delivery system for efficient cancer therapy. J. Mater. Chem. B 2017, 5, 3940–3944. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Zolotukhin, P.V.; Mukhanov, E.L.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Beseda, D.K.; Metelitsa, A.V.; Lukyanov, B.S. Novel molecular hybrids of indolinespiropyrans and α-lipoic acid as potential photopharmacological agents: Synthesis, structure, photochromic and biological properties. Bioorg. Med. Chem. Lett. 2021, 31, 127709. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Tkachev, V.V.; Lukyanov, B.S.; Mukhanov, E.L.; Rostovtseva, I.A.; Lukyanova, M.B.; Shilov, G.V.; Strekal, N.D.; Aldoshin, S.M.; Minkin, V.I. Synthesis, structure and photochromic properties of novel highly functionalized spiropyrans of 1,3-benzoxazin-4-one series. J. Mol. Struc. 2018, 1161, 18–25. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Pugachev, A.D.; Tkachev, V.V.; Kozlenko, A.S.; Chepurnoi, P.B.; Dmitriev, V.S.; Shilov, G.V.; Aldoshin, S.M.; Minkin, V.I.; Lukyanov, B.S. Synthesis and study of interconversions of new indoline spiropyrans based on 4-hydroxy-3,5-diformylbenzoic acid. Russ. Chem. Bull. 2022, 71, 1710–1719. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Chernyavina, V.V.; Lukyanov, B.S.; Malay, V.I.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Lukyanova, M.B.; Metelitsa, A.V.; Aldoshin, S.M. Synthesis and study of new photochromic spiropyrans modified with carboxylic and aldehyde substituents. J. Mol. Struc. 2019, 1196, 409–416. [Google Scholar] [CrossRef]
- Parker, C.A.; Rees, W.T. Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Magde, D.; Brannon, J.H.; Cremers, T.L.; Olmsted, J. Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 1979, 83, 696–699. [Google Scholar] [CrossRef]
- Gel’man, N.E.; Terent’eva, E.A.; Shanina, T.M.; Kiparenko, L.M. Metody Kolichestvennogo Organicheskogo Elementnogo Analiza; Khimiya: Moscow, Russia, 1987. (In Russian) [Google Scholar]



| Compound | Isomer | Absorption λmax, nm (ε·10−3, M−1·cm−1) | τ, s | Fluorescense λmax, nm (Φfl) |
|---|---|---|---|---|
| 1 | SP | 202 (33.4); 253 (47.1); 299 sh (11.2); 341 sh (3.5) | - | - |
| MC | 402; 536 | 105 | 611 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozhogin, I.V.; Pugachev, A.D.; Kozlenko, A.S.; Rostovtseva, I.A.; Makarova, N.I.; Borodkin, G.S.; El-Sewify, I.M.; Metelitsa, A.V.; Lukyanov, B.S. Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank 2023, 2023, M1549. https://doi.org/10.3390/M1549
Ozhogin IV, Pugachev AD, Kozlenko AS, Rostovtseva IA, Makarova NI, Borodkin GS, El-Sewify IM, Metelitsa AV, Lukyanov BS. Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank. 2023; 2023(1):M1549. https://doi.org/10.3390/M1549
Chicago/Turabian StyleOzhogin, Ilya V., Artem D. Pugachev, Anastasia S. Kozlenko, Irina A. Rostovtseva, Nadezhda I. Makarova, Gennady S. Borodkin, Islam M. El-Sewify, Anatoly V. Metelitsa, and Boris S. Lukyanov. 2023. "Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate" Molbank 2023, no. 1: M1549. https://doi.org/10.3390/M1549
APA StyleOzhogin, I. V., Pugachev, A. D., Kozlenko, A. S., Rostovtseva, I. A., Makarova, N. I., Borodkin, G. S., El-Sewify, I. M., Metelitsa, A. V., & Lukyanov, B. S. (2023). Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank, 2023(1), M1549. https://doi.org/10.3390/M1549