Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry
Abstract
:1. Introduction
2. Methods
2.1. Preparatory Phase: Obtaining Knowledge and Forming Green Practice Groups
2.2. Operation: Training Practical Green Chemistry Ability and Fostering the Spirit of Cooperation
2.3. Feedback and Summary: Calculation and Analysis of the Specific Capacitance Data and Finished Lab Reports
3. Hazards
4. Results and Discussion
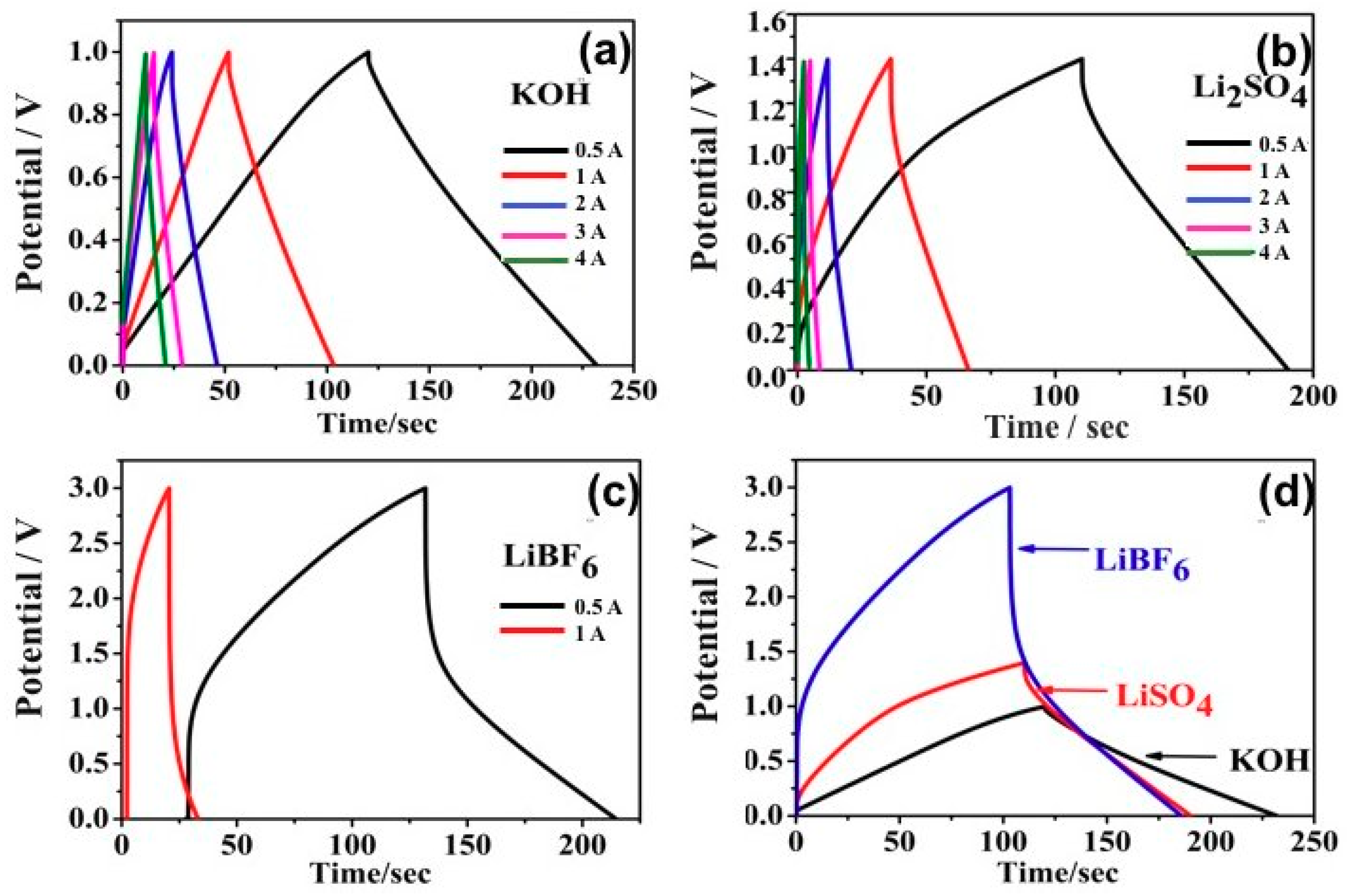
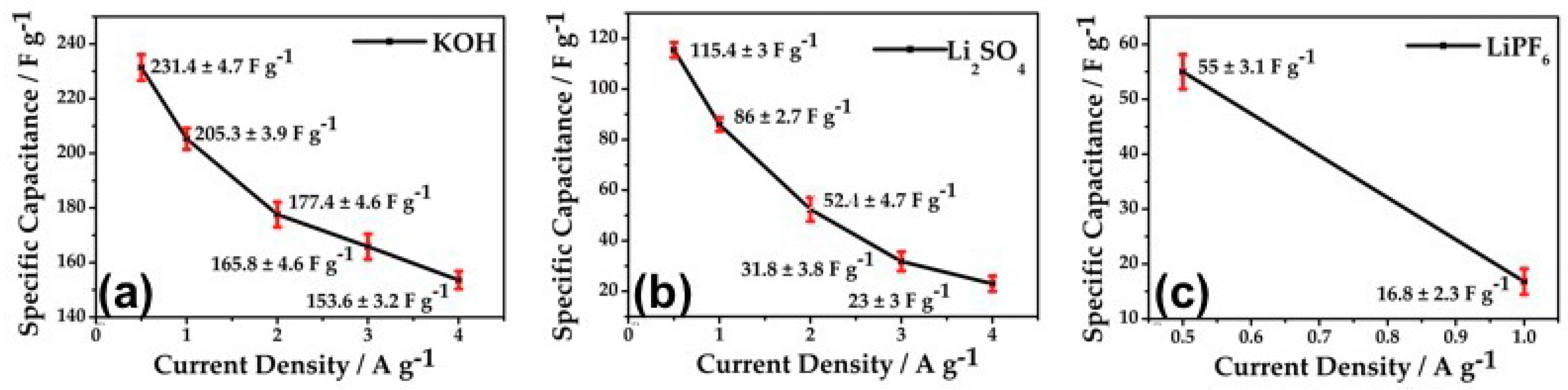
4.1. Independent Study
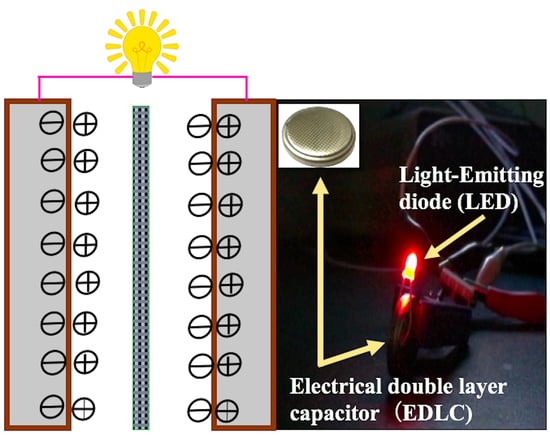
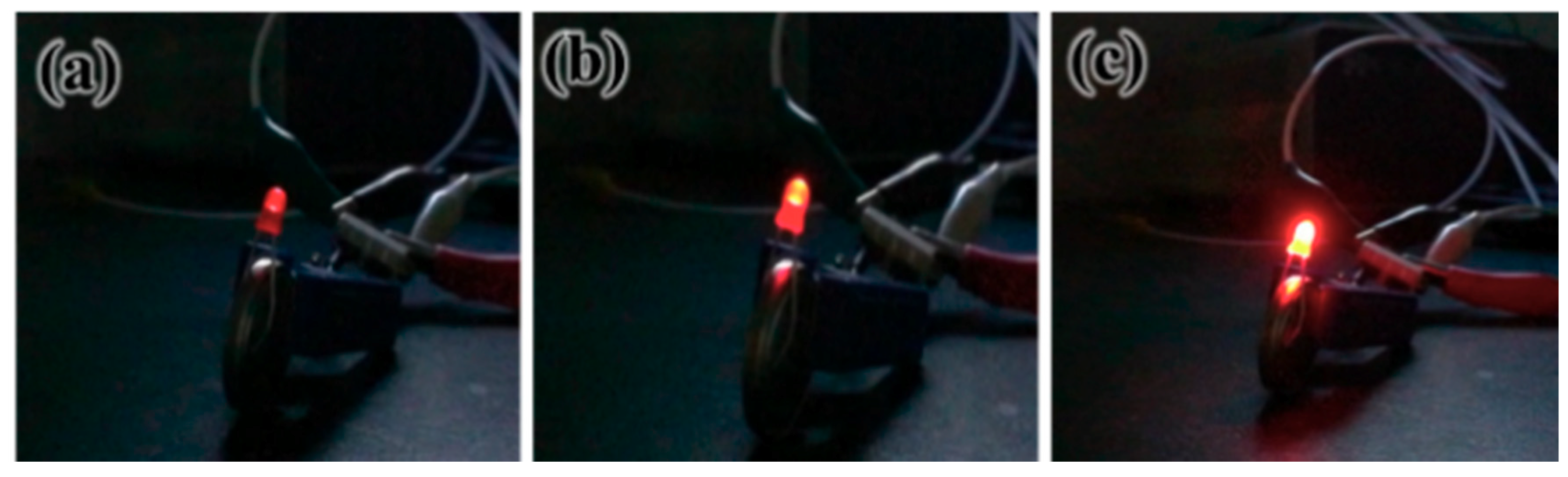
4.2. Experimental Illustration of Green Energy
4.3. Green Chemistry Application and Connecting Ideas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ge, S.G.; Li, B.; Sun, G.Z.; Mei, R.G.; Zheng, L.X. Three-Dimensional Porous LiFePO4: Design, Architectures and High Performance for Lithium Ion Batteries. Curr. Inorg. Chem. 2012, 2, 194–212. [Google Scholar] [CrossRef]
- Hofstein, A.; Lunetta, V.N. The laboratory in science education: Foundations for the twenty-first century. Sci. Educ. 2004, 88, 28–54. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of Gold Nanoparticles Using Tea: A Green Chemistry Experiment. J. Chem. Educ. 2012, 89, 1316–1318. [Google Scholar] [CrossRef]
- Bopegedera, A.M.R.P.; Perera, K.N.R. “Greening” a Familiar General Chemistry Experiment: Coffee Cup Calorimetry to Determine the Enthalpy of Neutralization of an Acid-Base Reaction and the Specific Heat Capacity of Metals. J. Chem. Educ. 2017, 94, 494–499. [Google Scholar] [CrossRef]
- Sjostrom, J.; Eilks, I.; Zuin, V.G. Towards Eco-Reflexive Science Education A Critical Reflection About Educational Implications of Green Chemistry. Sci. Educ. 2016, 25, 321–341. [Google Scholar] [CrossRef]
- Duarte, R.C.C.; Ribeiro, M.G.T.C.; Machado, A.A.S.C. Reaction Scale and Green Chemistry: Microscale or Macroscale, Which Is Greener? J. Chem. Educ. 2017, 94, 1255–1264. [Google Scholar] [CrossRef]
- Shamuganathan, S.; Karpudewan, M. Science writing heuristics embedded in green chemistry: A tool to nurture environmental literacy among pre-university students. Chem. Educ. Res. Pract. 2017, 18, 386–396. [Google Scholar] [CrossRef]
- Ji, H.X.; Zhao, X.; Qiao, Z.H.; Jung, J.; Zhu, Y.W.; Lu, Y.L.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dou, H.; Ding, B.; Wang, J.; Chang, Z.; Xu, Y.L.; Hao, X.D. Nanospace-confined synthesis of oriented porous carbon nanosheets for high-performance electrical double layer capacitors. J. Mater. Chem. 2016, A4, 16879–16885. [Google Scholar] [CrossRef]
- Lee, D.Y.; An, G.H.; Ahn, H.J. High-surface-area tofu based activated porous carbon for electrical double-layer capacitors. J. Ind. Eng. Chem. 2017, 52, 121–127. [Google Scholar] [CrossRef]
- Shen, H.H.; Hu, C.C. Determination of the upper and lower potential limits of the activated carbon/propylene carbonate system for electrical double-layer capacitors. J. Electroanal. Chem. 2016, 779, 161–168. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.-C.; Wang, F.; Wang, G.; Qi, P.-R.; Guo, X.-H.; Dai, B.; Yu, F. An activated carbon derived from tobacco waste for use as a supercapacitor electrode material. New Carbon Mater. 2017, 32, 592–599. [Google Scholar] [CrossRef]
- Murashko, K.; Nevstrueva, D.; Pihlajamaki, A.; Koiranen, T.; Pyrhonen, J. Cellulose and activated carbon based flexible electrical double-layer capacitor electrode: Preparation and characterization. Energy 2017, 119, 435–441. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhao, H.; Yu, F.; Yang, J. Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry. Sustainability 2018, 10, 3630. https://doi.org/10.3390/su10103630
Lin Y, Zhao H, Yu F, Yang J. Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry. Sustainability. 2018; 10(10):3630. https://doi.org/10.3390/su10103630
Chicago/Turabian StyleLin, Yannan, Hongxia Zhao, Feng Yu, and Jinfeng Yang. 2018. "Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry" Sustainability 10, no. 10: 3630. https://doi.org/10.3390/su10103630
APA StyleLin, Y., Zhao, H., Yu, F., & Yang, J. (2018). Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry. Sustainability, 10(10), 3630. https://doi.org/10.3390/su10103630