Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation from Ripe Plantain Peels
2.3. Characterization of Heterogeneous Catalyst
2.4. Experimental Design for the Transesterification Process
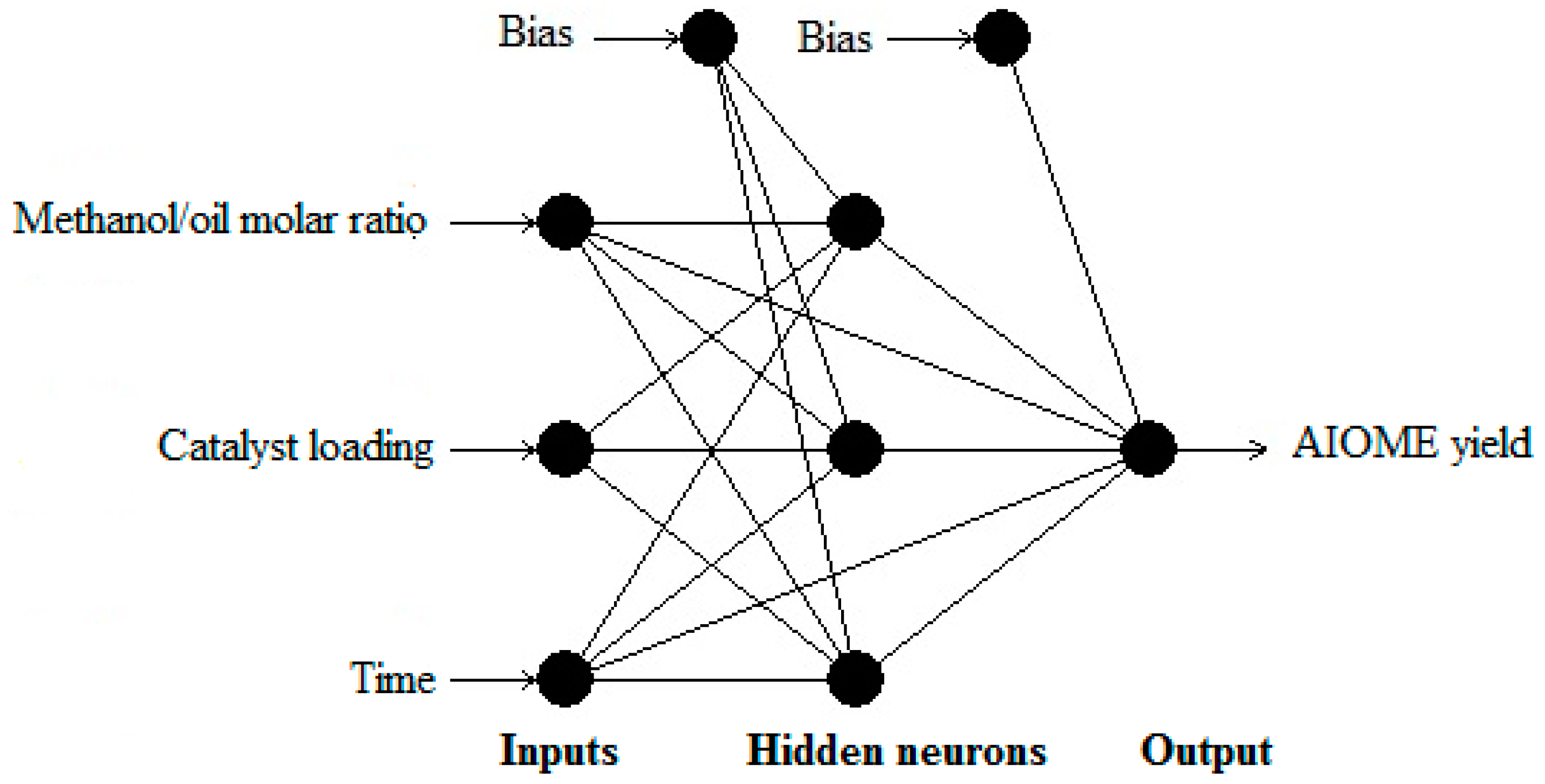
2.5. Model Development and Optimization
2.6. Biodiesel Synthesis via Two-Step Transesterification
2.7. Characterization of AIOME
3. Results and Discussion
3.1. CRPPA Characterization
3.2. AIOME Synthesis Using CRPPA
3.2.1. Parameters Optimization for AIOME Synthesis
3.2.2. Interactions of Independent Variables
3.3. AIOME Quality Characterization and Its Fatty Acid Composition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIOME | Azadirachta indica oil methyl esters |
| ANN | artificial neural network |
| ANOVA | analysis of variance |
| BET | Brunauer-Emmett-Teller |
| BJH | Barret-Joyner-Halenda |
| CCD | central composite design |
| CRPPA | calcined ripe plantain peel ash |
| FT-IR | Fourier transform infra-red |
| GA | genetic algorithm |
| IBP | incremental back propagation |
| MFFF | multilayer full feedforward |
| MNFF | multilayer normal feed forward |
| MRPD | mean relative percentage deviation |
| RPP | raw ripe plantain peel |
| R2 | coefficient of determination |
| RSM | response surface methodology |
| SEM | scanning electron microscope |
| XRD | X-ray diffraction |
References
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties†. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel derived from a model oil enriched in palmitoleic acid, macadamia nut oil. Energy Fuels 2010, 24, 2098–2103. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Daramola, M.O. Transesterification of rubber seed oil to biodiesel over a calcined waste rubber seed shell catalyst: Modeling and optimization of process variables. Energy Fuels 2017, 31, 6109–6119. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-step conversion of neem (Azadirachta indica) seed oil into fatty methyl esters using a heterogeneous biomass-based catalyst: An example of cocoa pod husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich-and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Deka, D.C.; Basumatary, S. High quality biodiesel from yellow oleander (Thevetia peruviana) seed oil. Biomass Bioenergy 2011, 35, 1797–1803. [Google Scholar] [CrossRef]
- Betiku, E.; Ajala, S.O. Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology. Ind. Crops Prod. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Vadery, V.; Narayanan, B.N.; Ramakrishnan, R.M.; Cherikkallinmel, S.K.; Sugunan, S.; Narayanan, D.P.; Sasidharan, S. Room temperature production of jatropha biodiesel over coconut husk ash. Energy 2014, 70, 588–594. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Biodiesel production from Jatropha curcas L. oil using Lemna perpusilla Torrey ash as heterogeneous catalyst. Biomass Bioenergy 2013, 55, 386–389. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Kaewpuang, T.; Yoosuk, B.; Promarak, V. Economical and green biodiesel production process using river snail shells-derived heterogeneous catalyst and co-solvent method. Bioresour. Technol. 2016, 209, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Muciño, G.G.; Romero, R.; Ramírez, A.; Martínez, S.L.; Baeza-Jiménez, R.; Natividad, R. Biodiesel production from used cooking oil and sea sand as heterogeneous catalyst. Fuel 2014, 138, 143–148. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Manique, M.C.; Lacerda, L.V.; Alves, A.K.; Bergmann, C.P. Biodiesel production using coal fly ash-derived sodalite as a heterogeneous catalyst. Fuel 2017, 190, 268–273. [Google Scholar] [CrossRef]
- Zobel, C.W.; Cook, D.F. Evaluation of neural network variable influence measures for process control. Eng. Appl. Artif. Intel. 2011, 24, 803–812. [Google Scholar] [CrossRef]
- Rajendra, M.; Jena, P.C.; Raheman, H. Prediction of optimized pretreatment process parameters for biodiesel production using ANN and GA. Fuel 2009, 88, 868–875. [Google Scholar] [CrossRef]
- Avramović, J.M.; Veličković, A.V.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of sunflower oil ethanolysis catalyzed by calcium oxide: RSM versus ANN-GA. Energy Convers. Manag. 2015, 105, 1149–1156. [Google Scholar] [CrossRef]
- Sarve, A.; Sonawane, S.S.; Varma, M.N. Ultrasound assisted biodiesel production from sesame (Sesamum indicum L.) oil using barium hydroxide as a heterogeneous catalyst: Comparative assessment of prediction abilities between response surface methodology (RSM) and artificial neural network (ANN). Ultrason. Sonochem. 2015, 26, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Okunsolawo, S.S.; Ajala, S.O.; Odedele, O.S. Performance evaluation of artificial neural network coupled with generic algorithm and response surface methodology in modeling and optimization of biodiesel production process parameters from shea tree (Vitellaria paradoxa) nut butter. Renew. Energy 2015, 76, 408–417. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ansari, A.; Bahari, F.; Ghaedi, A.; Vafaei, A. A hybrid artificial neural network and particle swarm optimization for prediction of removal of hazardous dye brilliant green from aqueous solution using zinc sulfide nanoparticle loaded on activated carbon. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Omilakin, O.R.; Ajala, S.O.; Okeleye, A.A.; Taiwo, A.E.; Solomon, B.O. Mathematical modeling and process parameters optimization studies by artificial neural network and response surface methodology: A case of non-edible neem (Azadirachta indica) seed oil biodiesel synthesis. Energy 2014, 72, 266–273. [Google Scholar] [CrossRef]
- FAOSTAT. Statistical Databases. Food and Agriculture Organization of the United Nations. Statistics Division. 2014. Available online: www.fao.org/faostat/en/#data/QC (accessed on 14 May 2017).
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel production from vegetable oil using heterogenous acid and alkali catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Olutoye, M.; Lee, S.; Hameed, B. Synthesis of fatty acid methyl ester from palm oil (Elaeis guineensis) with ky(MgCa)2xO3 as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 10777–10783. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Meher, L.; Naik, S.; Das, L. Production of biodiesel from high free fatty acid karanja (Pongamia pinnata) oil. Biomass Bioenergy 2008, 32, 354–357. [Google Scholar] [CrossRef]
- Olabanji, I.O.; Oluyemi, E.A.; Ajayi, O.S. Metal analyses of ash derived alkalis from banana and plantain peels (Musa spp.) in soap making. Afr. J. Biotechnol. 2012, 11, 16512–16518. [Google Scholar]
- Onyegbado, C.; Iyagba, E.; Offor, O. Solid soap production using plantain peel ash as source of alkali. J. Appl. Sci. Environ. Manag. 2002, 6, 73–77. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.; Tuli, D. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Nisar, J.; Razaq, R.; Farooq, M.; Iqbal, M.; Khan, R.A.; Sayed, M.; Shah, A.; Ur Rahman, I. Enhanced biodiesel production from jatropha oil using calcined waste animal bones as catalyst. Renew. Energy 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Lukić, I.; Krstić, J.; Jovanović, D.; Skala, D. Alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour. Technol. 2009, 100, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Wang, Y.; Komatsu, M.; Ookawa, M. Preparation of new solid bases derived from supported metal nitrates and carbonates. Catal. Surv. Jpn. 2002, 5, 81–89. [Google Scholar] [CrossRef]
- Genge, M.J.; Jones, A.P.; Price, G.D. An infrared and Raman study of carbonate glasses: Implications for the structure of carbonatite magmas. Geochim. Cosmochim. Acta 1995, 59, 927–937. [Google Scholar] [CrossRef]
- Piriou, B.; Mcmillan, P. The high-frequency vibrational spectra of vitreous and crystalline orthosilicates. Am. Mineral. 1983, 68, 426–443. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Muthu, H.; SathyaSelvabala, V.; Varathachary, T.; Kirupha Selvaraj, D.; Nandagopal, J.; Subramanian, S. Synthesis of biodiesel from neem oil using sulfated zirconia via tranesterification. Braz. J. Chem. Eng. 2010, 27, 601–608. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]







| Heterogeneous Catalyst Type | Feedstock | Surface Area (m2/g) | Catalyst Dosage (wt %) | Alcohol/Oil Ratio | Temperature (°C) | Time (min) | Yield (wt %) | Reference |
|---|---|---|---|---|---|---|---|---|
| Rubber seed shell | Rubber seed oil | 352.51 | 2.2 | 0.2 a | 60 | 60 | 83.11 b | [4] |
| Cocoa pod husks | Neem oil | 2.76 | 0.65 | 0.73 a | 65 | 57 | 99.3 b | [5] |
| Ostrich-egg shell | Waste cooking oil | 71.00 | 1.5 | 12 c | 65 | 120 | 96 b | [6] |
| Chicken-egg shell | Waste cooking oil | 54.60 | 1.5 | 12 c | 65 | 120 | 94 b | [6] |
| Unripe plantain peels | Yellow oleander oil | NR | 2.8 | 0.3 a | 60 | 75 | 94.97 b | [8] |
| Banana peels | Napoleon’s plume oil | 4.442 | 2.75 | 7.6 c | 65 | 69.02 | 98.50 b | [9] |
| Banana peels | Waste cooking oil | 14.036 | 2 | 6 c | 60 | 180 | 100 b | [10] |
| Coconut husk | Jatropha oil | — | 7 | 12 c | 45 | 45 | 99.86 d | [11] |
| Torrey ash | Jatropha oil | 9.622 | 5.0 | 9 c | 65 | 300 | 89.43 d | [12] |
| River snail shells | Palm oil | 3.495 | 5 | 12 c | 65 | 90 | 98.50 b | [13] |
| Waste chicken bones | Waste cooking oil | 98.54 | 5 | 15 c | 65 | 240 | 89.33 b | [14] |
| Sea sand | Soybean oil | 4.60 | 7.5 | 12 c | 60 | 360 | 97.50 d | [15] |
| Pyrolyzed rice husk | Waste cooking oil | 4.00 | 5 | 20 c | 110 | 900 | 87.57 b | [16] |
| Coal fly ash-derived sodalite | Soy oil | 10.00 | 4 | 12 c | 65 | 120 | 95.50 b | [17] |
| Factor | Unit | Coded Factor Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | ||
| Methanol/oil ratio (X1) | v/v | 0.23 | 0.40 | 0.65 | 0.90 | 1.07 |
| Catalyst loading (X2) | wt % | 0.65 | 1.50 | 2.75 | 4.00 | 4.85 |
| Reaction time (X3) | min | 24.77 | 35 | 50 | 65 | 75.23 |
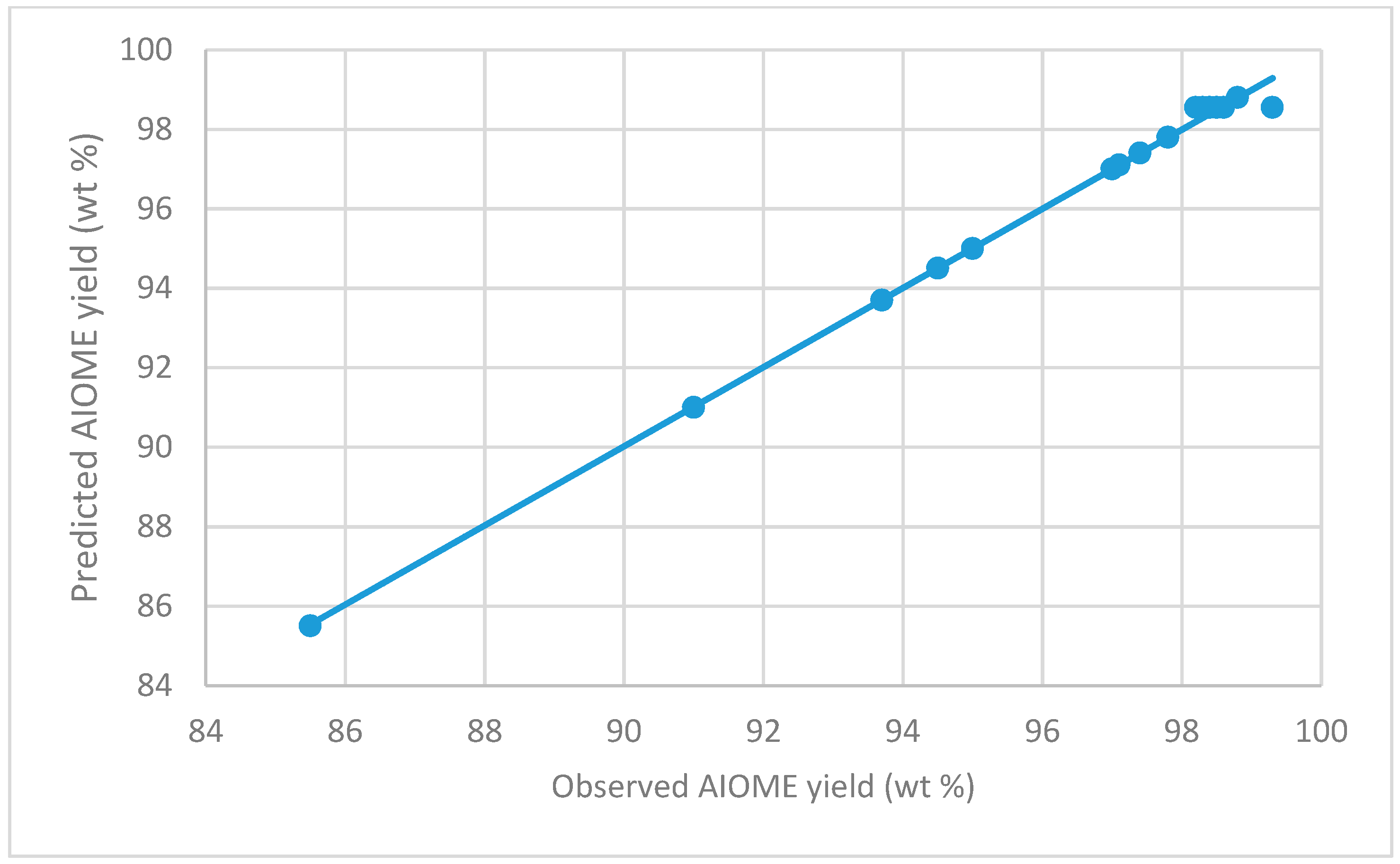
| Run | Methanol/Oil Ratio (v/v) | Catalyst Loading (wt %) | Reaction Time (min) | Observed AIOME (wt %) | Predicted AIOME (wt %) |
|---|---|---|---|---|---|
| 1 * | 0.65 (0) | 0.65 (−α) | 50.00 (0) | 97.00 | 97.00 |
| 2 | 0.65 (0) | 2.75 (0) | 50.00 (0) | 98.50 | 98.55 |
| 3 | 0.40 (−1) | 4.00 (1) | 65.00 (1) | 94.50 | 94.50 |
| 4 | 0.90 (1) | 1.50 (−1) | 65.00 (1) | 98.80 | 98.80 |
| 5 | 0.23 (−α) | 2.75 (0) | 50.00 (0) | 85.50 | 85.50 |
| 6 | 0.65 (0) | 4.85 (+α) | 50.00 (0) | 97.00 | 97.00 |
| 7 | 0.65 (0) | 2.75 (0) | 50.00 (0) | 98.30 | 98.55 |
| 8 * | 0.90 (1) | 4.00 (1) | 35.00 (−1) | 91.00 | 91.00 |
| 9 | 0.40 (−1) | 4.00 (1) | 35.00 (−1) | 97.40 | 97.40 |
| 10 | 0.65 (0) | 2.75 (0) | 75.23 (+α) | 97.10 | 97.10 |
| 11 | 0.65 (0) | 2.75 (0) | 50.00 (0) | 99.30 | 98.55 |
| 12 | 0.90 (1) | 4.00 (1) | 65.00 (1) | 93.70 | 93.70 |
| 13 | 0.65 (0) | 2.75 (0) | 24.77 (−α) | 97.40 | 97.40 |
| 14 | 0.90 (1) | 1.50 (−1) | 35.00 (−1) | 95.00 | 95.00 |
| 15 * | 1.07 (+α) | 2.75 (0) | 50.00 (0) | 95.00 | 95.00 |
| 16 | 0.40 (−1) | 1.50 (−1) | 35.00 (−1) | 97.80 | 97.80 |
| 17 | 0.65 (0) | 2.75 (0) | 50.00 (0) | 98.40 | 98.55 |
| 18 | 0.65 (0) | 2.75 (0) | 50.00 (0) | 98.60 | 98.55 |
| 19 | 0.40 (−1) | 1.50 | 65.00 (1) | 97.80 | 97.80 |
| 20 | 0.65 (0) | 2.75 | 50.00 (0) | 98.20 | 98.55 |
| Temperature | O | Mg | P | S | K | Ca | Si | Cl | Al |
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (wt %) | ||||||||
| 500 | 38.99 | 0.80 | 1.23 | 0.00 | 43.49 | 0.00 | 7.41 | 8.09 | 0.00 |
| 700 | 36.43 | 1.15 | 1.84 | 0.47 | 51.02 | 0.00 | 2.51 | 6.27 | 0.29 |
| 900 | 45.81 | 0.55 | 2.80 | 0.66 | 39.20 | 3.23 | 5.56 | 1.46 | 0.00 |
| 1100 | 40.91 | 0.41 | 5.38 | 1.39 | 47.38 | 0.00 | 1.78 | 2.29 | 0.46 |
| Name | Model | Learning Algorithm | Connection Type | Transfer Function Hidden Layer | Transfer Function Output Layer | R2 Whole Data | MRPD Whole Data (%) |
|---|---|---|---|---|---|---|---|
| H55 | 3-4-1 | IBP a | MFFF b | Sigmoid | Linear | 0.9962 | 8.10 |
| H54 | 3-4-1 | IBP | MNFF c | Sigmoid | Linear | 0.9962 | 8.10 |
| G33 | 3-3-1 | IBP | MNFF | Sigmoid | Linear | 0.9546 | 46.82 |
| G45 | 3-2-1 | IBP | MFFF | Sigmoid | Linear | 0.9220 | 71.76 |
| Parameter | Mean Value | ASTM D6751 | EN 14214 |
|---|---|---|---|
| Moisture content (%) | 0.01 | <0.03 | 0.02 |
| Specific gravity | 0.88 | 0.86–0.90 | 0.85 |
| Kinematic viscosity (mm2/s) at 40 °C | 5.0 | 1.9–6.0 | 3.5–5.0 |
| Acid value (mg KOH/g oil) | 0.45 | 0.5 max | 0.5 max |
| Iodine value (g I2/100 g oil) | 58.6 | NS | 120 max |
| Higher heating value (MJ/kg) | 48.7 | NS | NS |
| Cetane number | 81 | 47 min | 51 min |
| Group I metals (Na + K) (ppm) | 1.80 | 5.0 max | 5.0 max |
| Group II metals (Ca + Mg) (ppm) | 0.42 | 5.0 max | 5.0 max |
| Pour point (°C) | 9 | NS | NS |
| Cloud point (°C) | 21 | NS | NS |
| Flash point (°C) | 274 | 130 min | 101 min |
| Fatty Acid | Structure | % Composition |
|---|---|---|
| Saturated fatty acid | ||
| Palmitic | C16:0 | 14.25 |
| Stearic | C18:0 | 10.85 |
| Arachidic | C20:0 | 0.57 |
| Lignoceric | C24:0 | 0.55 |
| Total | 26.22 | |
| Unsaturated fatty acid | ||
| Palmitoleic | C16:1 | 0.05 |
| Oleic | C18:1 | 14.34 |
| Linoleic | C18:2 | 59.10 |
| Linolenic | C18:3 | 0.30 |
| Total | 73.79 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etim, A.O.; Betiku, E.; Ajala, S.O.; Olaniyi, P.J.; Ojumu, T.V. Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability 2018, 10, 707. https://doi.org/10.3390/su10030707
Etim AO, Betiku E, Ajala SO, Olaniyi PJ, Ojumu TV. Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability. 2018; 10(3):707. https://doi.org/10.3390/su10030707
Chicago/Turabian StyleEtim, Anietie O., Eriola Betiku, Sheriff O. Ajala, Peter J. Olaniyi, and Tunde V. Ojumu. 2018. "Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm" Sustainability 10, no. 3: 707. https://doi.org/10.3390/su10030707
APA StyleEtim, A. O., Betiku, E., Ajala, S. O., Olaniyi, P. J., & Ojumu, T. V. (2018). Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability, 10(3), 707. https://doi.org/10.3390/su10030707





