The Structure and Diversity of Bacterial Communities in Differently Managed Soils Studied by Molecular Fingerprinting Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Study Design
2.2. Total DNA Extraction
2.3. Automated Ribosomal Intergenic Spacer Analysis (ARISA)
2.4. Terminal-Restriction Fragment Length Polymorphism (T-RFLP)
2.5. Statistical Analyses
3. Results
3.1. Total Microbial Biomass and Bacterial Richness
3.2. Bacterial Genetic Diversity
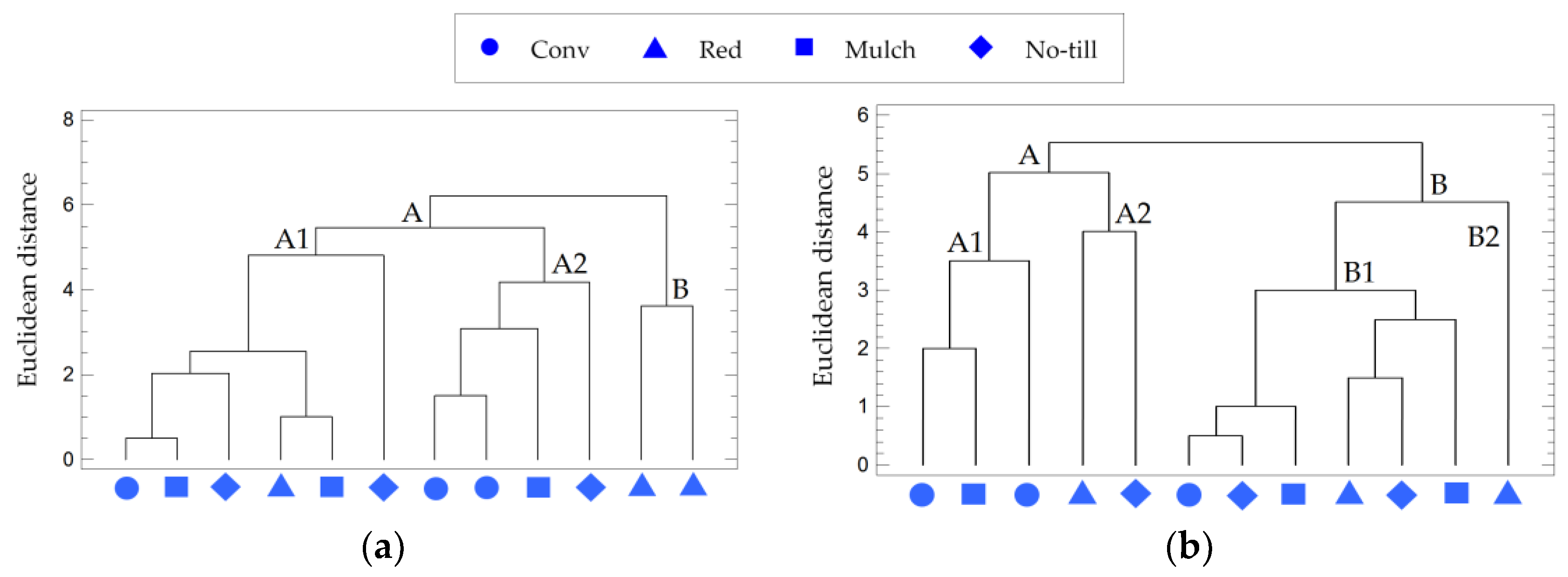
3.3. The Impact of Tillage on Soil Bacterial Communities
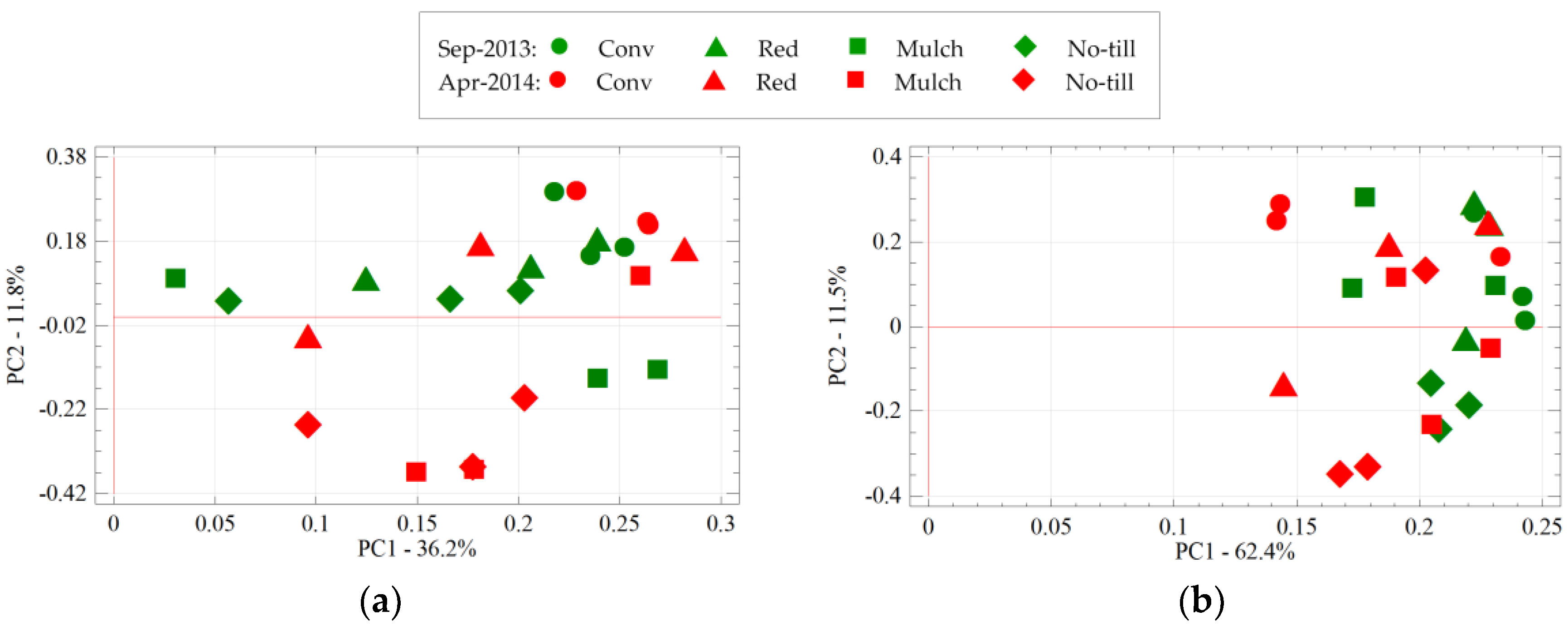
3.4. The Impact of Sampling Time and Tillage on Soil Bacterial Communities
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wall, D.H.; Virginia, R.A. Controls on soil biodiversity: Insights from extreme environments. Appl. Soil Ecol. 1999, 13, 137–150. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Marschner, H.; Jakobsen, I. Role of arbuscular mycorrhizal fungi in uptake of phosphorous and nitrogen from soil. Crit. Rev. Biotechnol. 1995, 15, 257–270. [Google Scholar] [CrossRef]
- Hartmann, M.; Widmer, F. Community structure analysis are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl. Environ. Microbiol. 2006, 72, 7804–7812. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, C.; Demyan, M.S.; Cavani, L.; Marzadori, C.; Ciavatta, C.; Kandeler, E. Chemical and microbiological soil quality indicators and their potential to differentiate fertilization in temperate agroecosystems. Appl. Soil Ecol. 2013, 64, 32–48. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Steenwerth, K.L.; Jackson, L.E.; Scow, K.M. Land use and climatic factors structure regional patterns in soil microbial communities. Glob. Ecol. Biogeogr. 2010, 19, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Collins, H.P. Management of organisms and their processes in soils. In Soil Microbiology, Ecology, and Biochemistry, 3rd ed.; Paul, E.A., Ed.; Academic Press: Oxford, UK, 2007; pp. 471–502. ISBN 9780080475141. [Google Scholar]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Gebhardt, M.R.; Daniel, T.C.; Schweizer, E.E.; Allmaras, R.R. Conservation tillage. Science 1985, 230, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.M.; Chaney, K.; Bradbury, R.B.; Wilcox, A. Non-inversion tillage and farmland birds: A review with special reference to the UK and Europe. IBIS 2004, 146, 192–202. [Google Scholar] [CrossRef]
- Jones, C.; Basch, G.; Bayliss, A.; Bazzoni, D.; Biggs, J.; Bradbury, R.; Chaney, K.; Deeks, L.; Field, R.; Gómez, J.; et al. Conservation Agriculture in Europe: An Approach to Sustainable Crop Production by Protecting Soil and Water? SOWAP: Bracknell, UK, 2006; p. 110. ISBN 9780955656507. [Google Scholar]
- Alletto, L.; Coquet, Y.; Benoit, P.; Heddadj, D.; Barriuso, E. Tillage management effects on pesticide fate in soil. A review. Agron. Sustain. Dev. 2010, 30, 367–400. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Locke, M.A.; Zablotowicz, R.M.; Weaver, M.A. Herbicide fate under conservation tillage, cover crop and edge of field management practices. In Handbook of Sustainable Weed Management; Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; Haworth: Binghampton, NY, USA, 2006; pp. 373–392. ISBN 9781560229575. [Google Scholar]
- Curci, M.; Pizzigallo, M.D.R.; Crecchio, C.; Mininni, R. Effects of conventional tillage on biochemical properties of soils. Biol. Fertil. Soils 1997, 25, 1–6. [Google Scholar] [CrossRef]
- Calderon, F.J.; Jackson, L.E.; Scow, K.M.; Rolston, D.E. Microbial responses to simulated tillage in cultivated and uncultivated soils. Soil Biol. Biochem. 2000, 32, 1547–1559. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Unno, Y.; Sayre, K.D.; Luna-Guido, M.; Vanherck, K.; Dendooven, L.; Deckers, J. Influence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversity. Appl. Soil Ecol. 2007, 37, 18–30. [Google Scholar] [CrossRef]
- Postma, J.; Veen, J.A. Habitable pore space and survival of Rhizobium leguminasorum biovar trifolii introduced into soil. Microb. Ecol. 1990, 19, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Kováč, K.; Kucharovic, A.; Žák, Š.; Hašana, R.; Bušo, R. Bilancia Energo-Materiálových Tokov v Poľnom Ekologicky Citlivom Agroekosystéme (Eng. Balance of Energy-Material Flows in the Ecologically Sensitive Agroecosystems); National Agricultural and Food Centre: Lužianky, Slovakia, 2014; p. 146. ISBN 9788089417537. [Google Scholar]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Zanardini, E.; Sorlini, C.; Corselli, C.; Daffonchio, D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes: Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Teske, A.; Wirsen, C.O. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 1995, 164, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 10 August 2017).
- Hammer, Ø.; Harper, D. Paleontological Data Analysis; Blackwell Publishing: Oxford, UK, 2006; p. 351. ISBN 1405115440. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–656. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Tate, R.L. Soil Microbiology, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000; p. 536. ISBN 9780471317913. [Google Scholar]
- Aislabie, J.; Deslippe, J.R. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand–Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. ISBN 9780478347364. [Google Scholar]
- Constancias, F.; Terrat, S.; Saby, N.P.A.; Horrigue, W.; Villerd, J.; Guillemin, J.P.; Biju-Duval, L.; Nowak, V.; Dequiedt, S.; Ranjard, L.; et al. Mapping and determinism of soil microbial community distribution across an agricultural landscape. Microbiol. Open 2015, 4, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Dequiedt, S.; Thioulouse, J.; Jolivet, C.; Saby, N.P.A.; Lelievre, M.; Maron, P.A.; Martin, M.P.; Prévost-Bouré, N.C.; Toutain, B.; Arrouays, D.; et al. Biogeographical patterns of soil bacterial communities. Environ. Microbiol. Rep. 2009, 1, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Dequiedt, S.; Saby, N.P.A.; Lelievre, M.; Jolivet, C.; Thioulouse, J.; Toutain, B.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Ranjard, L. Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob. Ecol. Biogeogr. 2011, 20, 641–652. [Google Scholar] [CrossRef]
- Enwall, K.; Throbäck, I.N.; Stenberg, M.; Söderström, M.; Hallin, S. Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Appl. Environ. Microbiol. 2010, 76, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; Veen, J.A.; Kowalchuk, G.A. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Wickings, K.; Grandy, A.S.; Kravchenko, A.N. Going with the flow: Landscape position drives differences in microbial biomass and activity in conventional, low input, and organic agricultural systems in the Midwestern U.S. Agric. Ecosyst. Environ. 2016, 218, 1–10. [Google Scholar] [CrossRef]
- Kara, Ö.; Bolat, I. The effect of different land uses on soil microbial biomass carbon and nitrogen in Bartin Province. Turk. J. Agric. For. 2008, 32, 281–288. [Google Scholar]
- Kallenbach, C.; Grandy, A.S. Controls over soil microbial biomass responses to carbon amendments in agricultural systems: A meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 241–252. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Frey, S.D.; Elliott, E.T.; Paustian, K. Bacterial and fungal abundance biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Drijber, R.A.; Doran, J.W.; Parkhurst, A.M.; Lyon, D.J. Changes in soil microbial community structure with tillage under long-term wheat-fallow management. Soil Biol. Biochem. 2000, 32, 1419–1430. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Bloem, J.; Baath, E.; Boeckx, P.; Rousk, J.; Bodé, S.; Forristal, D.; Jones, M.B. Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 2010, 42, 48–55. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Dowd, S.E.; Bell, C.W.; Lascano, R.; Booker, J.D. Microbial community composition as affected by dryland cropping systems and tillage in a semiarid sandy soil. Diversity 2010, 2, 910–931. [Google Scholar] [CrossRef]
- Cañizares, R.; Moreno, B.; Benitez, E. Biochemical characterization with detection and expression of bacterial β-glucosidase encoding genes of a Mediterranean soil under different long-term management practices. Biol. Fertil. Soil 2010, 48, 651–663. [Google Scholar] [CrossRef]
- Buckley, D.H.; Schmidt, T.M. The structure of microbial communities in soil and the lasting impact of cultivation. Microb. Ecol. 2001, 42, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Clegg, C.D.; Lovell, R.D.; Hobbs, P.J. The impact of grassland management regime on the community structure of selected bacterial groups in soils. FEMS Microb. Ecol. 2003, 43, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, E.; Yan, C.; Tian, J.; Zhang, H.; Zhang, Y. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Li, X.; Su, Z.; Li, X.; Xu, M. Effects of tillage and residue incorporation on composition and abundance of microbial communities of a fluvo-aquic soil. Eur. J. Soil Biol. 2014, 65, 70–78. [Google Scholar] [CrossRef]
- Hartmann, N.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Degrune, F.; Theodorakopoulos, N.; Dufrene, M.; Colinet, G.; Bodson, B.; Hiel, M.P.; Taminiau, B.; Nezer, C.; Daube, G.; Vandenbol, M. No favorable effect of reduced tillage on microbial community diversity in a silty loam soil (Belgium). Agric. Ecosyst. Environ. 2016, 224, 12–21. [Google Scholar] [CrossRef]
- Castañeda, L.E.; Godoy, K.; Manzano, M.; Marquet, P.A.; Barbosa, O. Comparison of soil microbial communities inhabiting vineyards and native sclerophyllous forests in central Chile. Ecol. Evol. 2015, 5, 3857–3868. [Google Scholar] [CrossRef] [PubMed]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Girvan, M.S.; Bullimore, J.; Pretty, J.N.; Osborn, A.M.; Ball, A.S. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl. Environ. Microb. 2003, 69, 1800–1809. [Google Scholar] [CrossRef]
- Bossio, D.A.; Girvan, M.S.; Verchot, L.; Bullimore, J.; Borelli, T.; Albrecht, A.; Scow, K.M.; Ball, A.S.; Pretty, J.N.; Osborn, A.M. Soil microbial community response to land use change in an agricultural landscape of western Kenya. Microb. Ecol. 2005, 49, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Bissett, A.; Abell, G.C.J.; Brown, M.; Thrall, P.H.; Bodrossy, L.; Smith, M.C.; Baker, G.H.; Richardsson, A.E. Land-use and management practices affect soil ammonia oxidiser community structure, activity and connectedness. Soil Biol. Biochem. 2014, 78, 138–148. [Google Scholar] [CrossRef]
- Banerjee, S.; Baah-Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Noya, Y.E.; Gómez-Acata, S.; Montoya-Ciriaco, N.; Rojas-Valdez, A.; Suárez-Arriaga, M.C.; Valenzuela-Encinas, C.; Jiménez-Bueno, N.; Verhulst, N.; Govaerts, B.; Dendooven, L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol. Biochem. 2013, 65, 86–95. [Google Scholar] [CrossRef]
- Anderson, C.; Beare, M.; Buckley, H.L.; Lear, G. Bacterial and fungal communities respond differently to varying tillage depth in agricultural soils. Peer J 2017, 5, e3930. [Google Scholar] [CrossRef] [PubMed]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBryun, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil. Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Rincon-Florez, V.A.; Ng, C.; Dang, Y.P.; Schenk, P.M.; Carvalhais, L.C. Short-term impact of an occasional tillage on microbial communities in a Vertosol after 43 years of no-tillage or conventional tillage. Eur. J. Soil Biol. 2016, 74, 32–38. [Google Scholar] [CrossRef]
- Vink, S.N.; Neilson, R.; Robinson, D.; Daniell, T.J. Temporal and land use effects on soil bacterial community structure of the machair, an EU Habitats Directive Annex I low-input agricultural system. Appl. Soil Ecol. 2014, 73, 116–123. [Google Scholar] [CrossRef]
- Habekost, M.; Eisenhauer, N.; Scheu, S.; Steinbeiss, S.; Weigelt, A.; Gleixner, G. Seasonal changes in the soil microbial community in a grassland plant diversity gradient four years after establishment. Soil Biol. Biochem. 2008, 40, 2588–2595. [Google Scholar] [CrossRef]
- Kennedy, N.M.; Gleeson, D.E.; Connolly, J.; Clipson, N.J.W. Seasonal and management influences on bacterial community structure in an upland grassland soil. FEMS Microbiol. Ecol. 2005, 53, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lukow, T.; Dunfield, P.F.; Liesack, W. Use of the T-RFLP technique to assess spatial and temporal changes in the bacterial community structure within an agricultural soil planted with transgenic and non-transgenic potato plants. FEMS Microbiol. Ecol. 2000, 32, 241–247. [Google Scholar] [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 2001, 67, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Influence of depth and sampling time on bacterial community structure in an upland grassland soil. FEMS Microbiol. Ecol. 2003, 43, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Žák, Š.; Beluský, J.; Bušo, R.; Gavurníková, S.; Hašana, R.; Macák, M.; Kováč, K.; Stanko, P. Pestovanie poľných Plodín s Orbou či Bez Orby? (Eng. Cultivation of Field Crops with or without Plows); Plant Production Research Centre Piešťany: Piešťany, Slovakia, 2011; p. 120. ISBN 9788071391494. [Google Scholar]
- Welbaum, G.A.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant Sci. 2010, 23, 175–193. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.K. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [PubMed]
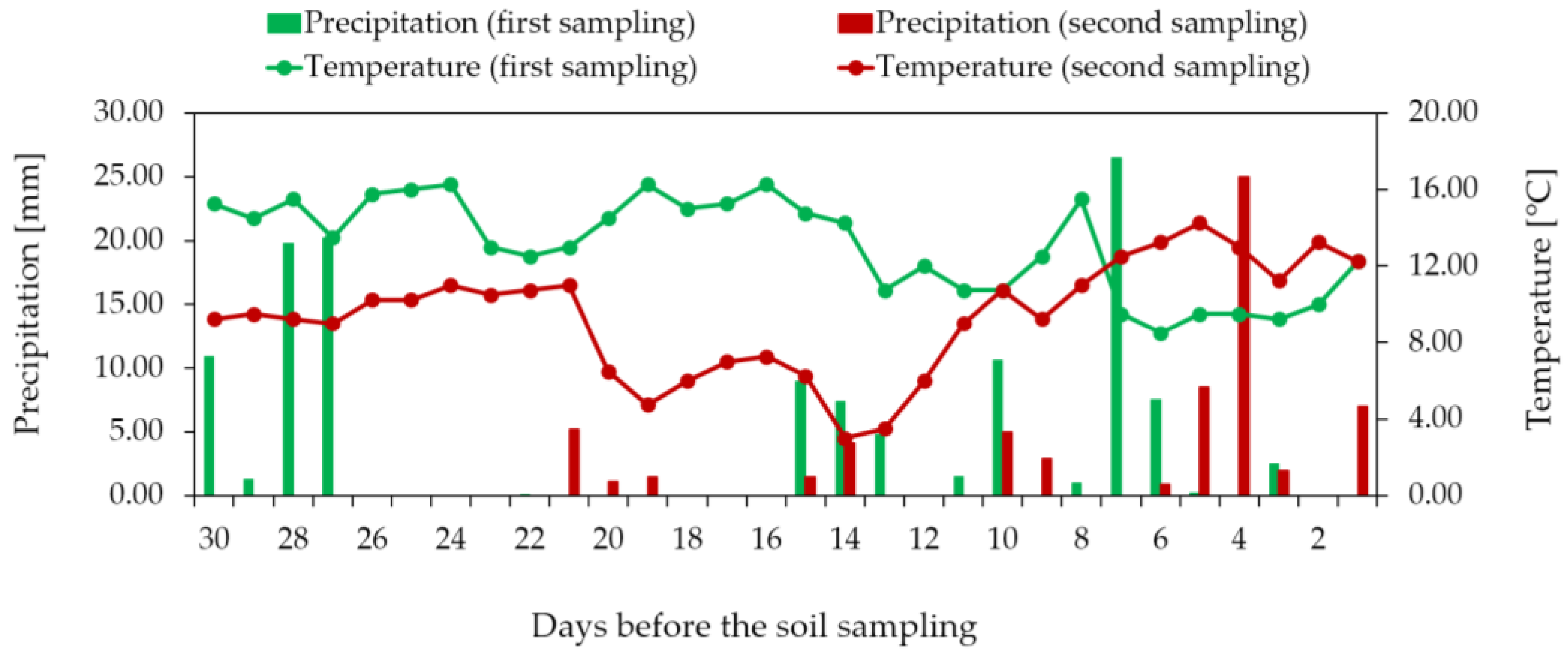

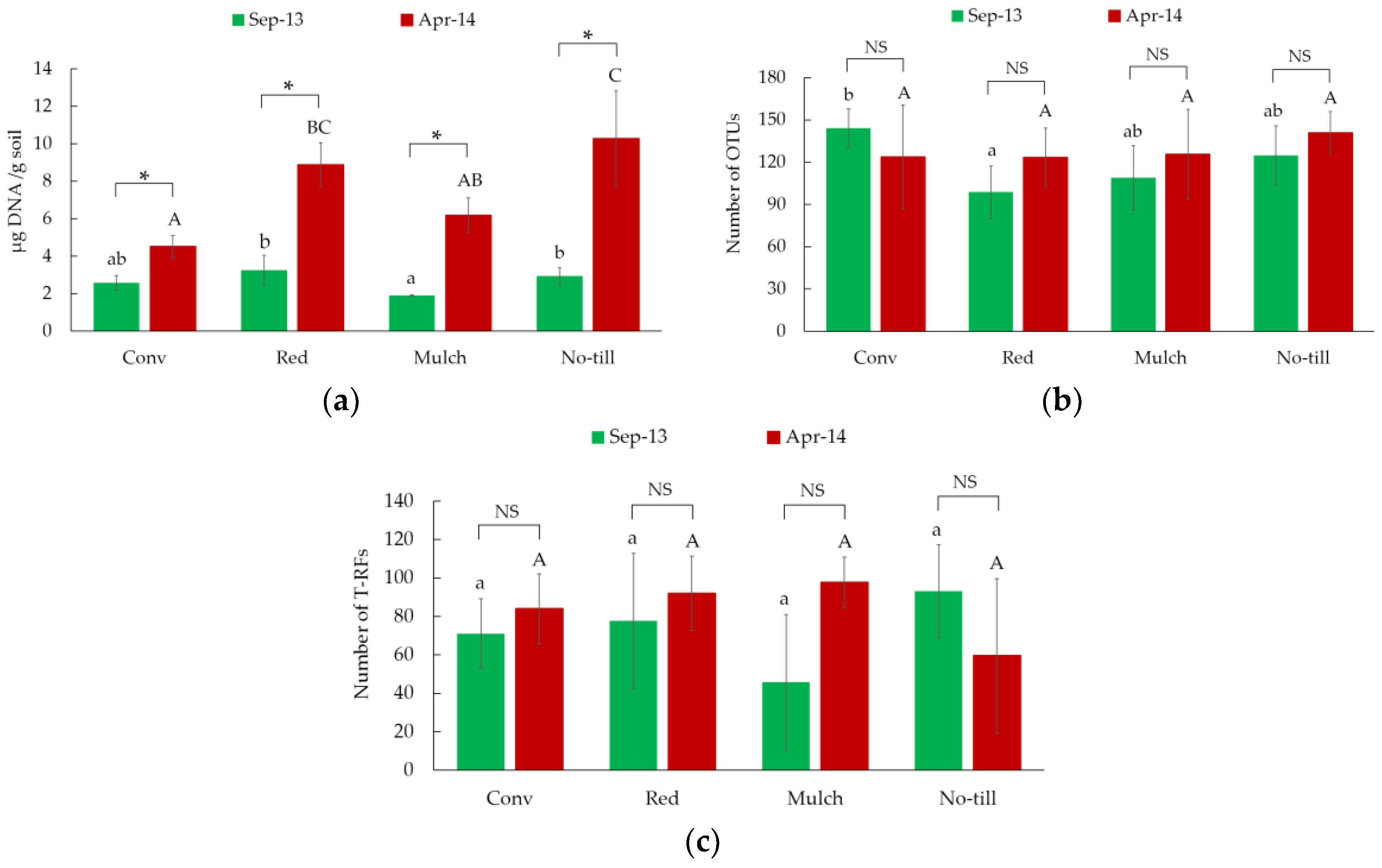

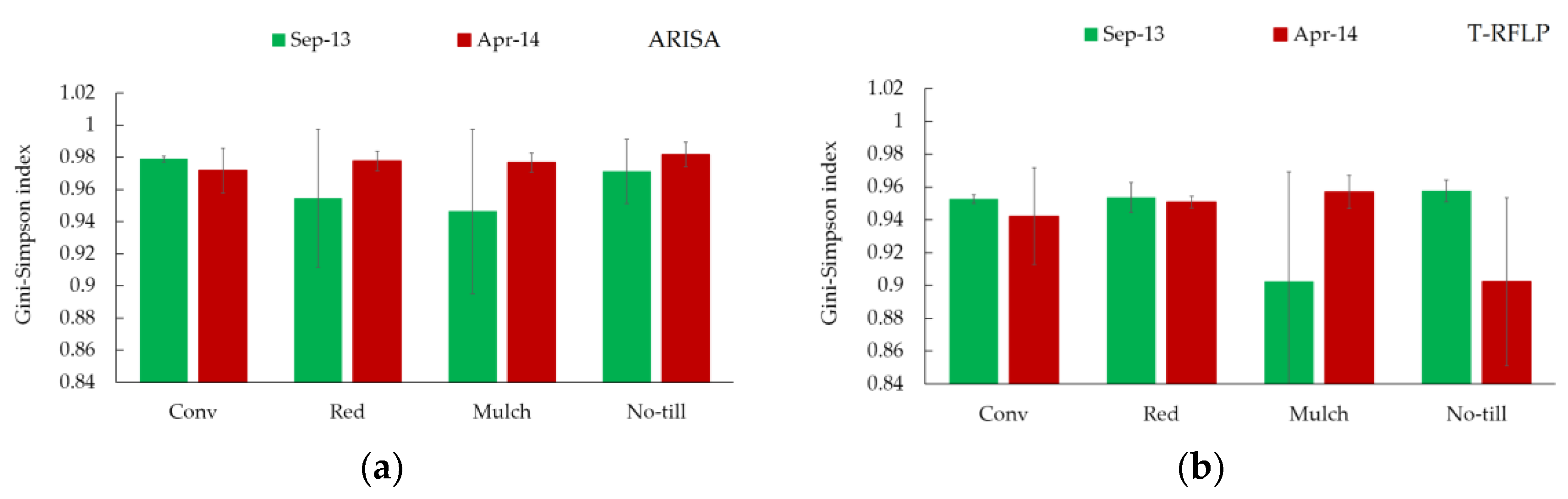
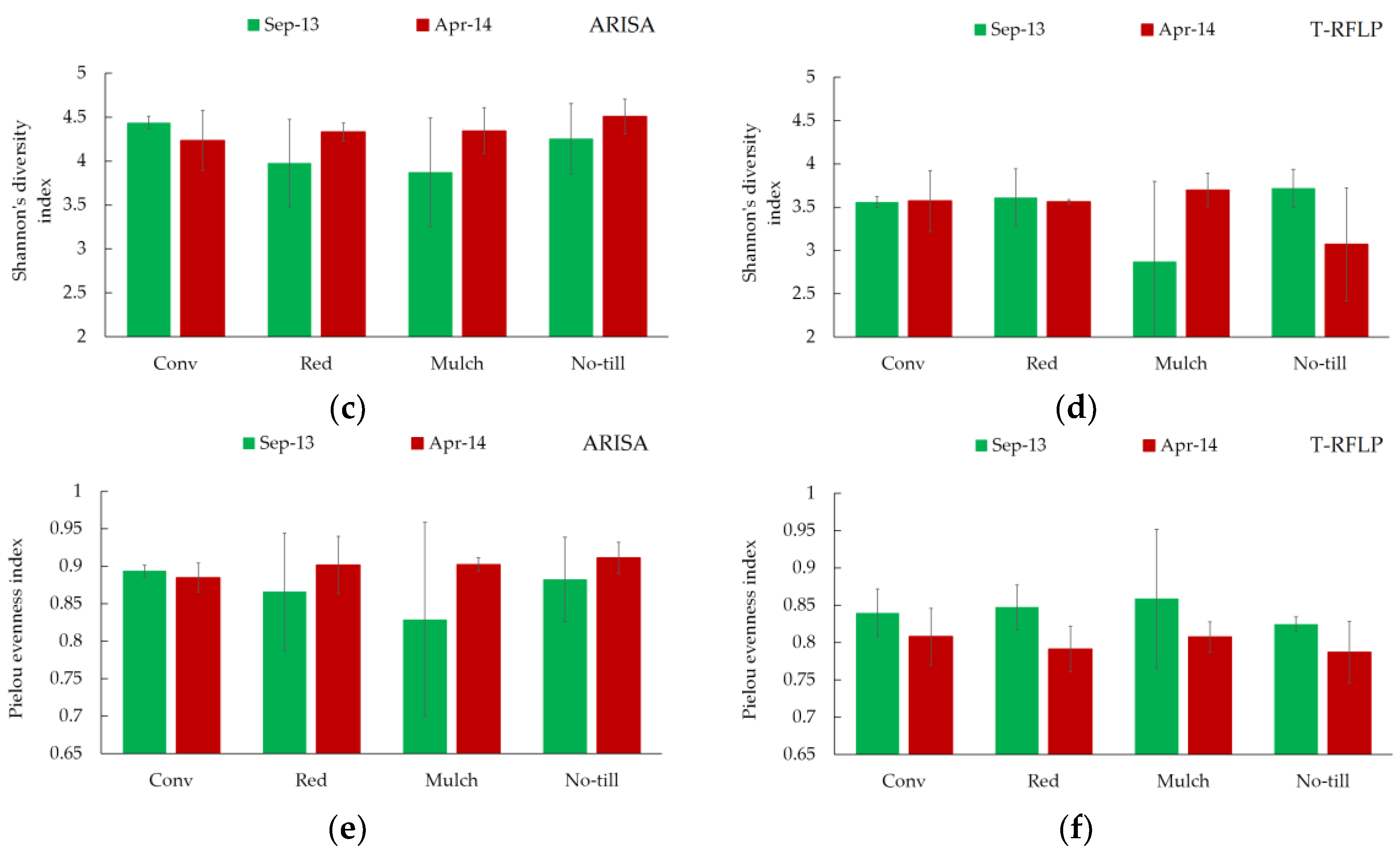


| Tillage System | Soil Temperature (°C) | Bulk Density (kg m−3) | Soil Porosity (%) | Soil Moisture (%) | Soil pH/KCl | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13 September | 14 April | 13 September | 14 April | 13 September | 14 April | 13 September | 14 April | 13 September | 14 April | |
| Conv | 12.60 | 11.37 | 1450 | 1490 | 46.26 | 44.73 | 19.79 | 19.17 | 6.46 | 6.74 |
| Red | 14.60 | 11.70 | 1330 | 1390 | 50.19 | 47.99 | 19.30 | 19.80 | 6.50 | 6.77 |
| Mulch | 14.78 | 10.87 | 1410 | 1400 | 47.16 | 47.42 | 19.95 | 19.80 | 6.48 | 6.71 |
| No-till | 13.39 | 10.49 | 1450 | 1450 | 45.93 | 45.86 | 19.92 | 19.58 | 6.93 | 6.78 |
| ARISA | T-RFLP | |
| Similarity index | Euclidean | Euclidean |
| Permutation N | 9999 | 9999 |
| p-value | ||
| Tillage | 0.5455 | 0.6643 |
| Season | 0.7356 | 0.5383 |
| Interaction | 0.4674 | 0.7006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondreičková, K.; Piliarová, M.; Bušo, R.; Hašana, R.; Schreiber, Ľ.; Gubiš, J.; Kraic, J. The Structure and Diversity of Bacterial Communities in Differently Managed Soils Studied by Molecular Fingerprinting Methods. Sustainability 2018, 10, 1095. https://doi.org/10.3390/su10041095
Ondreičková K, Piliarová M, Bušo R, Hašana R, Schreiber Ľ, Gubiš J, Kraic J. The Structure and Diversity of Bacterial Communities in Differently Managed Soils Studied by Molecular Fingerprinting Methods. Sustainability. 2018; 10(4):1095. https://doi.org/10.3390/su10041095
Chicago/Turabian StyleOndreičková, Katarína, Michaela Piliarová, Rastislav Bušo, Roman Hašana, Ľudovít Schreiber, Jozef Gubiš, and Ján Kraic. 2018. "The Structure and Diversity of Bacterial Communities in Differently Managed Soils Studied by Molecular Fingerprinting Methods" Sustainability 10, no. 4: 1095. https://doi.org/10.3390/su10041095





