Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Location and Climate
2.1.2. Morphology and Bathymetry
2.1.3. Geological and Hydrological Setting
2.1.4. Water Quality
2.1.5. Biodiversity
2.1.6. Socioeconomic Context and Anthropic Impacts
2.2. Previous Studies and Projects, and Datasets
2.3. Statistical and Other Methods
2.3.1. Time Series Assembly and Trend Analyses
2.3.2. Pairwise Pearson Correlation Analyses
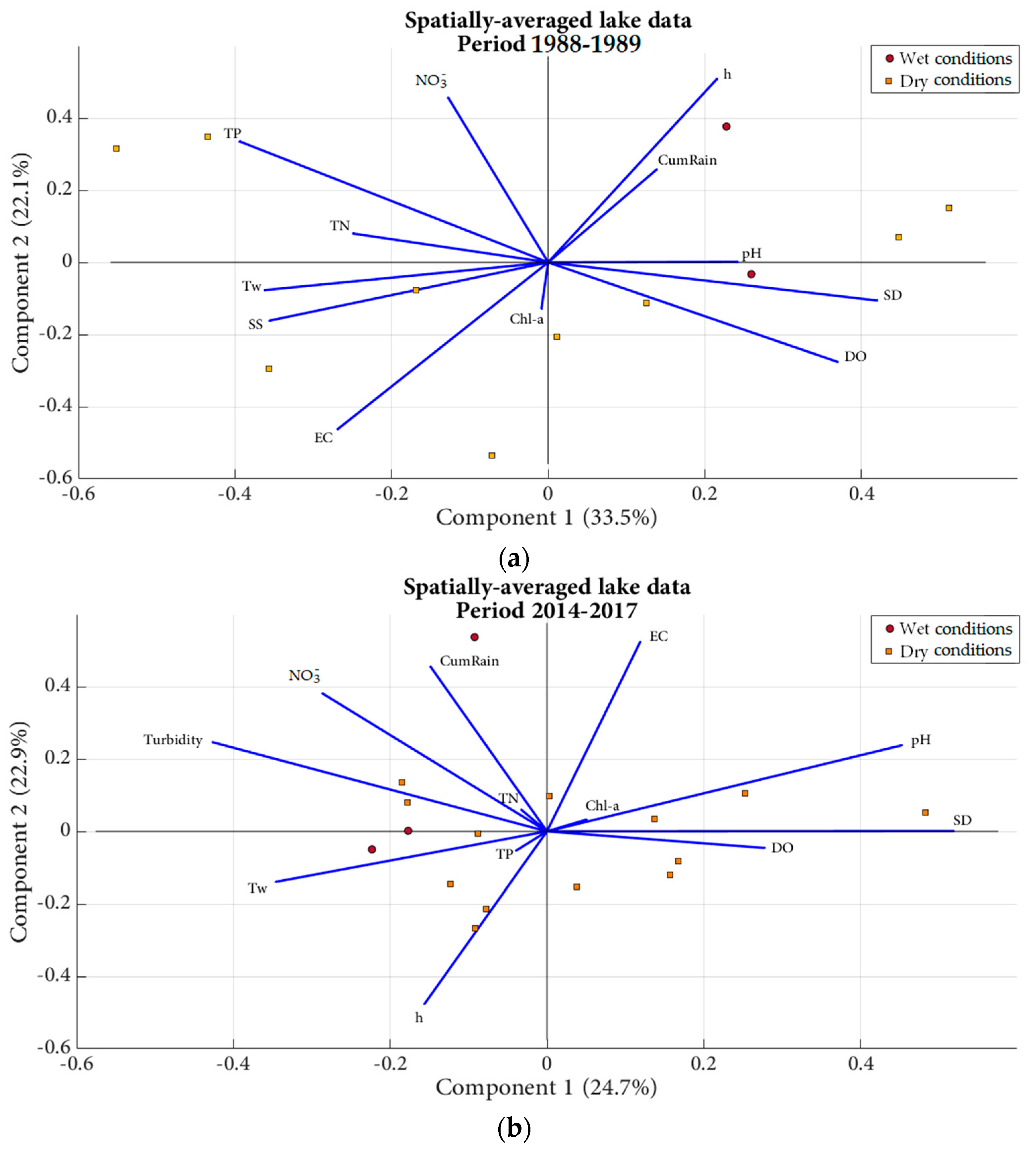
2.3.3. Principal Component Analyses and Simple Linear Regressions
2.3.4. Time Series Reconstruction of Trophic State Indices and Nutrient Limitation Assessment
3. Results
3.1. Scientific and Management-Oriented Research History
3.2. Statistical and Other Analyses
3.2.1. Time Series Assembly and Trend Analyses
3.2.2. Pairwise Pearson Correlation Analyses
3.2.3. Principal Component Analyses and Simple Linear Regressions
3.2.4. Time Series Reconstruction of Trophic State Indices and Nutrient Limitation Assessment
4. Discussion
4.1. Factors Affecting Primary Production
4.2. Complex Hydro-Morphological and Hydro-Ecological Conditions
4.3. Challenges for Restoration
4.4. Challenges for Management
4.5. Opportunities for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lewontin, R.C. The meaning of stability. In Diversity and Stability in Ecological Systems. Brookhaven Symposium in Biology; U.S. Department of Commerce: Springfield, VA, USA, 1969; Volume 22, pp. 13–24. [Google Scholar]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative stable states in shallow lakes. Trends Ecol. Evolut. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Scheffer, M. Alternative stable states in eutrophic, shallow freshwater systems: A minimal model. Hydrobiol. Bull. 1989, 23, 73–83. [Google Scholar] [CrossRef]
- Scheffer, M. Multiplicity of stable states in freshwater systems. Hydrobiologia 1990, 200, 475–486. [Google Scholar] [CrossRef]
- Janse, J.; De Senerpont Domis, L.N.; Scheffer, M.; Lijklema, L.; Van Lerie, L.; Klinge, M.; Mooij, W.M. Critical phosphorus loading of different types of shallow lakes and the consequences for management estimated with the ecosystem model PCLake. Limnologica 2008, 38, 203–219. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Nes, E.H. Shallow lakes theory revisited; various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 2007, 584, 455–466. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Rip, W.J.; Scheffer, M. A Theory for Cyclic Shifts between Alternative States in Shallow Lakes. Ecosystems 2007, 10, 17–27. [Google Scholar] [CrossRef]
- Hastings, A. Transients: The key to long-term ecological understanding? Trends Ecol. Evolut. 2004, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Van Geest, G.J.; Roozen, F.; Coops, H.; Roijackers, R.M.M.; Buijse, A.D.; Peeters, E.; Scheffer, M. Vegetation abundance in lowland flood plan lakes determined by surface area, age and connectivity. Freshw. Biol. 2003, 48, 440–454. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P. Pond or lake: Does it make any difference? Arch. Hydrobiol. 2005, 162, 143–165. [Google Scholar] [CrossRef]
- Janssen, A.B.G.; Teurlincx, S.; An, S.; Janse, J.H.; Paerl, H.W.; Mooij, W.M. Alternative stable states in large shallow lakes? J. Great Lakes Res. 2014, 40, 813–826. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M. Implications of spatial heterogeneity for catastrophic regime shifts in ecosystems. Ecology 2005, 86, 1797–1807. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M.; Van den Berg, M.S.; Coops, H. Dominance of charophytes in eutrophic shallow lakes—When should we expect it to be an alternative stable state? Aquat. Bot. 2002, 72, 275–296. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; Van Nes, E.H. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 1997, 78, 272–282. [Google Scholar] [CrossRef]
- Scheffer, M.; Szabó, S.; Gragnani, A.; Van Nes, E.H.; Rinaldi, S.; Kautsky, N.; Norberg, J.; Roijackers, R.M.M.; Franken, R.J.M. Floating plant dominance as a stable state. Proc. Natl. Acad. Sci. USA 2003, 100, 4040–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerhoff, M.; Mazzeo, N.; Moss, B.; Rodríguez-Gallego, L. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat. Ecol. 2003, 37, 377–391. [Google Scholar] [CrossRef]
- Meerhoff, M.; Teixeira-de Mello, F.; Kruk, C.; Alonso, C.; González-Bergonzoni, I.; Pacheco, J.P.; Lacerot, G.; Arim, M.; Beklioğlu, M.; Brucet, S.; et al. Environmental Warming in Shallow Lakes: A Review of Potential Changes in Community Structure as Evidenced from Space-for-Time Substitution Approaches. In Advances in Ecological Research: Global Change in Multispecies Systems, Part I; Jacob, U., Woodward, G., Eds.; Elsevier Ltd.: New York, NY, USA, 2012; Volume 46, pp. 259–349. [Google Scholar] [CrossRef]
- Kosten, S.; Jeppesen, E.; Huszar, V.L.M.; Mazzeo, N.; Van Nes, E.H.; Peeters, E.T.H.M.; Scheffer, M. Ambiguous climate impacts on competition between submerged macrophytes and phytoplankton in shallow lakes. Freshw. Biol. 2011, 56, 1540–1553. [Google Scholar] [CrossRef]
- Scheffer, M.; Straile, D.; Van Nes, E.H.; Hosper, H. Climatic warming causes regime shifts in lake food webs. Limnol. Oceanogr. 2001, 46, 1780–1783. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.M.; Teixeira de Mello, F.; Iglesias, C.; Pedersen, A.R.; Jeppesen, E. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Glob. Chang. Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Singh, M.S.; Kuang, Z.; Maloney, E.D.; Hannah, W.M.; Wolding, B.O. Increasing potential for intense tropical and subtropical thunderstorms under global warming. Proc. Natl. Acad. Sci. USA 2003, 114, 11657–11662. [Google Scholar] [CrossRef] [PubMed]
- Najibi, N.; Devineni, N. Recent trends in the frequency and duration of global floods. Earth Sys. Dynam. 2018, 9, 757–783. [Google Scholar] [CrossRef] [Green Version]
- Perkins-Kirkpatrick, S.E.; Gibson, P.B. Changes in regional heatwave characteristics as a function of increasing global temperature. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beklioğlu, M.; Meerhoff, M.; Søndergaard, M.; Jeppesen, E. Eutrophication and Restoration of Shallow Lakes from a Cold Temperate to a Warm Mediterranean and a (Sub)Tropical Climate. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Sarvajeet, S.G., Lanza, G.R., Rast, W., Eds.; Springer Science+Business Media: Berlin, Germany, 2011; pp. 91–108. [Google Scholar] [CrossRef]
- Schelske, C.L.; Lowe, E.F.; Kenney, W.F.; Battoe, L.E.; Brenner, M.; Coveney, M.F. How anthropogenic darkening of Lake Apopka induced benthic light limitation and forced the shift from macrophyte to phytoplankton dominance. Limnol. Oceanogr. 2010, 55, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Waters, M.N.; Schelske, C.L.; Brenner, M. Cyanobacterial dynamics in shallow Lake Apopka (Florida, U.S.A.) before and after the shift from a macrophyte-dominated to a phytoplankton-dominated state. Freshw. Biol. 2015, 60, 1571–1580. [Google Scholar] [CrossRef]
- Beaver, J.R.; Casamatta, D.A.; East, T.L.; Havens, K.E.; Rodusky, A.J.; James, R.T.; Taus, C.E.; Buccier, K.M. Extreme weather events influence the phytoplankton community structure in a large lowland subtropical lake (Lake Okeechobee, Florida, USA). Hydrobiologia 2013, 709, 213–226. [Google Scholar] [CrossRef]
- Rodusky, A.J. The influence of large water level fluctuations and hurricanes on periphyton and associated nutrient storage in subtropical Lake Okeechobee, USA. Aquat. Ecol. 2010, 44, 797–815. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, B.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- De Faria, D.M.; Cardoso, L.S.; da Motta Marques, D. Epiphyton dynamics during an induced succession in a large shallow lake: Wind disturbance and zooplankton grazing act as main structuring forces. Hydrobiologia 2017, 788, 267–280. [Google Scholar] [CrossRef]
- Da Rosa, L.M.; De Souza Cardozo, L.; Crossetti, L.O.; Da Motta-Marques, D. Spatial and temporal variability of zooplankton-phytoplankton interactions in a large subtropical shallow lake dominated by non-toxic cyanobacteria. Mar. Freshw. Res. 2017, 68, 226–243. [Google Scholar] [CrossRef]
- Scasso, F.; Mazzeo, N.; Gorga, J.; Kruk, C.; Lacerot, G.; Clemente, J.; Fabián, D.; Bonilla, S. Limnological changes in a sub-tropical shallow hypertrophic lake during its restoration: Two years of a whole-lake experiment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 31–44. [Google Scholar] [CrossRef]
- González-Madina, L.; Pacheco, J.P.; Yema, L.; De Tezanos, P.; Levrini, P.; Clemente, J.; Crisci, C.; Lagomarsino, J.J.; Méndez, G.; Fosalba, C.; et al. Drivers of cyanobacteria dominance, composition and nitrogen fixing behavior in a shallow lake with alternative regimes in time and space, Laguna del Sauce (Maldonado, Uruguay). Hydrobiologia 2018. [Google Scholar] [CrossRef]
- Mazzeo, N.; Gorga, J.; Crosa, D.; Ferrando, J.; Pintos, W. Spatial and temporal variation of physicochemical parameters in a shallow reservoir seasonally covered by Pistia stratiotes L. in Uruguay. J. Freshw. Ecol. 1995, 10, 141–149. [Google Scholar] [CrossRef]
- Pacheco, J.P.; Iglesias, C.; Meerhoff, M.; Fosalba, C.; Goyenola, G.; Teixeira-de Mello, F.; García, S.; Gelós, M.; García-Rodríguez, F. Phytoplankton community structure in five subtropical shallow lakes with different trophic status (Uruguay): A morphology-based approach. Hydrobiologia 2010, 646, 187–197. [Google Scholar] [CrossRef]
- Iglesias, C.; Meerhoff, M.; Johansson, L.S.; González-Bergonzoni, I.; Mazzeo, N.; Pacheco, J.P.; Teixeira-de Mello, F.; Goyenola, G.; Lauridsen, T.L.; Sondergaadr, M.; et al. Stable isotope analysis confirms substantial differences between subtropical and temperate shallow lake food webs. Hydrobiologia 2017, 784, 111–123. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Belda, M.; Holtanová, E.; Halenka, T.; Kalvová, J. Climate classification revisited: From Köppen to Trewartha. Clim. Res. 2014, 59, 1–13. [Google Scholar] [CrossRef]
- Directorate of Meteorology and Hydrology, National Directorate of Civil Aeronautics of Paraguay (DMH-DINAC). Meteorological Records for the Silvio Pettirossi International Airport (AISP) and the San Bernardino Nautical Club (CNSB) Stations; Database; DMH-DINAC: Asunción, Paraguay, 1959–2017.
- Grimm, A.M.; Barros, V.R.; Doyle, M.E. Climate variability in southern South America associated with El Niño and La Niña events. J. Clim. 2000, 13, 35–58. [Google Scholar] [CrossRef]
- Hirata, F.E.; Grimm, A.M. The role of synoptic and intraseasonal anomalies in the life cycle of summer rainfall extremes over South America. Clim. Dyn. 2016, 46, 3041–3055. [Google Scholar] [CrossRef]
- Hirata, F.E.; Grimm, A.M. The role of synoptic and intraseasonal anomalies on the life cycle of rainfall extremes over South America: Non-summer conditions. Clim. Dyn. 2017, 49, 313–326. [Google Scholar] [CrossRef]
- Pasquini, A.I.; Depetris, P.J. ENSO-triggered exceptional flooding in the Paraná River: Where is the excess water coming from? J. Hydrol. 2010, 383, 186–193. [Google Scholar] [CrossRef]
- Báez, J.; Monte Domecq, R. Analysis of drought episodes in Paraguay. Clim. Chang. 2014, 127, 15–25. [Google Scholar]
- Méndez, S. Estudio del Comportamiento del Viento en San Bernardino; Itaipú Binational Entity: Hernandarias, Paraguay, 2017.
- ANNP-Paraguay. Ypacaraí Lake: Water Level Records; Dataset; ANNP: Asunción, Paraguay, 1965–2016.
- Reservoir Division, Department of Reservoir and Protected Areas, Superintendence of Environmental Management, Directorate of Executive Coordination, Itaipú Binational Entity. Ypacaraí Lake: Water level Records, San Bernardino Nautical Club (CNSB) Station; Dataset; Itaipú Binational Entity: Hernandarias, Paraguay, 2016–2017.
- Reservoir Division, Department of Reservoir and Protected Areas, Superintendence of Environmental Management, Directorate of Executive Coordination, Itaipú Binational Entity. Bathymetric survey of the Ypacaraí Lake; Dataset; Itaipú Binational Entity: Hernandarias, Paraguay, 2014; Processed in 2017.
- Wetzel, R.G. Limnology. Lake and River Ecosystems, 3rd ed.; Academic Press (Elsevier): San Diego, CA, USA, 2001; p. 34. ISBN 978-0-12-744760-5. [Google Scholar]
- Riccomini, C.; Velázquez, V.F.; de Barros Gomes, C. Cenozoic lithospheric faulting in the Asunción Rift, eastern Paraguay. J. South Am. Earth Sci. 2001, 14, 625–630. [Google Scholar] [CrossRef]
- Velázquez, R.; Alviso, R.; Facetti, J. Sediments of the Ypacaraí Lake. J. Radioanal. Nucl. Chem. 1992, 161, 233–238. [Google Scholar] [CrossRef]
- Hinegk, L. Water and Nutrient Balance of Lake Ypacaraí and Salado River Basin (Paraguay): Data Analysis and Modeling. Master’s Thesis, University of Trento, Trento, Italy, 29 March 2018. [Google Scholar]
- Bendoricchio, G. Detención e Reversión del Proceso de Deterioro de la Cuenca del lago Ypacaraí. Misión de Identificación Para la Gestion Ecológica de la Cuenca del Lago Ypacaraí— Paraguay; Technical Report; ARPA Veneto: Padua, Italy, 2000. [Google Scholar]
- Beta Studio—Thetis S.p.A. Plan de Saneamiento Integral de la Cuenca del Lago Ypacaraí: Diagnóstico de la Situación Actual del Lago Ypacaraí y su Cuenca; Technical Report; Beta Studio S.R.L.: Padua, Italy; Thetis S.p.A.: Venice, Italy, 2015. [Google Scholar]
- Facetti Villasanti, F.S. Análisis físicoquímicos de las aguas del Lago Ypacaraí. Química y Colegio Químico del Paraguay 1945, 8/9, 19. [Google Scholar]
- Facetti, J.F.; Facetti Villasanti, F.S. Trace Elements in Ypacaraí lake water. In Proceedings of the Fifth InterAmerican Symposium on Nuclear Energy, Valparaiso, Chile, 9–13 March 1964; Pan American Union: Washington, DC, USA, 1965; p. 105. [Google Scholar]
- Escribá Ticoulat, J.; Escribá Sakoda, C. Informe de Evaluación de Nutrientes y Eficiencias de Depuración de los Humedales del Lago Ypacaraí; INYMA Consult S.R.L.: Asunción, Paraguay, 2016. [Google Scholar]
- Salvadore, A. Hydro-Thermodynamical Modelling of the Shallow Lake Ypacaraí (Paraguay). Master’s Thesis, University of Trento, Trento, Italy, 29 March 2018. [Google Scholar]
- Peralta López, I.; Benítez Rodas, G.A.; Ávalos, C.; Araujo Florentín, C.M.; Escobar, A.; Astigarraga Escobar, O.; Nuñez Zarate, I.B.; Villalba Duré, G.; Acosta Brítez, R.R.; Alcaraz, R.; et al. Monitoreo de Calidad de Agua por Campañas de Muestreo en el Lago Ypacaraí 2014; Technical Report; UNA: San Lorenzo, Paraguay, 2014. [Google Scholar]
- Peralta López, I.; Benítez Rodas, G.A.; Ávalos, C.; Araujo Florentín, C.M.; Escobar, A.; Astigarraga Escobar, O.; Nuñez Zarate, I.B.; Villalba Duré, G.; Acosta Brítez, R.R.; Alcaraz, R.; et al. Monitoreo de Calidad de Agua por Campañas de Muestreo en el Lago Ypacaraí 2016; UNA: San Lorenzo, Paraguay, 2016. [Google Scholar]
- López Arias, T.R.; Franco de Diana, D.; Fernández, V.; Benítez, C.; Sánchez, P.; López Vera, M.E.; Kurita, H.; Benítez, M.; Ramond, F.; Bobadill, N.; et al. Diangóstico Ecotoxicológico y Genotóxico de los Afluentes del Lago Ypacaraí Mediante bioensayos con Daphnia magna Straus, Danio rerio, Lactuca sativa L. y Allium cepa L. Investigaciones y Estudios—UNA; UNA: San Lorenzo, Paraguay, 2013. [Google Scholar]
- National Environmental Sanitation Service of Paraguay (SENASA); Japan International Cooperation Agency (JICA). Plan de Mejoramiento de la Calidad de Agua del Lago Ypacaraí y su Cuenca; SENASA: Asunción, Paraguay, 2001.
- Insaurralde, M.; Balbuena, E.; Frutos, A. Variedades de peces del lago Ypacaraí (Fish variety at Ypacaraí Lake). Compend. Cienc. Vet. 2013, 3, 13–21. [Google Scholar]
- Benítez Rodas, G.A.; Villalba Duré, G.; Ávalos de Enciso, C.; Araújo Florentín, C.; Acosta Brítez, R.; Escobar, A.; Astigarraga Escobar, O.; Peralta López, I.; Cardozo Román, C. Influencia de factores fisicoquímicos sobre la biodiversidad de cianobacterias en el Lago Ypacaraí durante el periodo 2012–2014. Steviana 2017, 9, 15–25. [Google Scholar]
- Benítez Rodas, G.; Dos Santos, M.; Núñez, A.; Villalba Duré, G.; Ávalos de Enciso, C.; Araújo Florentín, C.; Acosta Brítez, R.; Escobar, A.; Arenas, R.; Astigarraga Escobar, O.; et al. Primer reporte de floración por Ceratium furcoides (Levander) Langhans en el Lago Ypacaraí – Departamento Central, Paraguay. Steviana 2017, 9, 26–35. [Google Scholar]
- Benítez, G.A.; Multidisciplinary Centre for Technological Investigations, National University of Asunción, San Lorenzo, Paraguay. Personal communication, 2018.
- González Romero, N. Contribución al estudio del Fito y Zooplancton en el Paraguay. Rev. Soc. Cient. Parag. 1967, 5, 29–38. [Google Scholar]
- González Romero, N. Estudio Limnológico del Lago Ypacaraí; Revista del Instituto de Ciencias Básicas; Universidad Nacional de Asunción: San Lorenzo, Paraguay, 1986; 188p. [Google Scholar]
- Ritterbusch, B. Estudio limnológico del lago Ypacaraí. Revista de la Asociación de Ciencias Naturales del Litoral 1988, 19, 11–26. [Google Scholar] [CrossRef]
- Darrigran, G.; Escurra de Drago, I. Invasion of the exotic freshwater mussel Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) in South America. Nautilus 2000, 114, 169–173. [Google Scholar]
- Mereles, F. Contribución al estudio de la vegetación hidrófita de la cuenca del lago Ypacaraí. Rev. Soc. Cient. Parag. 1982, 17, 86–92. [Google Scholar]
- Mereles, F. Estudio de las comunidades vegetales de la cuenca del lago Ypacaraí. Rev. Soc. Cient. Parag. 1985, 5, 35–48. [Google Scholar]
- Mereles, F.; Basualdo, I.; Ortiz, M.; Soria, N. Contribución al estudio de la vegetación del valle del lago Ypacaraí II. Rev. Univ. Nac. Asunción 1991, 2, 56–59. [Google Scholar]
- Mereles, F.; Degen, R.; López de Kochalca, N. Humedales del Paraguay: Breve reseña de su vegetación. Amazoniana 1992, 12, 305–316. [Google Scholar]
- Mereles, F.; Department of Botany, Faculty of Chemistry, National University of Asunción, San Lorenzo, Paraguay. Personal communication, 2018.
- Weiler, A.; Núñez, K.; Airaldi Wood, K.; Caballero-Gini, A.; Bauer, F.; Cardozo, R. Aves de la Reserva de Recursos manejados Lago Ypacarai. Rev. Soc. Cient. Parag. 2015, 19, 63–73. [Google Scholar]
- General Directorate of Statistics, Surveys and Censuses of Paraguay (DGEEC). Proyección de la Población por Sexo y Edad Según Distrito (2000–2025); Technical Report; DGEEC: Asunción, Paraguay, 2012.
- Venohr, M.; Langhans, S.D.; Peters, O.; Hölker, F.; Arlinghaus, R.; Mitchel, L.; Wolter, C. The underestimated dynamics and impacts of water-based recreational activities on freshwater ecosystems. Environ. Rev. 2018, 26, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Public Works and Communications of Paraguay (MOPC). Plan Estratégico Metropolitano de Asunción (PEMA); Technical Report; MOPC: Asunción, Paraguay, 2014.
- Beta Studio—Thetis S.p.A. Plan de Saneamiento Integral de la Cuenca del Lago Ypacaraí: Modelación Matemática e Identificación de Acciones y Medidas de Mitigación; Technical Report; Beta Studio S.R.L.: Padua, Italy; Thetis S.p.A.: Venice, Italy, 2016. [Google Scholar]
- Mann, H.B. Non-parametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Measures; Charles Griffin: London, UK, 1975. [Google Scholar]
- McMahon, T.A.; Peel, M.C.; Lowe, L.; Srikanthan, R.; McVicar, T.R. Estimating actual, potential, reference crop and pan evaporation using standard meteorological data: A pragmatic synthesis. Hydrol. Earth Syst. Sci. 2013, 17, 1331–1363. [Google Scholar] [CrossRef]
- Murakami, M.; Oonisishi, Y.; Kunishi, H. A numerical simulation of the distribution of water temperature and salinity in the Seto Inland Sea. J. Oceanogr. Soc. Jpn. 1985, 41, 221–224. [Google Scholar] [CrossRef]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.P.D.; Talarico, M.; Chinez, S.J.; Agudo, E.G. A aplicação de modelos simplificados para avaliação do processo da eutrofização em lagos e reservatórios tropicais. In Proceedings of the 12th Congresso Brasileiro de Engenharia Sanitária e Ambiental, Camboriú, Brasil, 20–25 November 1983; pp. 1–34. [Google Scholar]
- Toledo, A.P., Jr. Informe Preliminar Sobre os Estudos Para a Obtenção de um Índice Para a Avaliação do Estado Trófico de Reservatórios de Regiões Quentes Tropicais; Technical Report; CETESB: São Paulo, Brazil, 1990.
- Salas, H.J.; Martino, P. A simplified phosphorus trophic state model for warm-water tropical lakes. Water Res. 1991, 25, 341–350. [Google Scholar] [CrossRef]
- Lamparelli, M.C. Graus de trofia em corpos d’água do Estado de São Paulo: Avaliação dos métodos de monitoramento. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2004. [Google Scholar]
- Cunha, D.G.F.; Do Carmo Calijuri, M.; Lamparelli, M.C. A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecol. Eng. 2013, 60, 126–134. [Google Scholar] [CrossRef]
- Kratzer, C.R.; Brezonik, P.L. A Carlson-type Trophic State Index for nitrogen in Florida lakes. J. Am. Water Resour. Assoc. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Havens, K.E. Secondary nitrogen limitation in a subtropical lake impacted by non-point source agricultural pollution. Environ. Pollut. 1995, 89, 241–246. [Google Scholar] [CrossRef]
- Håkanson, L.; Bryhn, A.C.; Hytteborn, J.K. On the issue of limiting nutrient and prediction of cyanobacteria in aquatic systems. Sci. Total Environ. 2007, 379, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. Available online: http://www.jstor.org/stable/27827150 (accessed on 2 July 2018).
- Willis, A.; Chuang, A.W.; Burford, M.A. Nitrogen fixation by the diazotroph Cylindrospermopsis raciborskii (Cyanophyceae). J. Phycol. 2016, 52, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zevenboom, W.; De Groot, G.J.; Mur, L.R. Effects of light on nitrate-limited Oscillatoria agardhii in chemostat cultures. Arch. Microbiol. 1989, 125, 59–65. [Google Scholar] [CrossRef]
- Das, R.; Samal, N.R.; Roy, P.K.; Mitra, D. Role of Electrical Conductivity as Indicator of Pollution in Shallow Lakes. Asian J. Water Environ. Pollut. 2016, 3, 143–146. [Google Scholar]
- Janssen, A.B.G.; De Jager, V.C.L.; Janse, J.H.; Kong, X.; Liu, S.; Ye, Q.; Mooij, W.M. Spatial identification of critical nutrient loads of large shallow lakes: Implications for Lake Taihu (China). Water Res. 2007, 219, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Cash, D.W.; Clark, W.C.; Alcock, F.; Dickson, N.M.; Eckley, N.; Guston, D.H.; Jäger, J.; Mitchell, R.B. Knowledge systems for sustainable development. Proc. Natl. Acad. Sci. USA 2003, 100, 8086–8091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölker, F.; Vanni, M.J.; Kuiper, J.J.; Meile, C.; Grossart, H.-P.; Stief, P.; Adrian, R.; Lorke, A.; Dellwig, O.; Brand, A.; et al. Tube-dwelling invertebrates: Tiny ecosystem engineers have large effects in lake ecosystems. Ecol. Monogr. 2015, 85, 333–351. [Google Scholar] [CrossRef] [Green Version]
- Havens, K.E.; Ji, G.; Beaver, J.R.; Fulton, R.S., III; Teacher, C.E. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia 2017, 1–17. [Google Scholar] [CrossRef]
- Havens, K.E.; Ji, G. Multiyear oscillations in depth affect water quality in Lake Apopka. Inland Waters 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Brothers, S.; Köhler, J.; Attermeyer, K.; Grossart, H.P.; Mehner, T.; Meyer, N.; Scharnweber, K.; Hilt, S. A feedback loop links brownification and anoxia in a temperate, shallow lake. Limnol. Oceanogr. 2014, 59, 1388–1398. [Google Scholar] [CrossRef] [Green Version]
- Kaczorowska, A.; Kornijów, R. Palaeoecological evidence for changes over the past 200 years in chironomid communities of a shallow lake exposed to cyanobacterial toxins. Aquat. Ecol. 2012, 46, 465–473. [Google Scholar] [CrossRef]
- Facetti, M.J.F.; Lozano, A.F.; Schade, F.; Flores, O.F.; Delgado, M.; Dávalos, A. Estudios Hídricos en el Lago Ypacaraí—I. Rev. Soc. Cient. Parag. 2005, 10, 85–97. [Google Scholar]
- Japan International Cooperation Agency (JICA) for the Technical Secretariat of Planning of Paraguay (STP). The Study on Water Pollution Control Plan for the Lake Ypacaraí and its Basin; Technical Reports; JICA: Tokyo, Japan, 1989.
- Undersecretariat of Environment and Natural Resources of the Ministry of Agriculture and Livestock of Paraguay (MAG). Environmental Evaluation of the Lake Ypacaraí and the Bay of Asunción; Technical Report; Dames & Moore, Inc.: Tampa, FL, USA, 1995. [Google Scholar]
- Wise Use International BV. La Limpieza Bioquímica del Contaminado Lago Ypacaraí, Paraguay; Technical Report; Wise Use International BV: Woerden, The Netherlands, 2008. [Google Scholar]












| Characteristic | Value | Units |
|---|---|---|
| Gauging station 1 reference level | 62.28 | m a.s.l. |
| Mean lake level | +0.20 | m |
| Average depth | 1.72 | m |
| Maximum depth | 2.53 | m |
| Maximum length | 14 | km |
| Maximum width | 6 | km |
| Surface area | 60 | km2 |
| Stored water volume | 101 × 106 | m3 |
| Shoreline length | 42 | km |
| Sub-Basin | Area (km2) |
|---|---|
| Pirayú | 367 |
| Yukyry | 343 |
| West shore | 51 |
| East shore | 65 |
| Subtotal | 826 |
| Ypacaraí Lake mean surface area | 60 |
| Ypacaraí Lake (sub-basin) | 886 |
| Salado wetlands (incremental sub-basin) | 203 |
| Salado River Basin | 1089 |
| Dataset | Owner 1 | Funded by | Covered Period | Variables 2 |
|---|---|---|---|---|
| JICA 3 | STP-Paraguay 4 | Japan | 16 February 1988–3 March 1989 | Tw, SD, SS, TP, TN, NO3−, DO, Chl-a, pH, EC |
| CEMIT-UNA I | CEMIT-UNA 5 | UNA | 5 October 2012–29 April 2014 | Tw, SD, Turb, TP, TN |
| CEMIT-UNA II | Itaipú Binational Entity | Itaipú Binational Entity | 1 December 2014–ongoing | Tw, SS, SD, Turb, TP, TN, DO, Chl-a |
| CIH-Itaipú 6 | Itaipú Binational Entity | Itaipú Binational Entity | 11 May 2016–ongoing | Tw, h, EC |
| ANNP 7 | ANNP-Paraguay | Paraguay | 1 January 1965–31 December 2007 1 February 2013–10 May 2016 | h |
| DMH-DINAC 8 | DMH-DINAC-Paraguay | Paraguay | 1 September 1959–ongoing | Ta, Precip, u10, RH |
| Variable (Units) 1 | Dataset 2 | Arithmetic Mean | Standard Deviation | Kendall’s τ | Sen’s Slope, S |
|---|---|---|---|---|---|
| Tw (°C) | JICA | 24.7 | 5.75 | n.s. 3 | n.s. |
| CEMIT-UNA I/II | 24.7 | 4.76 | |||
| h (m) | ANNP | 1.59 | 0.140 | −0.654 | −338 |
| ANNP/CIH-Itaipú | 0.643 | 0.336 | |||
| SS (mg·L−1) | JICA | 23.2 | 13.6 | n.s. | n.s. |
| CEMIT-UNA II | 40.2 | 36.1 | |||
| SD (m) | JICA | 0.513 | 0.187 | −0.482 | −392 |
| CEMIT-UNA I/II | 0.248 | 0.152 | |||
| TP (mg·L−1) | JICA | 0.124 | 0.0531 | +0.315 | +258 |
| CEMIT-UNA I/II | 0.227 | 0.148 | |||
| TN (mg·L−1) | JICA | 1.91 | 0.854 | n.s. | n.s. |
| CEMIT-UNA I/II | 1.75 | 1.19 | |||
| DO (mg·L−1) | JICA | 9.27 | 1.50 | −0.394 | −171 |
| CEMIT-UNA II | 7.57 | 0.824 | |||
| Chl-a (µg·L−1) | JICA | 54.3 | 24.6 | −0.589 | −445 |
| CEMIT-UNA II | 9.25 | 6.41 |
| 1988–1989 | Tw | h | SD | SS | TP | TN | NO3− | Chl-a | DO | pH | EC | CumRain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tw | 1.00 | −0.43 | −0.48 | 0.22 | 0.54 | 0.12 | 0.26 | 0.29 | −0.51 | −0.27 | 0.65 2 | −0.12 |
| h | 1.00 | 0.15 | −0.53 | 0.13 | −0.30 | 0.41 | −0.29 | −0.02 | −0.01 | −0.79 3 | 0.48 | |
| SD | 1.00 | −0.76 | −0.82 | −0.25 | −0.28 | −0.17 | 0.49 | 0.47 | −0.33 | −0.09 | ||
| SS | 1.00 | 0.47 | 0.48 | −0.14 | 0.24 | −0.27 | −0.17 | 0.41 | 0.16 | |||
| TP | 1.00 | 0.35 | 0.54 | 0.08 | −0.80 | −0.31 | 0.01 | 0.15 | ||||
| TN | 1.00 | 0.32 | −0.10 | −0.50 | 0.13 | −0.02 | −0.31 | |||||
| NO3− | 1.00 | 0.05 | −0.43 | 0.11 | −0.37 | 0.08 | ||||||
| Chl-a | 1.00 | 0.37 | 0.63 | 0.14 | 0.41 | |||||||
| DO | 1.00 | 0.44 | −0.03 | −0.23 | ||||||||
| pH | 1.00 | −0.42 | 0.22 | |||||||||
| EC | 1.00 | −0.41 | ||||||||||
| CumRain | 1.00 |
| 2014–2017 | Tw | h | SD | Turb | TP | TN | NO3− | Chl-a | DO | pH | EC | CumRain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tw | 1.00 | 0.26 | −0.35 | 0.28 | 0.45 | 0.08 | 0.04 | 0.00 | −0.37 | −0.47 | −0.07 | −0.12 |
| h | 1.00 | −0.29 | −0.31 | −0.01 | 0.01 | −0.19 | −0.18 | −0.16 | −0.34 | −0.82 3 | −0.32 | |
| SD | 1.00 | −0.62 2 | 0.13 | 0.00 | −0.46 | 0.02 | 0.33 | 0.67 | 0.24 | −0.26 | ||
| Turb | 1.00 | −0.05 | 0.15 | 0.51 | 0.06 | −0.17 | −0.53 | 0.14 | 0.36 | |||
| TP | 1.00 | 0.16 | −0.05 | 0.01 | 0.01 | −0.07 | 0.05 | −0.04 | ||||
| TN | 1.00 | −0.11 | 0.52 | −0.37 | 0.17 | 0.07 | 0.05 | |||||
| NO3− | 1.00 | −0.26 | −0.19 | −0.03 | 0.37 | 0.61 | ||||||
| Chl-a | 1.00 | −0.40 | 0.27 | −0.01 | −0.11 | |||||||
| DO | 1.00 | 0.18 | −0.03 | −0.18 | ||||||||
| pH | 1.00 | 0.46 | 0.17 | |||||||||
| EC | 1.00 | 0.50 | ||||||||||
| CumRain | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Moreira M., G.A.; Hinegk, L.; Salvadore, A.; Zolezzi, G.; Hölker, F.; Monte Domecq S., R.A.; Bocci, M.; Carrer, S.; De Nat, L.; Escribá, J.; et al. Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay). Sustainability 2018, 10, 2426. https://doi.org/10.3390/su10072426
López Moreira M. GA, Hinegk L, Salvadore A, Zolezzi G, Hölker F, Monte Domecq S. RA, Bocci M, Carrer S, De Nat L, Escribá J, et al. Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay). Sustainability. 2018; 10(7):2426. https://doi.org/10.3390/su10072426
Chicago/Turabian StyleLópez Moreira M., Gregorio Alejandro, Luigi Hinegk, Andrea Salvadore, Guido Zolezzi, Franz Hölker, Roger Arturo Monte Domecq S., Martina Bocci, Sebastiano Carrer, Luca De Nat, Juan Escribá, and et al. 2018. "Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay)" Sustainability 10, no. 7: 2426. https://doi.org/10.3390/su10072426
APA StyleLópez Moreira M., G. A., Hinegk, L., Salvadore, A., Zolezzi, G., Hölker, F., Monte Domecq S., R. A., Bocci, M., Carrer, S., De Nat, L., Escribá, J., Escribá, C., Benítez, G. A., Ávalos, C. R., Peralta, I., Insaurralde, M., Mereles, F., Sekatcheff, J. M., Wehrle, A., Facetti-Masulli, J. F., ... Toffolon, M. (2018). Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay). Sustainability, 10(7), 2426. https://doi.org/10.3390/su10072426






