Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review
Abstract
:1. Introduction
2. CaO/CaCO3 and CaO/Ca(OH)2 TES
2.1. CaO/CaCO3 TES
2.2. CaO/Ca(OH)2 TES
2.3. Coupling of CaO/Ca(OH)2 and CaO/CaCO3 TES
3. Effect of Energy Storage Conditions on CaO/Ca(OH)2 TES
3.1. Effect of Form of Water in Hydration and Dehydration Atmosphere
3.2. Effect of Temperature and Vapor Pressure
- (a)
- with increasing the dehydration temperature, the dehydration rate (the ratio of dehydration conversion to time) of Ca(OH)2 was accelerated and the dehydration conversion was greatly improved;
- (b)
- when the initial sample temperature of the hydration reaction increased, both the hydration rate (the ratio of hydration conversion to time) and the hydration conversion of CaO decreased sharply.
3.3. Effect of Reactor Type
4. Improvement on Performance of CaO-Based Materials in CaO/Ca(OH)2 TES
4.1. CaO-Based Materials Modified with Al2O3 in CaO/Ca(OH)2 TES
4.2. CaO-Based Materials Modified with LiOH in CaO/Ca(OH)2 TES
4.3. CaO-Based Materials Modified with Na2Si3O7 and Nano-SiO2 in CaO/Ca(OH)2 TES
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Nomenclature | |
| t | reaction time, s |
| A | pre-exponential factor, s−1 |
| E | apparent activation energy, J/mol |
| R | ideal gas constant, 8.314 J/(mol·K) |
| T | thermodynamic temperature, K |
| f(·) | differential mechanism function |
| G(·) | integral mechanism function |
| d | particle size, μm |
| P | steam pressure, MPa |
| P* | steam pressure at equilibrium, MPa |
| ex | global exergy destruction, W |
| z | exergy impedance, W−1 |
| q | thermal power consumed by the reaction, W |
| m | mass, kg |
| X | conversion, % |
| k | kinetic constant |
| P | water partial pressure, Pa |
| T | temperature, K |
| Qin | thermal power input from CSP used for the calcination of CaCO3, MW |
| Qout | thermal power output from the carbonation reactor or hydrator, MW |
| Estored | rate of energy stored, MW |
| mmedia | rate of media stored, kg/s |
| ρmedia | density of energy storage media, kg/m3 |
| Dv | energy density, kWh/m3 |
| Dm | specific energy, kJ/kg |
| Greek letters | |
| α | conversion rate, indicating the degree of heterogeneous system reaction, % |
| β | heating rate, K/min |
| v | steam volume fraction, % |
| Subscripts | |
| p | particle |
| H2O | water |
| d | destruction |
| Hy | hydration |
| eq | equilibrium |
| Dehy | dehydration |
References
- Asif, M.; Muneer, T. Energy Supply, its Demand and Security Issues for Developed and Emerging Economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Simon, S.; Naegler, T.; Gils, H. Transformation towards a Renewable Energy System in Brazil and Mexico-Technological and Structural Options for Latin America. Energies 2018, 11, 907. [Google Scholar] [CrossRef]
- Kim, T.; Jeong, Y. Analysis of Energy-Related Greenhouse Gas Emission in the Korea’s Building Sector: Use National Energy Statistics. Energies 2018, 11, 855. [Google Scholar] [CrossRef]
- Jones, G.A.; Warner, K.J. The 21st Century Population-Energy-Climate Nexus. Energy Policy 2016, 93, 206–212. [Google Scholar] [CrossRef]
- Bartłomiejczyk, M. Potential Application of Solar Energy Systems for Electrified Urban Transportation Systems. Energies 2018, 11, 954. [Google Scholar] [CrossRef]
- Mendecka, B.; Lombardi, L.; Gładysz, P.; Stanek, W. Exergo-Ecological Assessment of Waste to Energy Plants Supported by Solar Energy. Energies 2018, 11, 773. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, R.; Yang, Y.; Reyes-Belmonte, M. Techno-Economic Analysis of Solar Tower Aided Coal-Fired Power Generation System. Energies 2017, 10, 1392. [Google Scholar] [CrossRef]
- Schmidt, M.; Linder, M. Power Generation Based on the Ca(OH)2/CaO Thermochemical Storage System-Experimental Investigation of Discharge Operation Modes in Lab Scale and Corresponding Conceptual Process Design. Appl. Energy 2017, 203, 594–607. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of Thermochemical Systems Based on Solid-Gas Reversible Reactions for High Temperature Solar Thermal Energy Storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Sun, F.; Peng, H.; Ling, X. Progress in Medium to High Temperature Thermochemical Energy Storage Technologies. Energy Storage Sci. Technol. 2015, 4, 577–584. [Google Scholar]
- Aydin, D.; Casey, S.P.; Riffat, S. The Latest Advancements on Thermochemical Heat Storage Systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal Energy Storage: Recent Developments and Practical Aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A Review on High Temperature Thermochemical Heat Energy Storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical Energy Storage and Conversion: A-state-of-the-art Review of the Experimental Research under Practical Conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A Review of Promising Candidate Reactions for Chemical Heat Storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of Technology: Thermochemical Energy Storage for Concentrated Solar Power Plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical Heat Storage for High Temperature Applications—A Review. Green 2013, 3, 113–123. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.Z.; Li, Y. A Review of Available Technologies for Seasonal Thermal Energy Storage. Sol. Energy 2014, 103, 610–638. [Google Scholar] [CrossRef]
- Deutsch, M.; Müller, D.; Aumeyr, C.; Jordan, C.; Gierl-Mayer, C. Systematic Search Algorithm for Potential Thermochemical Energy Storage Systems. Appl. Energy 2016, 183, 113–120. [Google Scholar] [CrossRef]
- Erlund, R.; Zevenhoven, R. Hydration of Magnesium Carbonate in a Thermal Energy Storage Process and its Heating Application Design. Energies 2018, 11, 170. [Google Scholar] [CrossRef]
- Mastronardo, E.; Kato, Y.; Bonaccorsi, L.; Piperopoulos, E.; Milone, C. Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies 2017, 10, 70. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical Characterizations of Novel Vermiculite-LiCl Composite Sorbents for Low-Temperature Heat Storage. Energies 2016, 9, 854. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the Art on High Temperature Thermal Energy Storage for Power Generation. Part 1—Concepts, Materials and Modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Tamme, R.; Bauer, T.; Buschle, J.; Laing, D.; Müller-Steinhagen, H.; Steinhagen, W.D. Latent Heat Storage Above 1208 °C for Applications in the Industrial Process Heat Sector and Solar Power Generation. Int. J. Energy Res. 2008, 32, 264–271. [Google Scholar] [CrossRef]
- Pincemin, S.; Olives, R.; Py, X.; Christ, M. Highly Conductive Composites Made of Phase Change Materials and Graphite for Thermal Storage. Sol. Energy Mater. Sol. Cells 2008, 92, 603–613. [Google Scholar] [CrossRef]
- Hoshi, A.; Mills, D.R.; Bittar, A.; Saitoh, T.S. Screening of High Melting Point Phase Change Materials (PCM) in Solar Thermal Concentrating Technology Based on CLFR. Sol. Energy 2005, 79, 332–339. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierres, N.; Luo, L. A Review on Long-Term Sorption Solar Energy Storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Kyaw, K.; Matsuda, H.; Hasatani, M. Applicability of Carbonation/Decarbonation Reactions to High-Temperature Thermal Energy Storage and Temperature Upgrading. J. Chem. Eng. Jpn. 1996, 29, 119–125. [Google Scholar] [CrossRef]
- Funken, K.H. Solar Chemistry: Classification, Criteria and Identification of R&D Deficits. Sol. Energy Mater. 1991, 24, 370–385. [Google Scholar]
- André, L.; Abanades, S. Evaluation and Performances Comparison of Calcium, Strontium and Barium Carbonates during Calcination/Carbonation Reactions for Solar Thermochemical Energy Storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Obermeier, J.; Sakellariou, K.G.; Tsongidis, N.I.; Baciu, D.; Charalambopoulou, G.; Steriotis, T.; Müller, K.; Karagiannakis, G.; Konstandopoulos, A.G.; Stubos, A.; et al. Material Development and Assessment of an Energy Storage Concept Based on the CaO-looping Process. Sol. Energy 2017, 150, 298–309. [Google Scholar] [CrossRef]
- Chacartegui, R.; Alovisio, A.; Ortiz, C.; Valverde, J.M.; Verda, V.; Becerra, J.A. Thermochemical Energy Storage of Concentrated Solar Power by Integration of the Calcium Looping Process and a CO2 Power Cycle. Appl. Energy 2016, 173, 589–605. [Google Scholar] [CrossRef]
- Baker, E.H. The Calcium Oxide-Carbon Dioxide System in the Pressure Range 1–300 Atmospheres. J. Chem. Soc. 1962, 70, 464–470. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C. CO2 Capture Capacity of CaO in Long Series of Carbonation/Calcination Cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C.; Alonso, M.; González, B. Reactivity of Highly Cycled Particles of CaO in a Carbonation/Calcination Loop. Chem. Eng. J. 2008, 137, 561–567. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C. Development of a CaO-based Sorbent with Improved Cyclic Stability for CO2 Capture in Pressurized Carbonation. Chem. Eng. J. 2011, 171, 197–205. [Google Scholar] [CrossRef]
- Valverde, J.M.; Barea-López, M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Effect of Thermal Pretreatment and Nanosilica Addition on Limestone Performance at Calcium-Looping Conditions for Thermochemical Energy Storage of Concentrated Solar Power. Energy Fuels 2017, 31, 4226–4236. [Google Scholar] [CrossRef] [Green Version]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D. Solar Chemical Reactor Technology for Industrial Production of Lime. Sol. Energy 2006, 80, 1355–1362. [Google Scholar] [CrossRef]
- Meier, A.; Gremaud, N.; Steinfeld, A. Economic Evaluation of the Industrial Solar Production of Lime. Energy Convers. Manag. 2005, 46, 905–926. [Google Scholar] [CrossRef]
- Edwards, S.E.B.; Materić, V. Calcium Looping in Solar Power Generation Plants. Sol. Energy 2012, 86, 2494–2503. [Google Scholar] [CrossRef]
- Müller, B.; Arlta, W.; Wasserscheid, P. A New Concept for the Global Distribution of Solar Energy: Energy Carrying Compounds. Energy Environ. Sci. 2011, 4, 4322–4331. [Google Scholar] [CrossRef]
- Obermeier, J.; Müller, K.; Karagiannakis, G.; Stubos, A.; Arlt, A.W. A Novel Thermochemical Energy Storage and Transportation Concept Based on Concentrated Solar Irradiation-Aided CaO-looping. AIP Conf. Proc. 2016, 1734, 050033. [Google Scholar] [CrossRef]
- Sarrion, B.; Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.A.; Sanchez-Jimenez, P.E. On the Multicycle Activity of Natural Limestone/Dolomite for Cheap, Efficient and Non-Toxic Thermochemical Energy Storage of Concentrated Solar Power. Available online: https://www.researchgate.net/publication/301705419 (accessed on 2 May 2018).
- Valverde, J.M.; Miranda-Pizarro, J.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Calcium-Looping Performance of Steel and Blast Furnace Slags for Thermochemical Energy Storage in Concentrated Solar Power Plants. J. CO2 Util. 2017, 22, 143–154. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, S.; Zhao, R.; He, J.; Zhao, L. Energy-Saving Pathway Exploration of CCS Integrated with Solar Energy: A Review of Innovative Concepts. Renew. Sustain. Energy Rev. 2017, 77, 652–669. [Google Scholar] [CrossRef]
- Xu, Y.B.; Zhao, M.; Qiu, Y.; Zhou, C.; Gu, P.; Xiang, W. Performance of Coal Power Generation Integrating CaO Based Solar Energy Storage with CO2 Capture. Acta Energiae Solaris Sin. 2017, 38, 180–185. [Google Scholar]
- Zhou, C.; Xiang, W.G.; Chen, S.Y.; Xu, Y.B. Simulation of High-Temperature Energy Storage System for Wind Power Accommodation with CO2 Negative Emissions. Autom. Electr. Power Syst. 2016, 40, 116–121. [Google Scholar]
- Vandersickel, A.; Field, R.P.; Chen, W.; Mancini, N.D.; Mitsos, A. CaO-Based Energy and CO2 Storage System for the Flexibilization of an IGCC Plant with Carbon Capture. Ind. Eng. Chem. Res. 2014, 53, 12032–12043. [Google Scholar] [CrossRef]
- Siefert, N.; Narburgh, S.; Chen, Y. Comprehensive Exergy Analysis of Three IGCC Power Plant Configurations with CO2 Capture. Energies 2016, 9, 669. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Karagiannakis, G.; Criado, Y.A.; Konstandopoulos, A.G. Calcium Oxide Based Materials for Thermochemical Heat Storage in Concentrated Solar Power Plants. Sol. Energy 2015, 122, 215–230. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal Energy Storage Technologies and Systems for Concentrating Solar Power Plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Linder, M.; Roßkopf, C.; Schmidt, M.; Wörner, A. Thermochemical Energy Storage in kW-scale Based on CaO/Ca(OH)2. Energy Procedia 2014, 49, 888–897. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Experimental Study of CaO/Ca(OH)2 in a Fixed-Bed Reactor for Thermochemical Heat Storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Dutta, S.; Shirai, T. Experimental Investigation on a Fast and Exothermic Solid–Liquid Reaction System. Chem. Eng. Sci. 1980, 35, 209–216. [Google Scholar] [CrossRef]
- Irabien, A.; Toquero, A.; Ortiz, M.I. Kinetic Behaviour of Non-Isothermal Lime Hydration. Chem. Eng. J. 1989, 40, 93–99. [Google Scholar] [CrossRef]
- Matsuda, H.; Ishizu, T.; Lee, S.K.; Hasatani, M. Kinetic Study of Ca(OH)2/CaO Reversible Thermochemical Reaction for Thermal Energy Storage by Means of Chemical Reaction. Kagaku Kogaku Ronbunshu 1985, 11, 542–548. [Google Scholar] [CrossRef]
- Galwey, A.K.; Laverty, G.M. A Kinetic and Mechanistic Study of the Dehydroxylation of Calcium Hydroxide. Thermochim. Acta 1993, 228, 359–378. [Google Scholar] [CrossRef]
- Lin, S.; Harada, M.; Suzuki, Y.; Hatano, H. CaO Hydration Rate at High Temperature (∼1023 K). Energy Fuels 2006, 20, 903–908. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Wörner, A.; Müller-Steinhagen, H. A Thermodynamic and Kinetic Study of the De- and Rehydration of Ca(OH)2 at High H2O Partial Pressures for Thermo-Chemical Heat Storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Murthy, M.S.; Raghavendrachar, P.; Sriram, S.V. Thermal Decomposition of Doped Calcium Hydroxide for Chemical Energy Storage. Sol. Energy 1985, 36, 53–62. [Google Scholar] [CrossRef]
- Irabien, A.; Viguri, J.R.; Ortiz, I. Thermal Dehydration of Calcium Hydroxide. 1. Kinetic Model and Parameters. Ind. Eng. Chem. Res. 1990, 29, 1599–1606. [Google Scholar] [CrossRef]
- Mikhail, R.S.; Brunauer, S.; Copeland, L.E. Kinetics of the Thermal Decomposition of Calcium Hydroxide. J. Colloid Interface Sci. 1966, 21, 394–404. [Google Scholar] [CrossRef]
- Dutta, S.; Shirai, T. Kinetics of Drying and Decomposition of Calcium Hydroxide. Chem. Eng. Sci. 1974, 29, 2000–2003. [Google Scholar] [CrossRef]
- Chen, D.; Gao, X.; Dollimore, D. The Application of Non-Isothermal Methods of Kinetic Analysis to the Decomposition of Calcium Hydroxide. Thermochim. Acta 1993, 215, 65–82. [Google Scholar] [CrossRef]
- Wang, W.; Kolditz, O.; Nagel, T. Parallel Finite Element Modelling of Multi-Physical Processes in Thermochemical Energy Storage Devices. Appl. Energy 2017, 185, 1954–1964. [Google Scholar] [CrossRef]
- Nagel, T.; Shao, H.; Roßkopf, C.; Linder, M.; Wörner, A.; Kolditz, O. The Influence of Gas-Solid Reaction Kinetics in Models of Thermochemical Heat Storage under Monotonic and Cyclic Loading. Appl. Energy 2014, 136, 289–302. [Google Scholar] [CrossRef]
- Long, X.; Wu, J. Thermal Decomposition Kinetics of Thermochemical Energy Storage System Ca(OH)2/CaO. J. South China Univ. Technol. (Nat. Sci. Ed.) 2014, 42, 75–80. [Google Scholar]
- Neveu, P.; Tescari, S.; Aussel, D.; Mazet, N. Combined Constructal and Exergy Optimization of Thermochemical Reactors for High Temperature Heat Storage. Energy Convers. Manag. 2013, 71, 186–198. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH)2 Hydration/Dehydration Reaction for Thermochemical Energy Storage Applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef]
- Nagel, T.; Shao, H.; Singh, A.K.; Watanabe, N.; Roßkopf, C.; Linder, M.; Wörner, A.; Kolditz, O. Non-Equilibrium Thermochemical Heat Storage in Porous Media: Part 1—Conceptual Model. Energy 2013, 60, 254–270. [Google Scholar] [CrossRef]
- Serris, E.; Favergeon, L.; Pijolat, M.; Soustelle, M.; Nortier, P.; Gärtner, R.S.; Chopin, T.; Habib, Z. Study of the Hydration of CaO Powder by Gas-Solid Reaction. Cem. Concr. Res. 2011, 41, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Watanabe, T.; Kanzawa, A. Proposal of a Chemical Heat Pump with Paraldehyde Depolymerization for Cooling System. Appl. Therm. Eng. 1999, 19, 133–143. [Google Scholar] [CrossRef]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A Review of Chemical Heat Pump Technology and Applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Chan, C.W.; Ling-Chin, J.; Roskilly, A.P. A Review of Chemical Heat Pumps, Thermodynamic Cycles and Thermal Energy Storage Technologies for Low Grade Heat Utilisation. Appl. Therm. Eng. 2013, 50, 1257–1273. [Google Scholar] [CrossRef]
- Ogura, H.; Kubota, M.; Suzuki, H.; Yamakawa, T. Fundamental Experimental Study on Chemical Heat Pump for Storing Low-Temperature Waste Heat and Releasing Cold-Heat. Kagaku Kogaku Ronbunshu 2009, 35, 506–510. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Rossides, S.D.; Haj Khalil, R. Thermal Energy Storage Using Thermo-Chemical Heat Pump. Energy Convers. Manag. 2013, 65, 721–724. [Google Scholar] [CrossRef]
- Obermeier, J.; Müller, K.; Arlt, W. Thermodynamic Analysis of Chemical Heat Pumps. Energy 2015, 88, 489–496. [Google Scholar] [CrossRef]
- He, Z.; Huang, H.; Wang, N.; Liu, X.; Osaka, Y. A Review on Development of Middle-Low Temperature Heat Source Chemical Heat Pump. Huaxue Gongcheng/Chem. Eng. (China) 2015, 43, 1–6. [Google Scholar]
- Kinnarinen, T.; Golmaei, M.; Jernström, E.; Häkkinen, A. Separation, Treatment and Utilization of Inorganic Residues of Chemical Pulp Mills. J. Clean. Prod. 2016, 133, 953–964. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshizawa, Y. Kinetic Study of the Hydration of Magnesium Oxide for a Chemical Heat Pmp. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- Ogura, H.; Yamamoto, T.; Kage, H.; Matsuno, Y.; Mujumdar, A.S.; Yoshizo Matsuno A, A.S.M. Effects of Heat Exchange Condition on Hot Air Production by a Chemical Heat Pump Dryer Using CaO/H2O/Ca(OH)2 Reaction. Chem. Eng. J. 2002, 86, 3–10. [Google Scholar] [CrossRef]
- Fujimoto, S.; Bilgen, E.; Ogura, H. Dynamic Simulation of CaO/Ca(OH)2 Chemical Heat Pump Systems. Exergy Int. J. 2002, 2, 6–14. [Google Scholar] [CrossRef]
- Ogura, H. Energy Recycling System Using Chemical Heat Pump Container. Energy Procedia 2012, 14, 2048–2053. [Google Scholar] [CrossRef]
- Ogura, H.; Shimojyo, R.; Kage, H.; Matsuno, Y.; Mujumdar, A.S. Simulation of Hydration/Dehydration of CaO/Ca(OH)2 Chemical Heat Pump Reactor for Cold/Hot Heat Generation. Dry Technol. 1999, 17, 1579–1592. [Google Scholar] [CrossRef]
- Zhang, H.; Ogura, H. Studies on Hydration Reaction Rates of Various Size CaO Particles for Chemical Heat Storage/Pump. J. Chem. Eng. Jpn. 2014, 47, 587–594. [Google Scholar] [CrossRef]
- Matsuda, H. Temperature Response of the Solid Particle Bed in a High-Temperature Chemical Heat Pump Using Ca(OH)2/CaO Reversible Thermochemical Reaction. Kagaku Kogaku Ronbunshu 1988, 14, 769–778. [Google Scholar] [CrossRef]
- Odukoya, A.; Naterer, G.F. Calcium Oxide/Steam Chemical Heat Pump for Upgrading Waste Heat in Thermochemical Hydrogen Production. Int. J. Hydrog. Energy 2015, 40, 11392–11398. [Google Scholar] [CrossRef]
- Kito, T.; Kobayashi, N. Evaluation of Output Power Characteristics of Chemical Heat Pump by Using CaO-LiCl Compound Reactant. Kagaku Kogaku Ronbunshu 2012, 38, 172–175. [Google Scholar] [CrossRef]
- Arjmand, M.; Liu, L.; Neretnieks, I. Exergetic Efficiency of High-Temperature-Lift Chemical Heat Pump (CHP) Based on CaO/CO2 and CaO/H2O Working Pairs. Int. J. Energy Res. 2013, 37, 1122–1131. [Google Scholar] [CrossRef]
- Aihara, M.; Yoshii, T.; Shimazaki, Y.; Takeuchi, T.; Habuka, H. Decarbonation and Pore Structural Change of Ca-solid Reactant for CaO/CO2 Chemical Heat Pump. J. Chem. Eng. Jpn. 2008, 41, 513–518. [Google Scholar] [CrossRef]
- Ogura, H.; Kanamori, M.; Matsuda, H.; Hasatani, M.; Yanadori, M.; Hiramatsu, M. Heat Storage Characteristics of Chemical Heat Pump Using Ca(OH)2/CaO Reversible Reaction. Kagaku Kogaku Ronbunshu 1993, 19, 553–557. [Google Scholar] [CrossRef]
- Ogura, H.; Fujimoto, S.; Iwamoto, H.; Kage, H.; Matsuno, Y.; Kanamaru, Y. Basic Performance of CaO/H2O/Ca(OH)2 Chemical Heat Pump Unit for Night-Electric Heat-Storage and Cold/Hot Heat-Recovering. Kagaku Kogaku Ronbunshu 1998, 24, 856–861. [Google Scholar] [CrossRef]
- Fujimoto, S.; Bilgen, E.; Ogura, H. CaO/Ca(OH)2 Chemical Heat Pump System. Chem. Eng. Sci. 2002, 43, 947–960. [Google Scholar] [CrossRef]
- Ogura, H.; Yamamoto, T.; Kage, H. Efficiencies of CaO/H2O/Ca(OH)2 Chemical Heat Pump for Heat Storing and Heating/Cooling. Energy 2003, 28, 1479–1493. [Google Scholar] [CrossRef]
- Ogura, H.; Miyazaki, M.; Matsuda, H.; Hasatani, M.; Yanadori, M.; Hiramatsu, M. Experimental Study on Heat Transfer Enhancement of the Solid Reactant Particle Bed in a Chemical Heat Pump Using Ca(OH)2/CaO Reaction. Kagaku Kogaku Ronbunshu 1991, 17, 916–923. [Google Scholar] [CrossRef]
- Ogura, H.; Kanamori, M.; Matsuda, H.; Hasatani, M. Generation of Low-Temperature Heat by Use of CaO/H2O/Ca(OH)2 Reaction. Kagaku Kogaku Ronbunshu 1993, 19, 941–946. [Google Scholar] [CrossRef]
- Ogura, H.; Fujimoto, S.; Sato, S.; Kage, H.; Matsuno, Y. Low-Temperature Heat-Generation by Chemical Heat Pump Using CaO/H2O/Ca(OH)2 Reversible Reaction-Effect of Reactor Design and Heat-Exchange Condition. Kagaku Kogaku Ronbunshu 1997, 23, 397–403. [Google Scholar] [CrossRef]
- Ogura, H.; Miyazaki, M.; Matsuda, H.; Hasatani, M.; Yanadori, M.; Hiramatsu, M. Numerical Analysis of Heat Transfer in Particle-Bed Reactor with Fins in Chemical Heat Pump Using Ca(OH)2/CaO Reaction. Kagaku Kogaku Ronbunshu 1992, 18, 669–676. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Zamengo, M.; Ryu, J.; Kato, Y. Energy Density Enhancement of Chemical Heat Storage Material for Magnesium Oxide/Water Chemical Heat Pump. Appl. Therm. Eng. 2015, 91, 377–386. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Ryu, J.; Kato, Y. Dehydration Kinetic Study of a Chemical Heat Storage Material with Lithium Bromide for a Magnesium Oxide/Water Chemical Heat Pump. Prog. Nucl. Energy 2015, 82, 153–158. [Google Scholar] [CrossRef]
- Ogura, H.; Nagura, A.; Matsuda, H.; Hasatani, M.; Yanadori, M.; Hiramatsu, M. Studies on Improving the Efficiency of Chemical Heat Pump Using CaO/Ca(OH)2 Reversible Reaction. In Proceedings of the 4th World Congress of Chemical Engineering, Karlsruhe, Germany, 16–21 June 1991. [Google Scholar]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental Results of a 10 kW High Temperature Thermochemical Storage Reactor Based on Calcium Hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Azpiazu, M.N.; Morquillas, J.M.; Vazquez, A. Heat Recovery From a Thermal Energy Storage Based on the Ca(OH)2/CaO Cycle. Appl. Therm. Eng. 2003, 23, 733–741. [Google Scholar] [CrossRef]
- Schaube, F.; Schaube, F.; Worner, A.; Tamme, R. High Temperature Thermochemical Heat Storage for Concentrated Solar Power Using Gas-Solid Reactions. J. Sol. Energy 2011, 133, 1–7. [Google Scholar] [CrossRef]
- Brown, D.R.; La Marche, J.L.; Spanner, G.E. Chemical Energy Storage System for Solar Electric Generating System (SEGS) Solar Thermal Power Plant. J. Sol. Energy 1992, 114, 212–218. [Google Scholar] [CrossRef]
- Kanamori, M.; Matsuda, H.; Hasatani, M. Heat Storing/Releasing Characteristics of a Chemical Heat Storage Unit of Electricity Using a Ca(OH)2/CaO Reaction. Heat Transf. Res. 1996, 25, 400–409. [Google Scholar] [CrossRef]
- Materić, V.; Edwards, S.; Smedley, S.I.; Holt, R. Ca(OH)2 Superheating as a Low-Attrition Steam Reactivation Method for CaO in Calcium Looping Applications. Ind. Eng. Chem. Res. 2010, 49, 12429–12434. [Google Scholar] [CrossRef]
- Schaube, F.; Kohzer, A.; Schütz, J.; Wörner, A.; Müller-Steinhagen, H. De- and Rehydration of Ca(OH)2 in a Reactor with Direct Heat Transfer for Thermo-Chemical Heat Storage. Part A: Experimental Results. Chem. Eng. Res. Des. 2013, 91, 856–864. [Google Scholar] [CrossRef]
- Hanak, D.P.; Biliyok, C.; Manovic, V. Calcium Looping with Inherent Energy Storage for Decarbonisation of Coal-Fired Power Plant. Energy Environ. Sci. 2016, 9, 971–983. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Steam Reactivation of Spent CaO-Based Sorbent for Multiple CO2 Capture Cycles. Environ. Sci. Technol. 2007, 41, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ramkumar, S.; Li, S.; Wong, D.; Iyer, M.; Sakadjian, B.B.; Statnick, R.M.; Fan, L.S. Subpilot Demonstration of the Carbonation−Calcination Reaction (CCR) Process: High-Temperature CO2 and Sulfur Capture from Coal-Fired Power Plants. Ind. Eng. Chem. Res. 2010, 49, 5094–5101. [Google Scholar] [CrossRef]
- Materić, V.; Symonds, R.; Lu, D.; Holt, R.; Manović, V. Performance of Hydration Reactivated Ca Looping Sorbents in a Pilot-Scale, Oxy-Fired Dual Fluid Bed Unit. Energy Fuels 2014, 28, 5363–5372. [Google Scholar] [CrossRef]
- Nikulshina, V.; Hirsch, D.; Mazzotti, M.; Steinfeld, A. CO2 Capture From Air and Co-Production of H2 Via the Ca(OH)2-CaCO3 Cycle Using Concentrated Solar power-Thermodynamic Analysis. Energy 2006, 31, 1715–1725. [Google Scholar] [CrossRef]
- Nikulshina, V.; Steinfeld, A. CO2 Capture From Air Via CaO-carbonation Using a Solar-Driven Fluidized Bed Reactor-Effect of Temperature and Water Vapor Concentration. Chem. Eng. J. 2009, 155, 867–873. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, C.S.; Qu, C.R.; Duan, L.B.; Li, Q.Z.; Liang, C. CO2 Capture Using CaO Modified with Ethanol/Water Solution during Cyclic Calcination/Carbonation. Chem. Eng. Technol. 2008, 31, 237–244. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Composite Material for Thermochemical Energy Storage Using CaO/Ca(OH)2. Ind. Eng. Chem. Res. 2015, 54, 9314–9327. [Google Scholar] [CrossRef]
- Knoll, C.; Müller, D.; Artner, W.; Welch, J.M.; Werner, A.; Harasek, M.; Weinberger, P. Probing Cycle Stability and Reversibility in Thermochemical Energy Storage-CaC2O4·H2O as Perfect Match? Appl. Energy 2017, 187, 1–9. [Google Scholar] [CrossRef]
- Shi, H.S.; Zhao, Y.J.; Li, W.W. Effects of Temperature on the Hydration Characteristics of Free Lime. Cem. Concr. Res. 2002, 32, 789–793. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C.; Anxionnaz-Minvielle, Z. Conceptual Process Design of a CaO/Ca(OH)2 Thermochemical Energy Storage System Using Fluidized Bed Reactors. Appl. Therm. Eng. 2014, 73, 1087–1094. [Google Scholar] [CrossRef]
- Pardo, P.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cognet, P.; Cabassud, M. Ca(OH)2/CaO Reversible Reaction in a Fluidized Bed Reactor for Thermochemical Heat Storage. Sol. Energy 2014, 107, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Fennell, P.S.; Pacciani, R.; Dennis, J.S.; Davidson, J.F.; Hayhurst, A.N. The Effects of Repeated Cycles of Calcination and Carbonation on a Variety of Different Limestones, as Measured in a Hot Fluidized Bed of Sand. Energy Fuels 2007, 21, 2072–2081. [Google Scholar] [CrossRef]
- Arias, B.; Criado, Y.A.; Sanchez-Biezma, A.; Abanades, J.C. Oxy-Fired Fluidized Bed Combustors with a Flexible Power Output Using Circulating Solids for Thermal Energy Storage. Appl. Energy 2014, 132, 127–136. [Google Scholar] [CrossRef]
- Ma, Z.W.; Glatzmaier, G.; Mehos, M. Fluidized Bed Technology for Concentrating Solar Power with Thermal Energy Storage. J. Sol. Energy 2014, 136, 1–9. [Google Scholar] [CrossRef]
- Ma, Z.; Glatzmaier, G.C.; Mehos, M. Development of Solid Particle Thermal Energy Storage for Concentrating Solar Power Plants that Use Fluidized Bed Technology. Energy Procedia 2014, 49, 898–907. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental Investigation and Model Validation of a CaO/Ca(OH)2 Fluidized Bed Reactor for Thermochemical Energy Storage Applications. Chem. Eng. J. 2016. [Google Scholar] [CrossRef]
- Schmidt, M.; Gollsch, M.; Giger, F.; Grün, M.; Linder, M. Development of a Moving Bed Pilot Plant for Thermochemical Energy Storage with CaO/Ca(OH)2. AIP Conf. Proc. 2016, 1734, 050041. [Google Scholar] [CrossRef]
- Kribus, A.; Doron, P.; Rubin, R.; Karni, J.; Reuven, R.; Duchan, S.; Taragan, E. A Multistage Solar Receiver: The Route to High Temperature. Sol. Energy 1999, 67, 3–11. [Google Scholar] [CrossRef]
- Iguchi, Y.; Narushima, T.; Izumi, C. Calorimetric Study on Hydration of CaO-based Oxides. J. Alloys Compd. 2001, 321, 276–281. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. First-Principle Study of CaO/Ca(OH)2 Thermochemical Energy Storage System by Li Or Mg Cation Doping. Chem. Eng. Sci. 2014, 117, 293–300. [Google Scholar] [CrossRef]
- Sakellarious, K.G.; Tsongidis, N.I.; Karagiannaki, G.; Konstandopoulos, A.G.; Baciu, D.; Charalambopoulou, G.; Steriotis, T.; Stubos, A.; Arlt, W. Development and Evaluation of Materials for Thermochemical Heat Storage Based On the CaO/CaCO3 Reaction Couple. AIP Conf. Proc. 2016, 1734, 050040. [Google Scholar] [CrossRef]
- Mohamed, B.M.; Sharp, J.H. Kinetics and Mechanism of Formation of Tricalcium Aluminate, Ca3Al2O6. Thermochim. Acta 2002, 388, 105–114. [Google Scholar] [CrossRef]
- Rivas Mercury, J.M.; De Aza, A.H.; Turrillas, X.; Pena, P. The Synthesis Mechanism of Ca3Al2O6 from Soft Mechanochemically Activated Precursors Studied by Time-Resolved Neutron Diffraction Up to 1000 °C. J. Solid State Chem. 2004, 177, 866–874. [Google Scholar] [CrossRef]
- Ruszak, M.; Witkowski, S.; Pietrzyk, P.; Kotarba, A.; Sojka, Z. The Role of Intermediate Calcium Aluminate Phases in Solid State Synthesis of Mayenite (Ca12Al14O33). Funct. Mater. Lett. 2011, 04, 183–186. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and Kinetic Study of the Dehydration Process of CaO/Ca(OH)2 Thermochemical Heat Storage System with Li Doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 Based Material for Thermochemical Energy Storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Roßkopf, C.; Afflerbach, S.; Schmidt, M.; Görtz, B.; Kowald, T.; Linder, M.; Trettin, R. Investigations of Nano Coated Calcium Hydroxide Cycled in a Thermochemical Heat Storage. Energy Convers. Manag. 2015, 97, 94–102. [Google Scholar] [CrossRef]
- Roßkopf, C.; Haas, M.; Faik, A.; Linder, M.; Wörner, A. Improving Powder Bed Properties for Thermochemical Storage by Adding Nanoparticles. Energy Convers. Manag. 2014, 86, 93–98. [Google Scholar] [CrossRef]
- Jaskula, B.W. 2014 Minerals Yearbook-Lithium [Advance Release]. Sci. Chang. World 2016, 44, 1–12. [Google Scholar]
- Fujii, I.; Ishino, M.; Akiyama, S.; Murthy, M.S.; Rajanandam, K.S. Behavior of Ca(OH)2/CaO Pellet Under Dehydration and Hydration. Sol. Energy 1994, 53, 329–341. [Google Scholar] [CrossRef]
- Martínez, I.; Grasa, G.; Murillo, R.; Arias, B.; Abanades, J.C. Evaluation of CO2 Carrying Capacity of Reactivated CaO by Hydration. Energy Fuels 2011, 25, 1294–1301. [Google Scholar] [CrossRef] [Green Version]

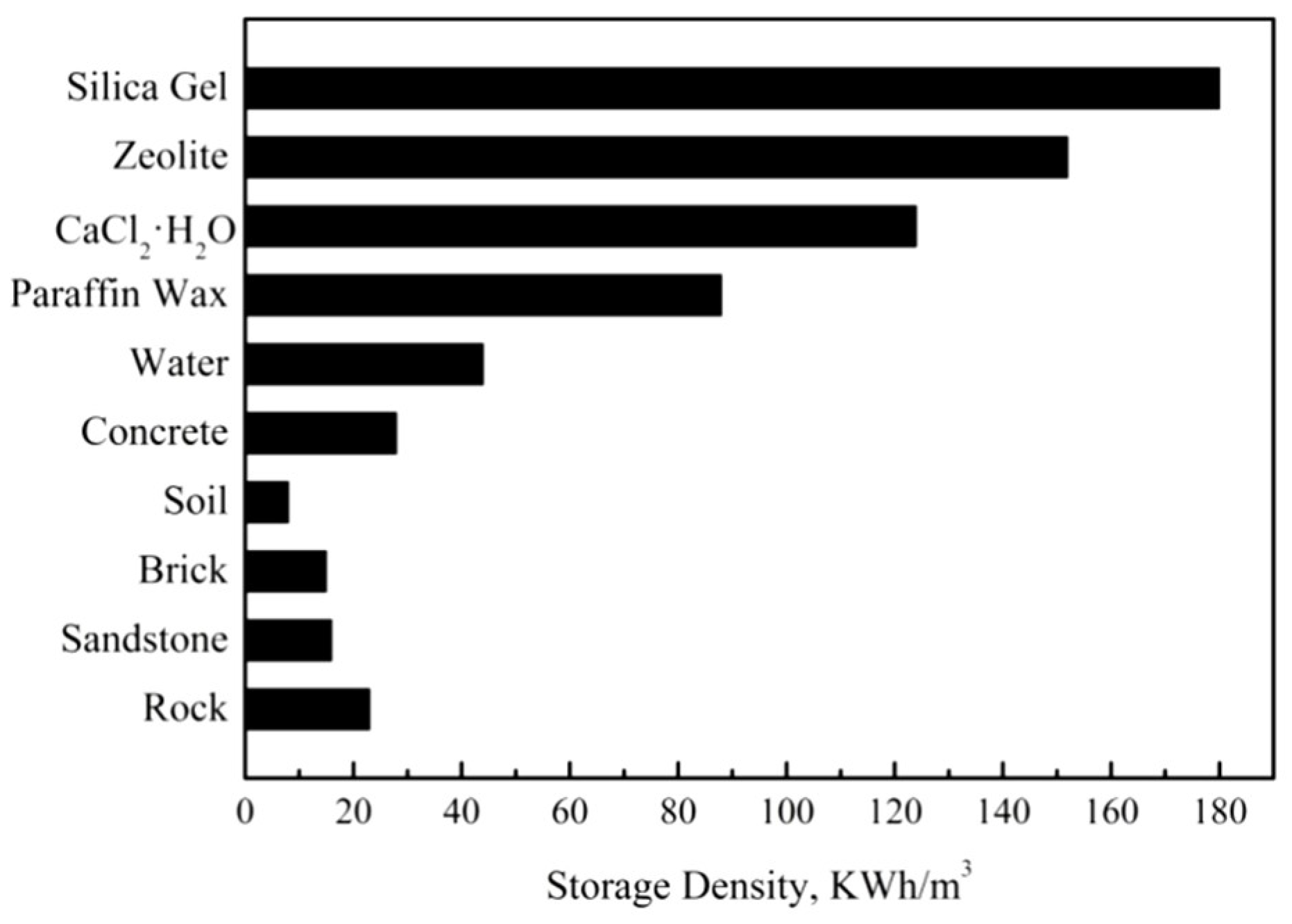
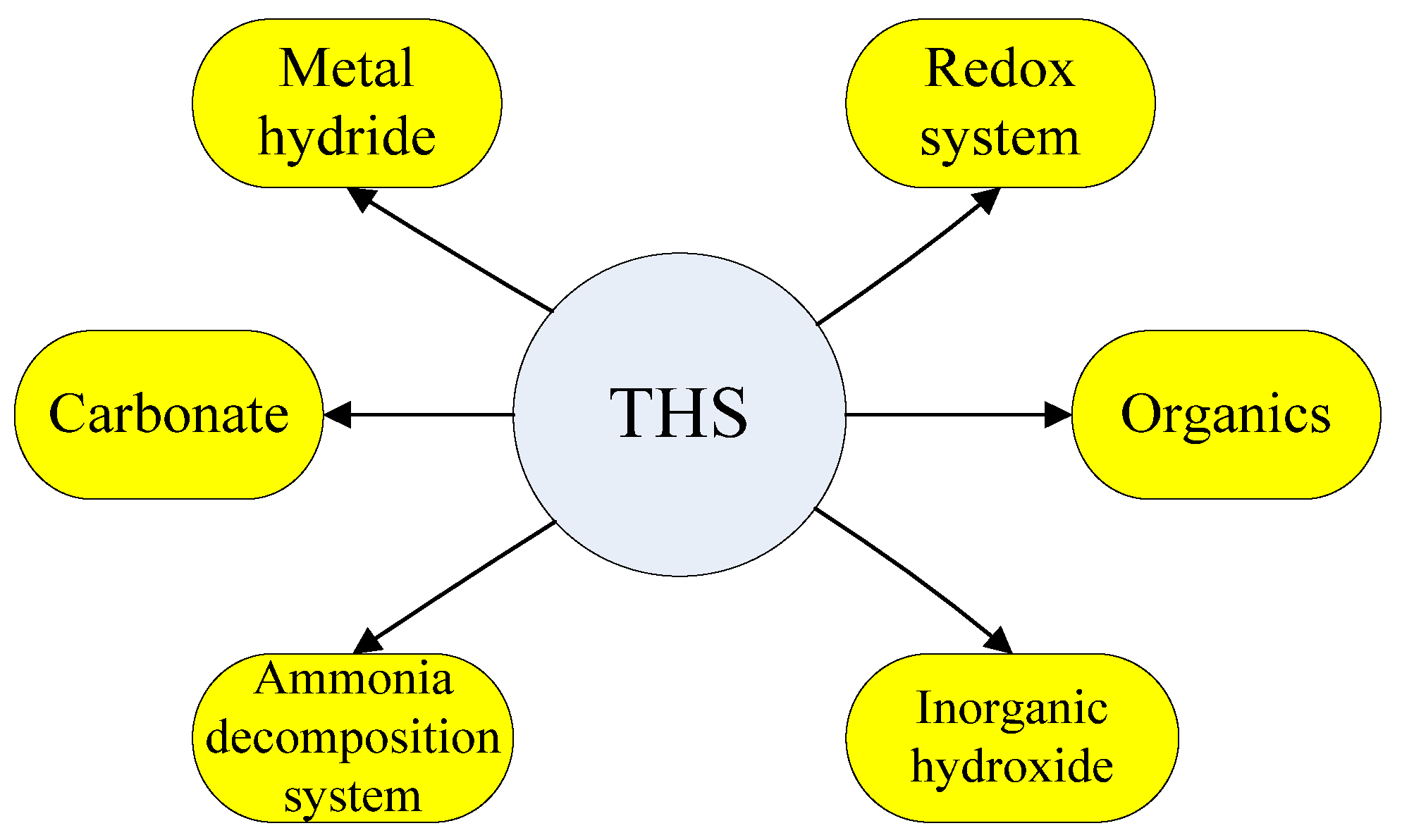

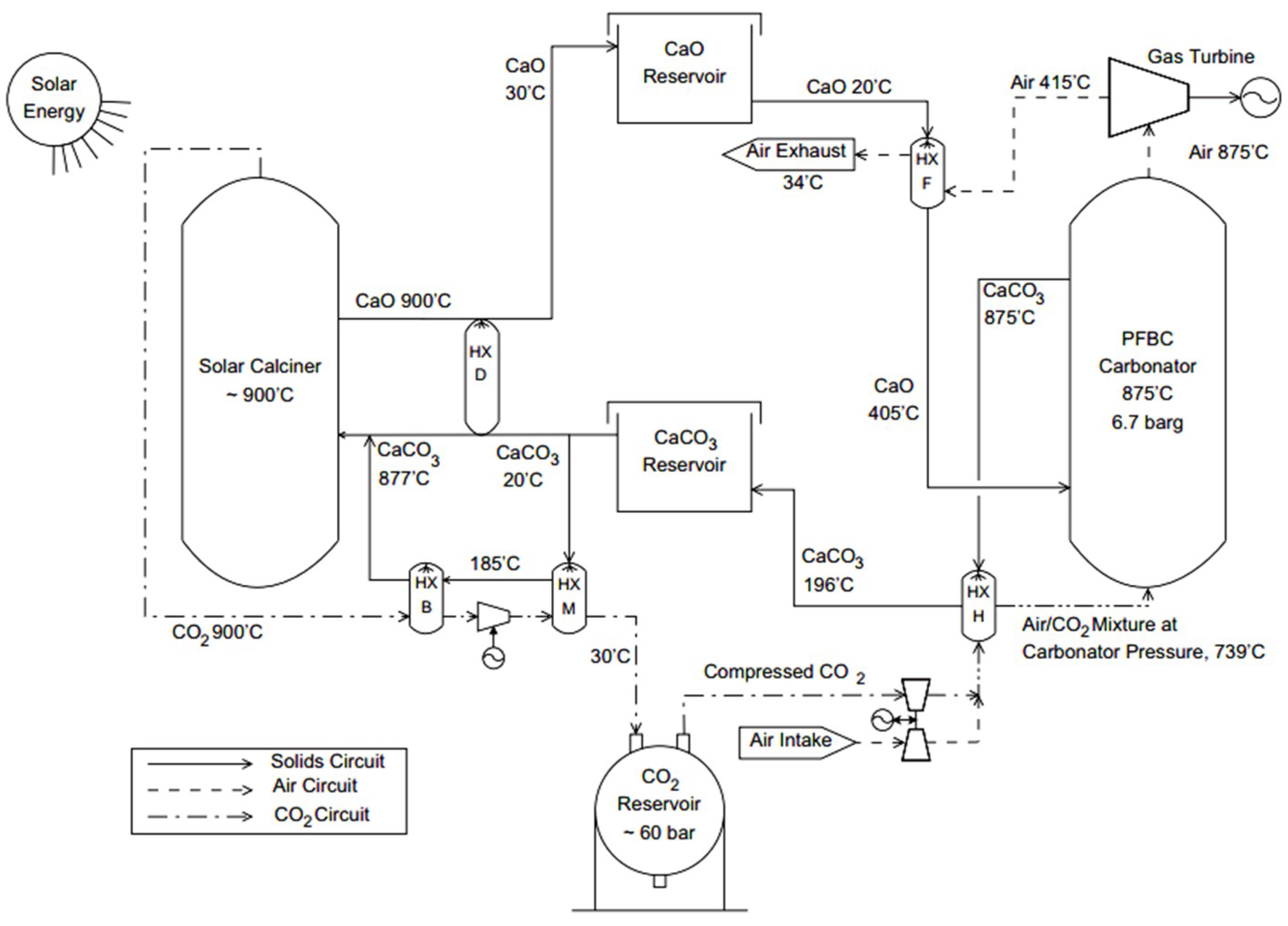
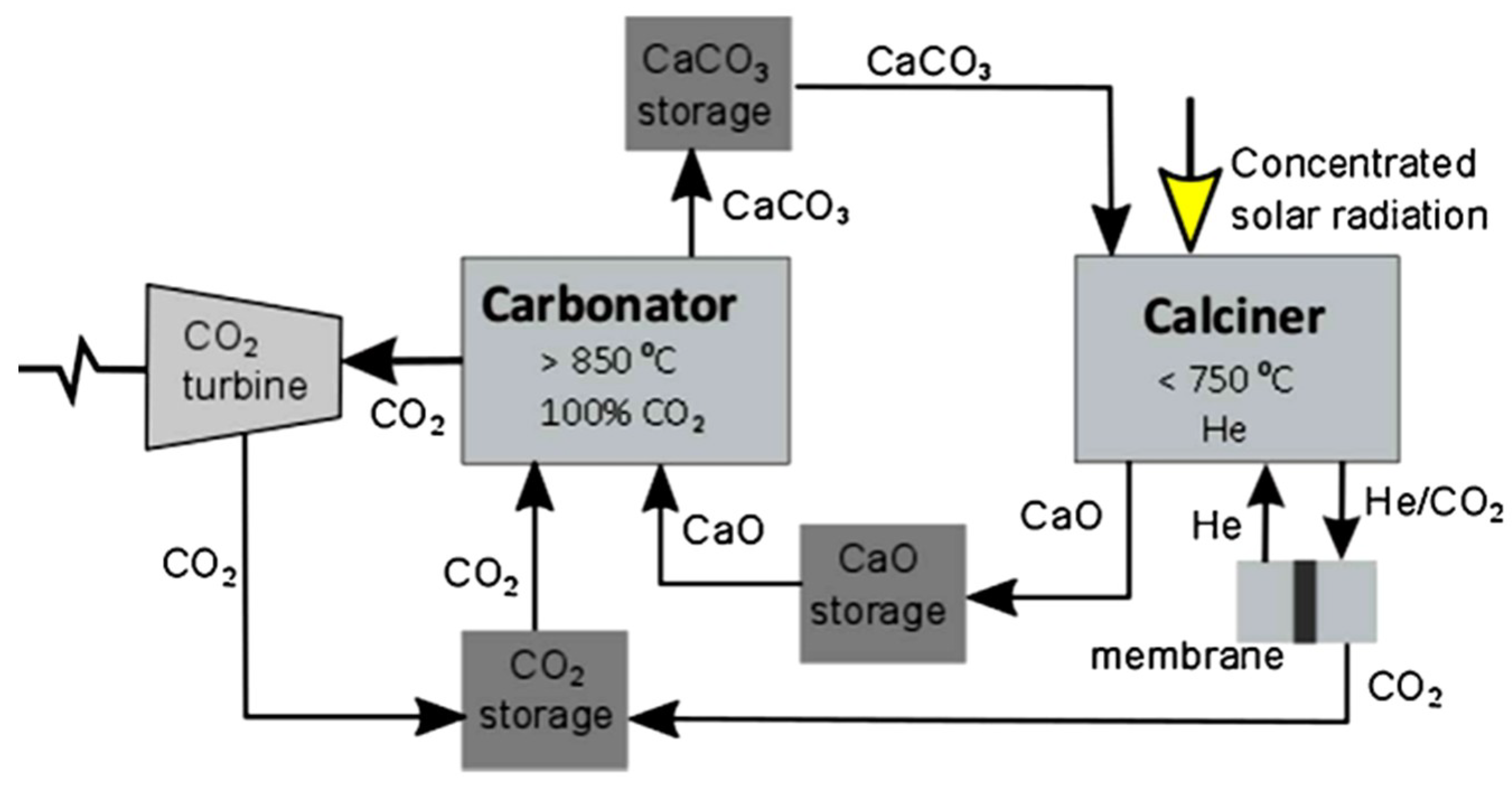



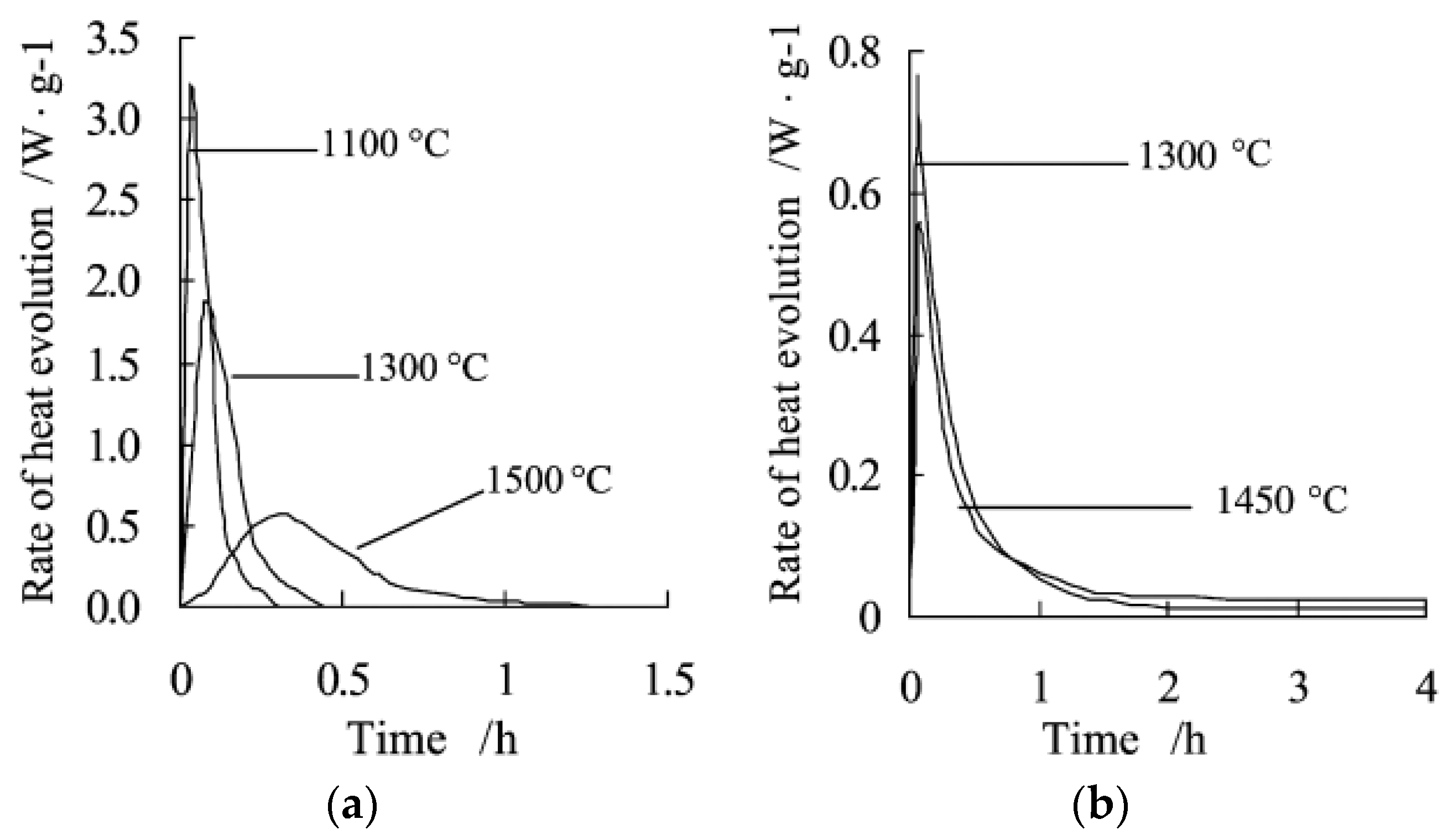
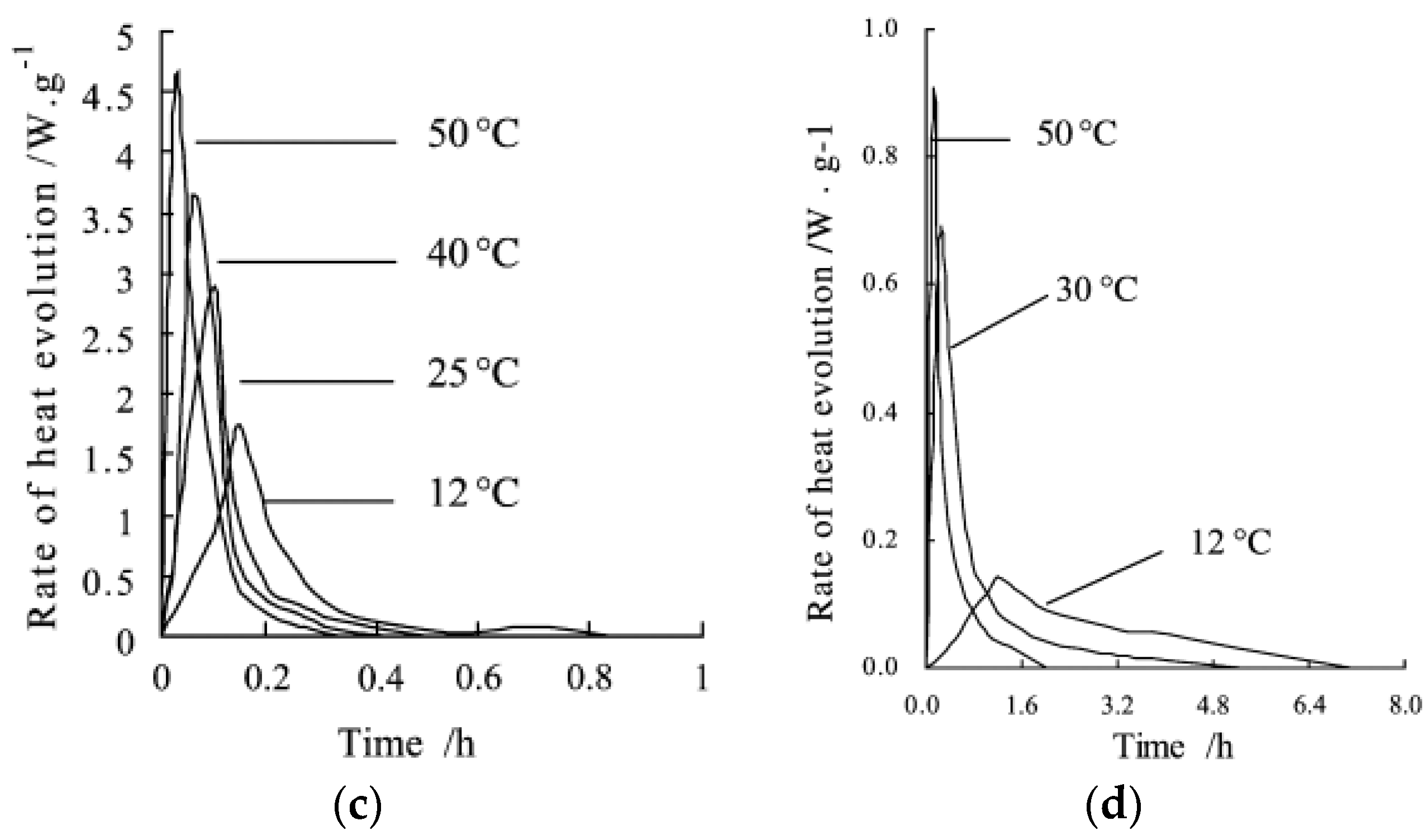

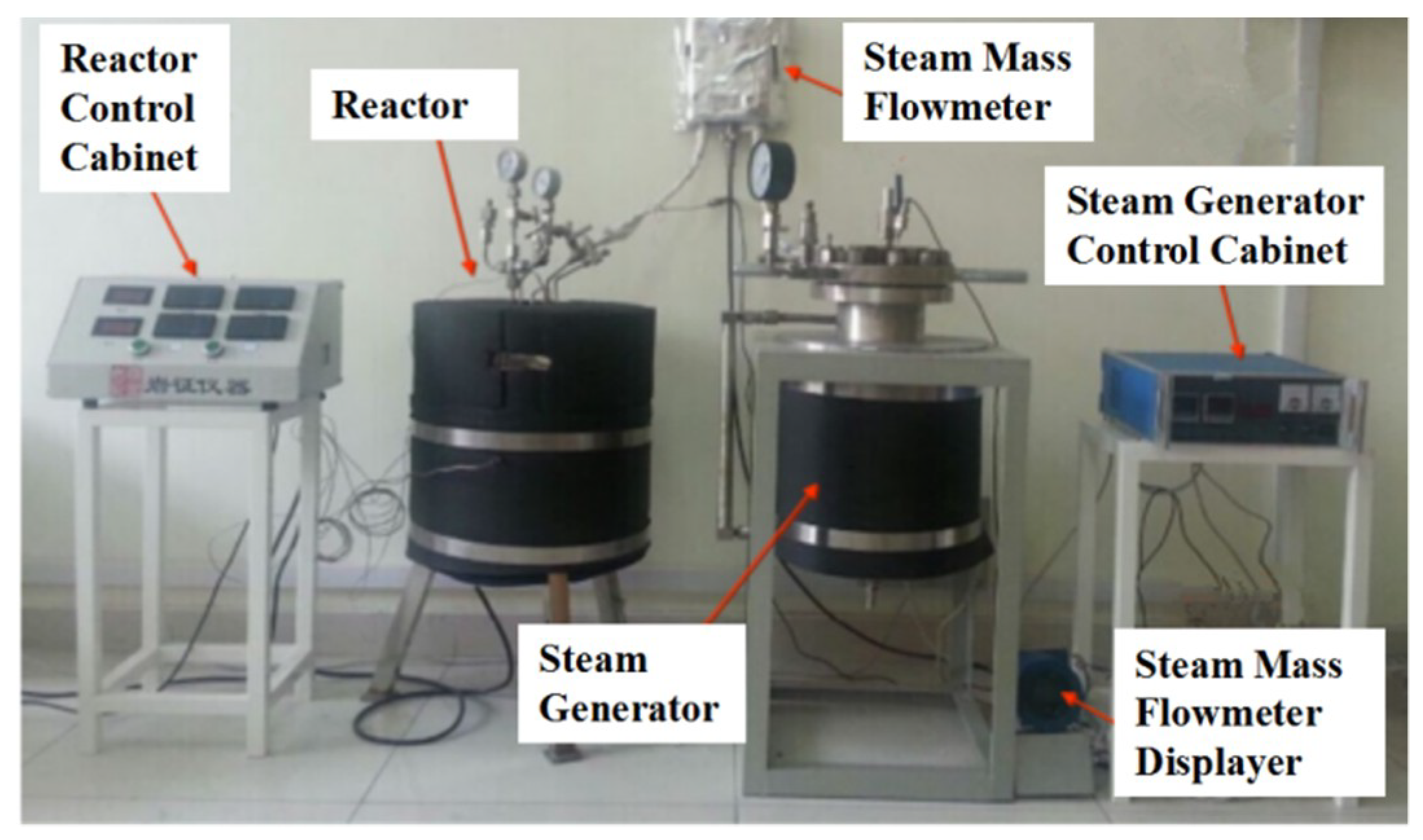
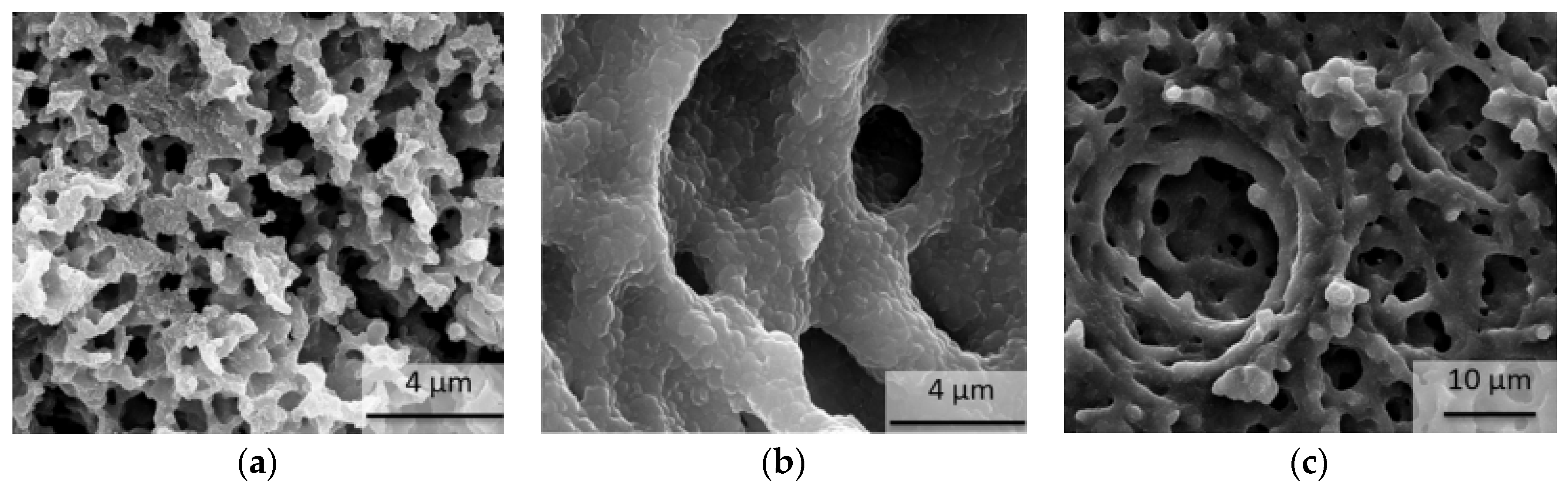
| Type of TES | Material | Turning Temperature (°C) | Energy Density(MJ/m3) | Reference |
|---|---|---|---|---|
| SHS | Silicone oil | 300–400 | 189 | [23] |
| SHS | Nitrite salts | 250–450 | 548 | [23] |
| SHS | Nitrate salts | 265–565 | 898 | [23] |
| SHS | Carbonate salts | 450–850 | 1512 | [23] |
| SHS | Liquid sodium | 270–530 | 287 | [23] |
| LHS | Sodium nitrite | 270 | 373 | [24] |
| LHS | Sodium nitrate | 307 | 389 | [23] |
| LHS | Potassium nitrate | 333 | 561 | [25] |
| LHS | Sodium carbonate | 854 | 701 | [26] |
| THS | FeO/CO2 | 180 | 2600 | [27] |
| THS | CaO/H2O | 500 | 3000 | [23] |
| THS | CaO/CO2 | 800–900 | 4400 | [23] |
| THS | NH4HSO4/NH3 | 467 | 3082 | [28] |
| THS | SrO/CO2 | 1108 | 3948 | [28] |
| Reference | Method | Results |
|---|---|---|
| [67] |
|
|
| [58] | High pressure TGA | CaO hydration rate (R): |
| [68] |
|
|
| [69] | TGA | Hydration rates: Dehydration rates: |
| [70] |
|
|
| [71] |
|
|
| [59] |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Li, Y.; Zhao, J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability 2018, 10, 2660. https://doi.org/10.3390/su10082660
Yuan Y, Li Y, Zhao J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability. 2018; 10(8):2660. https://doi.org/10.3390/su10082660
Chicago/Turabian StyleYuan, Yi, Yingjie Li, and Jianli Zhao. 2018. "Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review" Sustainability 10, no. 8: 2660. https://doi.org/10.3390/su10082660
APA StyleYuan, Y., Li, Y., & Zhao, J. (2018). Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability, 10(8), 2660. https://doi.org/10.3390/su10082660







