Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. Spatial Distribution and Classification of Hydroelectric Dams
2.1.2. Fishway Survey
2.1.3. River Connectivity
2.1.4. Bioecological Aspects and Conservation Status of Migratory Species
2.2. Data Analysis
3. Results
3.1. Hydropower Development
3.2. Fishway by Hydropower
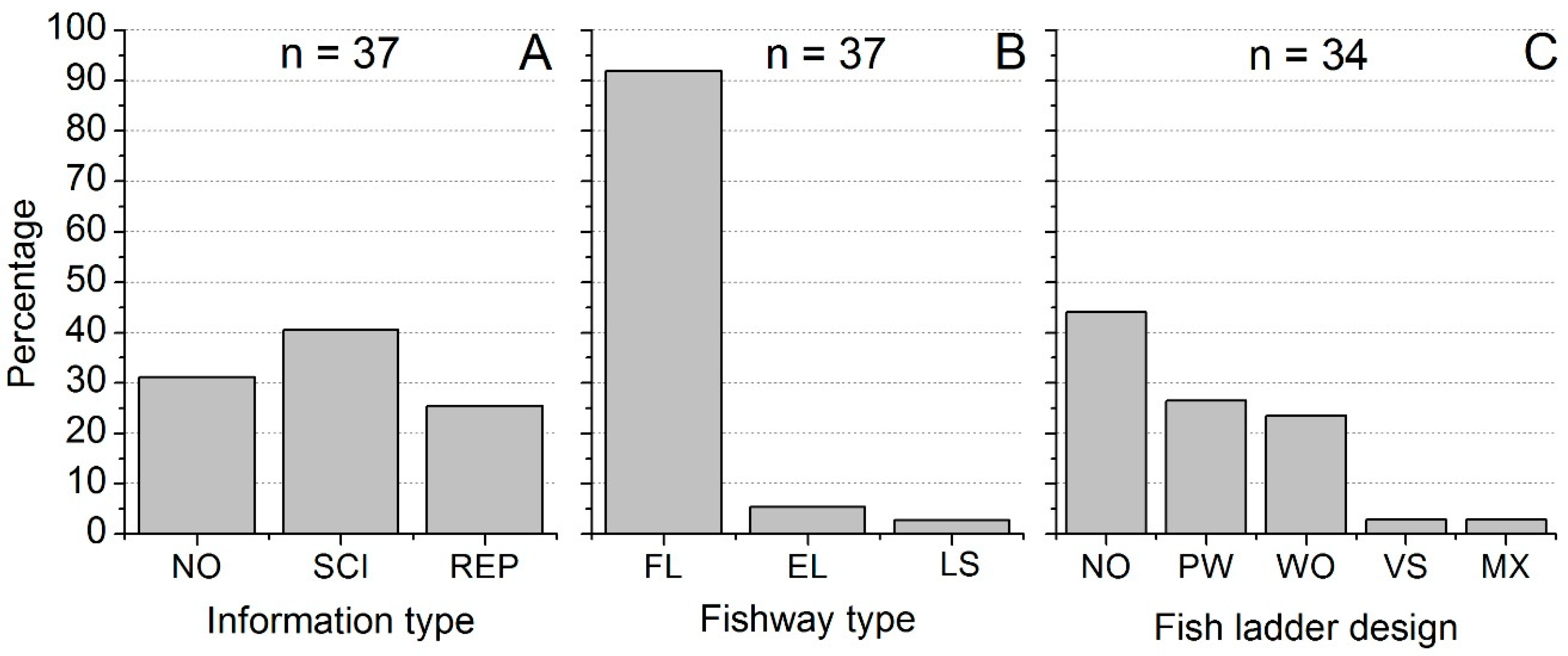
3.3. Fishway Information Profile
3.4. Fishway Types
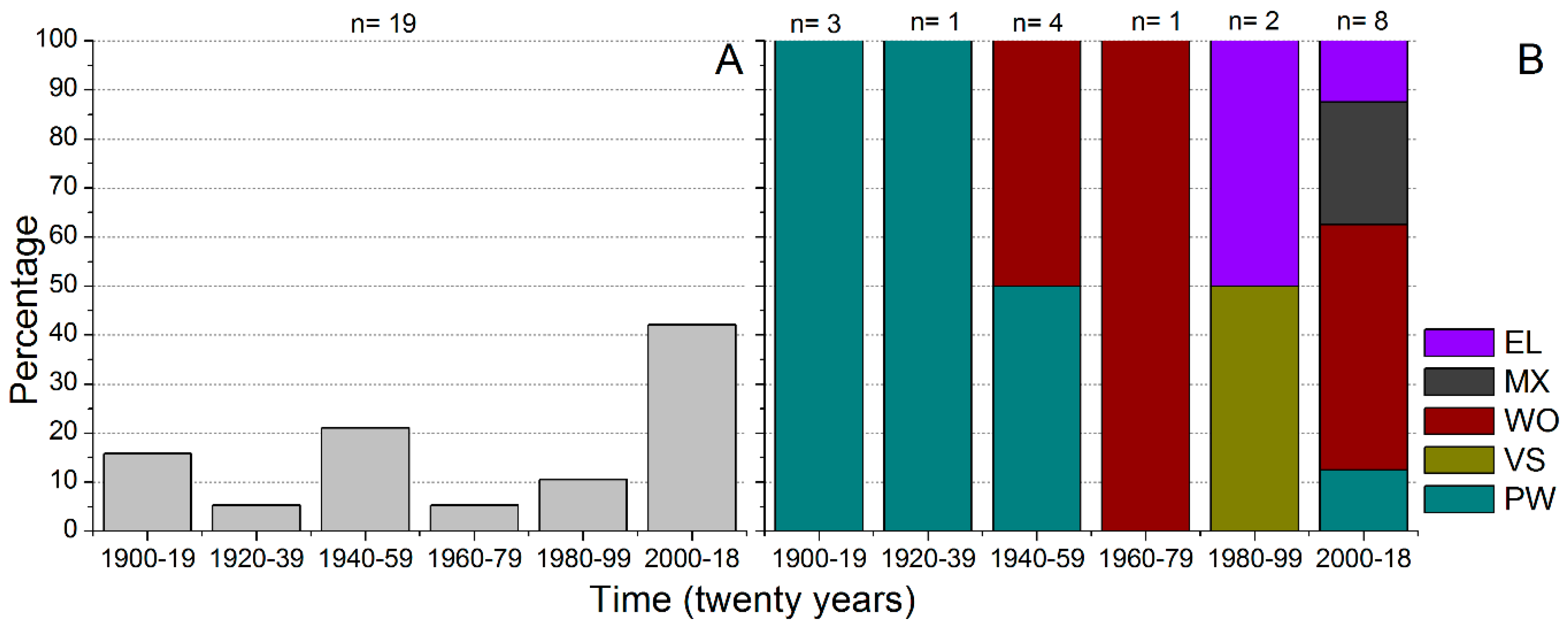
3.5. History of Fishways in the Upper Paraná River Basin
3.6. Structural Connectivity by Fishways
3.7. Bioecological Aspects and Conservation Status of Migratory Species
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Albins, M.; Evans, A.; Ismail, G.; Neilsen, B.; Pusack, T.; Schemmel, E.; Smith, W.; Stoike, S.; Li, H.W.; Noakes, D.L.G. Can humans coexist with fishes? Environ. Biol. Fishes 2013, 96, 1301–1313. [Google Scholar] [CrossRef]
- Timpe, K.; Kaplan, D. The changing hydrology of a dammed Amazon. Sci. Adv. 2017, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, A.; Pelicice, F.; Gomes, L. Dams and the fish fauna of the Neotropical region: Impacts and management related to diversity and fisheries. Brazi. J. Biol. 2008, 68, 1119–1132. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regul. Rivers Res. Manag. 1995, 11, 105–119. [Google Scholar] [CrossRef]
- Barletta, M.; Jaureguizar, A.J.; Baigun, C.; Fontoura, N.F.; Agostinho, A.A.; Almeida-Val, V.M.F.; Val, A.L.; Torres, R.A.; Jimenes-Segura, L.F.; Giarrizzo, T.; et al. Fish and aquatic habitat conservation in South America: A continental overview with emphasis on neotropical systems. J. Fish Biol. 2010, 76, 2118–2176. [Google Scholar] [CrossRef]
- Gouskov, A.; Reyes, M.; Wirthner-Bitterlin, L.; Vorburger, C. Fish population genetic structure shaped by hydroelectric power plants in the upper Rhine catchment. Evol. Appl. 2016, 9, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.C.; Baras, E. Migration of Freshwater Fishes; Wiley Online Library: Oxford, UK, 2001. [Google Scholar]
- Moraes, P.; Deverat, F. An Introduction to Fish Migration; Moraes, P., Deverat, F., Eds.; Taylor & Francis Group: London, UK, 2016; ISBN 978-1-4987-1874-5. [Google Scholar]
- Langeani, F.; Oyakawa, O.T.; Shibatta, A.O.; Pavanelli, C.S.; Casatti, L. Diversidade da ictiofauna do Alto Rio Paraná: Composição atual e perspectivas futuras. Biota Neotrop. 2007, 7, 1–18. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Gomes, L.C.; Suzuki, H.I.; Júlio-Júnior, H.F. Migratory fishes of the Upper Paraná River Basin, Brazil. In Migratory Fishes of South America; Carolsfeld, J., Harvey, B., Ross, C., Baer, A., Eds.; World Bank: Washington, DC, USA, 2003; ISBN 0-9683958-2-12. [Google Scholar]
- Suzuki, H.I.; de Vazzoler, A.E.A.M.; Marques, E.E.; Lizama, M.L.A.P.; Inada, P. Reproductive Ecology of the Fish Assemblages. In Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation; Thomaz, S.M., Agostinho, A.A., Hahn, N.S., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2004; pp. 271–292. [Google Scholar]
- Makrakis, M.C.; Miranda, L.E.; Makrakis, S.; Fontes, H.M.; Morlis, W.G.; Dias, J.H.P.; Garcia, J.O. Diversity in migratory patterns among Neotropical fishes. J. Fish Biol. 2012, 81, 866–881. [Google Scholar] [CrossRef]
- Godinho, A.L.; Kynard, B. Migratory fishes of Brazil: Life history and fish passage needs. River Res. Appl. 2008, 25, 702–712. [Google Scholar] [CrossRef]
- Godoy, M.P. Peixes do Brasil, Subordem Characoidei: Bacia do Rio Mogí Guassú; Franciscana: Piracicaba, Brazil, 1975. [Google Scholar]
- Cowx, I.G.; Welcomme, R.L. Rehabilitation of Rivers for Fish; Food & Agriculture Organization: Rome, Italy, 1998. [Google Scholar]
- Da Silva, P.S.; Makrakis, M.C.; Miranda, L.E.; Makrakis, S.; Assumpção, L.; Paula, S.; Dias, J.H.P.; Marques, H. Importance of reservoir tributaries to spawning of migratory fish in the upper Paraná River. River Res. Appl. 2015, 31, 313–322. [Google Scholar] [CrossRef]
- Quirós, R. The Paraná river basin development and the changes in the lower basin fisheries. Interciencia 1990, 15, 442–451. [Google Scholar]
- Hoeinghaus, D.J.; Agostinho, A.A.; Gomes, L.C.; Pelicice, F.M.; Okada, E.K.; Latini, J.D.; Kashiwaqui, E.A.L.; Winemiller, K.O. Effects of river impoundment on ecosystem services of large tropical rivers: Embodied energy and market value of artisanal fisheries. Conserv. Biol. 2009, 23, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Porcher, J.P.; Travade, F. Fishways: Biological basis, limits and legal considerations. Bull. Français la Pêche la Piscic. 2002, 364, 9–20. [Google Scholar] [CrossRef]
- Clay, C.H. Design of Fishways and Other Fish Facilities, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Celestino, L.F.; Sanz-Ronda, F.J.; Miranda, L.E.; Makrakis, M.C.; Dias, J.H.P.; Makrakis, S. Bidirectional connectivity via fish ladders in a large Neotropical river. River Res. Appl. 2019, 35, 236–246. [Google Scholar] [CrossRef]
- Larinier, M. Fishways-General consideration. Bull. Français La Pêche La Piscic. 2002, 364, 21–27. [Google Scholar] [CrossRef]
- DVWK. Fish Passages—Design, Dimension and Monitoring; FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; ISBN 92-5-104894-0. [Google Scholar]
- Castro-Santos, T.; Cotel, A.; Webb, P. Fishway evaluations for better bioengineering: An Integrative approach a framework for fishway. Am. Fish. Soc. 2009, 69, 557–575. [Google Scholar]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The future of fish passage science, engineering, and practice. Fish Fish. 2018, 19, 340–362. [Google Scholar] [CrossRef]
- Larinier, M. Environmental Issues, Dams and Fish Migration. In Dams, Fish and Fisheries: Opportunities, Challenges and Conflict Resolution; Marmulla, G., Ed.; Fisheries Techinical Paper; FAO Food and Agriculture Organization: Rome, Italy, 2001; pp. 45–90. ISBN 92-5-104694-8. [Google Scholar]
- Gustafsson, S.; Österling, M.; Skurdal, J.; Schneider, L.D.; Calles, O. Macroinvertebrate colonization of a nature-like fishway: The effects of adding habitat heterogeneity. Ecol. Eng. 2013, 61, 345–353. [Google Scholar] [CrossRef]
- Dodd, J.R.; Cowx, I.G.; Bolland, J.D. Efficiency of a nature-like bypass channel for restoring longitudinal connectivity for a river-resident population of brown trout. J. Environ. Manag. 2017, 204, 318–326. [Google Scholar] [CrossRef]
- Calles, O.; Greenberg, L. Connectivity is a two-way street-the need for a holistic approach to fish passage problems in regulated rivers. River Res. Appl. 2009, 25, 1268–1286. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Geist, J. Ecological functions of fish bypass channels in streams: Migration corridor and habitat for rheophilic spceies. River Res. Appl. 2013, 29, 441–450. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Van der Vinne, G.; Katopodis, C. Hydraulics of Vertical Slot Fishways. J. Hydraul. Eng. 1986, 112, 909–927. [Google Scholar] [CrossRef]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Gutfreund, C.; Makrakis, S.; Castro-Santos, T.; Celestino, L.F.; Dias, J.H.P.; Makrakis, M.C. Effectiveness of a fish ladder for two Neotropical migratory species in the Paraná River. Mar. Freshw. Res. 2018, 1–9. [Google Scholar] [CrossRef]
- Quirós, R. Structures Assisting the Migrations of Non-Salmonid Fish: Latin America; Quirós, R., Ed.; Techinical Paper. N°5; COPESCAL: Rome, Italy, 1989; ISBN 92-5-102683-1. [Google Scholar]
- Fernandez, D.R.; Agostinho, A.A.; Bini, L.M.; Pelicice, F.M. Diel variation in the ascent of fishes up an experimental fish ladder at Itaipu Reservoir: Fish size, reproductive stage and taxonomic group influences. Neotrop. Ichthyol. 2007, 5, 215–222. [Google Scholar] [CrossRef]
- De Santos, H.A.; Viana, E.M.F.; Pompeu, P.S.; Martinez, C.B. Optimal swim speeds by respirometer: An analysis of three neotropical species. Neotrop. Ichthyol. 2012, 10, 805–811. [Google Scholar] [CrossRef]
- Assumpção, L.; Makrakis, M.C.; Makrakis, S.; Wagner, R.L.; Da Silva, P.S.; De Lima, A.F.; Kashiwaqui, E.A.L. The use of morphometric analysis to predict the swimming efficiency of two Neotropical long-distance migratory species in fish passage. Neotrop. Ichthyol. 2012, 10, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Bido, A.F.; Urbinati, E.C.; Makrakis, M.C.; Celestino, L.F.; Serra, M.; Makrakis, S. Stress indicators for Prochilodus lineatus (Characiformes: Prochilodontidae) breeders during passage through a fish ladder. Mar. Freshw. Res. 2018, 1–8. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Haro, A. Fish Guidance and Passage at Barriers. In Fish Locomotion: An Eco-Ethological Perspective; Domenici, P., Kapoor, B.G., Eds.; Science Publisher: Enfield, NH, USA, 2010; pp. 62–89. [Google Scholar]
- Fontes, H.M., Jr.; Castro-santos, T.; Makrakis, S.; Gomes, L.C.; Latini, J.D. A barrier to upstream migration in the fish passage of Itaipu Dam (Canal da Piracema), Paraná River basin. Neotrop. Ichthyol. 2012, 10, 697–704. [Google Scholar] [CrossRef]
- Oldani, N.O.; Baigún, C.R.M. Performance of a fishway system in a major South American dam on the Parana River (Argentina-Paraguay). River Res. Appl. 2002, 18, 171–183. [Google Scholar] [CrossRef]
- Almeida, L.E.G.; Mattos, M.E.; Tanaka, H.R. Modernização da PCH Gavião Peixoto: Gestão, controle ambiental e mecanismo de desenvolvimento limpo. Semin. Nac. PRODUÇÃO E Transm. Energ. ELÉTRICA 2010, 7. [Google Scholar]
- Lira, N.A.; Pompeu, P.S.; Agostinho, C.S.; Agostinho, A.A.; Arcifa, M.S.; Pelicice, F.M. Fish passages in South America: An overview of studied facilities and research effort. Neotrop. Ichthyol. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecol. Eng. 2013, 58, 123–132. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Haro, A.; Walk, S. A passive integrated transponder (PIT) tag system for monitoring fishways. Fish. Res. 1996, 28, 253–261. [Google Scholar] [CrossRef]
- Energy, D. ABC da Energia. 2013. Available online: https://abc-da-energia.webnode.com/ (accessed on 5 July 2019).
- Britto, S.G.D.C.; Carvalho, E.D. Reproductive migration of fish and movement in a series of reservoirs in the Upper Parana River basin, Brazil. Fish. Manag. Ecol. 2013, 20, 426–433. [Google Scholar] [CrossRef]
- Auffret, A.G.; Plue, J.; Cousins, S.A.O. The spatial and temporal components of functional connectivity in fragmented landscapes. Ambio 2015, 44, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfeo, O.; Stevaux, J. Hydraulic and morphological characteristics of middle and upper reaches of the Paraná River (Argentina and Brazil). Geomorphology 2002, 44, 309–322. [Google Scholar] [CrossRef]
- QGIS Development Team. Development Geographic Information System User Guide. Open Source Geospatial Foundation Project 2019; QGIS Development Team: Grut, Switzerland, 2019. [Google Scholar]
- ANEEL—Agência Nacional de Energia Elétrica. Resolução Normativa N° 687, de 24 de Novembro de 2015; Gência Nacional de Energia Elétrica: Brasilia, Brazil, 2015.
- ANEEL—Agência Nacional de Energia Elétrica Resolução Normativa N° 673, de 4 de Agosto de 2015; Gência Nacional de Energia Elétrica: Brasilia, Brazil, 2015.
- ANEEL. Atlas de Energia Elétrica do Brasil, 3rd ed.; de Elétrica, A.N.E., Ed.; Agência Nacional de Energia Elétrica: Brasilia, Brazil, 2008; ISBN 978-85-87491-10-7.
- São Paulo. Decreto N° 63.853, de 27 de Novembro de 2018. São Paulo. 2018. Available online: https://www.al.sp.gov.br/repositorio/legislacao/decreto/2018/decreto-63853-27.11.2018.html (accessed on 5 July 2019).
- Brasil Portaria do Ministério do Meio Ambiente (MMA). 445, de 17 de Dezembro de 2014. Reconhece Como Espécies de Peixes e Invertebrados Aquáticos da Fauna Brasileira Ameaçadas de Extinção Aquelas Constantes da Lista Nacional Oficial de Espécies da Fauna Ameaçada; Brasil Portaria do Ministério do Meio Ambiente: Brasilia, Brazil, 2014; pp. 126–130.
- Da Ingenito, L.F.S.; Duboc, L.F.; Abilhoa, V. Contribuição ao conhecimento da ictiofauna da bacia do alto rio Iguaçu, Paraná, Brasil. Arq. Ciências Veterinárias e Zool. da Unipar 2004, 7, 23–36. [Google Scholar]
- De Vazzoler, A.E.A.M. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática; Eduem: Maringá, Brazil, 1996; ISBN 85-85545-16-X. [Google Scholar]
- Ota, R.R.; de Deprá, G.C.; da Graça, W.J.; Pavanelli, C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: Revised, annotated and updated. Neotrop. Ichthyol. 2018, 16, 1–111. [Google Scholar] [CrossRef]
- Hahn, N.S.; Fugi, R.; Andrian, I.F. Trophic Ecology of Fish Assemblages. In The Upper Paraná River Floodplain: Physical Aspects, Ecology and Conservation; Thomaz, S.M., Agostinho, A.A., Hahn, N.S., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2004; pp. 247–269. [Google Scholar]
- De Resende, E.K. Migratory Fishes of the Paraguay-Paraná Basin. In Migratoty Fishes of South America: Biology, Fisheries, and Conservation Status; Carolsfeld, J., Harvey, B., Ross, C., Baer, A., Eds.; World Fisheries Trust: Washington, DC, USA, 2003; pp. 98–155. [Google Scholar]
- Agostinho, A.A.; Gomes, L.C.; Pelicice, F.M. Ecologia e Manejo de Recursos Pesqueiros em Rereservatórios do Brasil; EDUEM: Maringá, Brazil, 2007. [Google Scholar]
- Godinho, A.L.; Lamas, I.R.; Godinho, H.P. Reproductive ecology of Brazilian freshwater fishes. Environ. Biol. Fishes 2010, 87, 143–162. [Google Scholar] [CrossRef]
- Bowen, M.D.; Marques, S.; Silva, L.G.M.; Vono, V.; Godinho, H.P. Comparing on site human and video counts at Igarapava fish ladder, Southeastern Brazil. Neotrop. Ichthyol. 2006, 4, 291–294. [Google Scholar] [CrossRef]
- Bizzotto, P.M.; Godinho, A.L.; Vono, V.; Kynard, B.; Godinho, H.P. Influence of seasonal, diel, lunar, and other environmental factors on upstream fish passage in the Igarapava Fish Ladder, Brazil. Ecol. Freshw. Fish 2009, 18, 461–472. [Google Scholar] [CrossRef]
- Lopes, C.M.; De Almeida, F.S.; Orsi, M.L.; Britto, S.G.D.C.; Sirol, R.N.; Sodré, L.M.K. Fish passage ladders from Canoas Complex—Paranapanema River: Evaluation of genetic structure maintenance of Salminus brasiliensis (Teleostei: Characiformes). Neotrop. Ichthyol. 2007, 5, 131–138. [Google Scholar] [CrossRef]
- Britto, S.G.D.C. A estratégia reprodutiva dos peixes migradores frente às escacda do complexo canoas (rio Paranapanema, bacia do alto Paraná). Ph.D. Thesis, Universidade Estadual Paulista, Sao Paolo, Brazil, 2009. [Google Scholar]
- Arcifa, M.S.; Luiz, A.; Esguícero, H. The fish fauna in the fish passage at the Ourinhos Dam, Paranapanema River. Neotrop. Ichthyol. 2012, 10, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.L. Sistemas Para a Transposição de Peixes Neotropicais Potamódromos. Ph.D. Thesis, Universidade Estadual Paulista, Sao Paolo, Brazil, 2005. [Google Scholar]
- De Viana, E.M.F. Mapeamento do Campo de Velocidades em Mecanismos de Transposição de Peixes do Tipo Slot Vertical em Diferentes Escale. Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2005. [Google Scholar]
- Godoy, M.P. Aqüicultira—Atividade Multidisciplinar e Outras Facilidades Para Passagens de Peixes, Estações de Piscicultura; Eletrosul: Florianópolis, Brazil, 1985.
- Eletrosul. 5° Relatório Semestral de Andamento dos Programas e Planos Ambietais; Fase de Operação UHE São Domingos; Eletrosul: Florianópolis, Brazil, 2015; Volume 1.
- Makrakis, S.; Makrakis, M.C.; Wagner, R.L.; Dias, J.H.P.; Gomes, L.C. Utilization of the fish ladder at the Engenheiro Sergio Motta Dam, Brazil, by long distance migrating potamodromous species. Neotrop. Ichthyol. 2007, 5, 197–204. [Google Scholar] [CrossRef]
- Wagner, R.L.; Makrakis, S.; Castro-Santos, T.; Makrakis, M.C.; Dias, J.H.P.; Belmont, R.F. Passage performance of long-distance upstream migrants at a large dam on the Paraná River and the compounding effects of entry and ascent. Neotrop. Ichthyol. 2012, 10, 785–795. [Google Scholar] [CrossRef]
- Volpato, G.L.; Barreto, R.E.; Marcondes, A.L.; Andrade Moreira, P.S.; de Barros Ferreira, M.F. Fish ladders select fish traits on migration–still a growing problem for natural fish populations. Mar. Freshw. Behav. Physiol. 2009, 42, 307–313. [Google Scholar] [CrossRef]
- CESP. Programa de Manejo Pesqueiro: Plano de Trabalho 2000–2001; CESP: São Paulo, Brazil, 2000. [Google Scholar]
- CESP. Programa de Manejo Pesqueiro: Plano de Trabalho 2002–2003; CESP: São Paulo, Brazil, 2002. [Google Scholar]
- CESP. Programa de Manejo Pesqueiro: Plano de Trabalho 2003–2004; CESP: São Paulo, Brazil, 2003. [Google Scholar]
- Makrakis, S.; Makrakis, M.C.; Da Silva, P.S.; Celestino, L.F. Monitoramento do Ictioplâncton em Tributários do Reservatório de Porto Primavera e Monitoramento da Transposição; Unioeste: Toledo, Brazil, 2015. [Google Scholar]
- Makrakis, S.; Gomes, L.C.; Makrakis, M.C.; Fernandez, D.R.; Pavanelli, C.S. The Canal da Piracema at Itaipu Dam as a fish pass system. Neotrop. Ichthyol. 2007, 5, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Hahn, L.; English, K.; Carosfeld, J.; Gustavo, L.; Latini, J.D.; Agostinho, A.A.; Fernandez, D.R. Preliminary study on the application of radio-telemetry techniques to evaluate movements of fish in the Lateral canal at Itaipu Dam, Brazil. Neotrop. Ichthyol. 2007, 5, 103–108. [Google Scholar] [CrossRef]
- Makrakis, S.; Miranda, L.E.; Gomes, L.C.; Makrakis, M.C.; Junior, H.M.F.J. Ascent of neotropical fish in the Itaipu reservoir fish pass. River Res. Appl. 2011, 27, 511–519. [Google Scholar] [CrossRef]
- Google Maps. Available online: https://www.google.com.br/maps (accessed on 15 February 2018).
- Suzuki, F.M.; Pires, L.V.; Pompeu, P.S. Passage of fish larvae and eggs through the Funil, Itutinga and Camargos Reservoirs on the upper Rio Grande (Minas Gerais, Brazil). Neotrop. Ichthyol. 2011, 9, 617–622. [Google Scholar] [CrossRef]
- Aliança Usina de Funil. Available online: https://aliancaenergia.com.br/br/nossas-usinas/usina-de-funil (accessed on 24 April 2019).
- Shibatta, O.A.; Gealh, A.M.; Bennemann, S.T. Ictiofauna dos trechos alto e médio da bacia do rio Tibagi, Paraná, Brasil. Biota Neotrop. 2007, 7, 125–134. [Google Scholar] [CrossRef]
- Godinho, H.P.; Godinho, A.L.; Formagio, P.S.; Torquato, V.C. Fish ladder efficiency in a southeastern Brazilian river. Cienc. Cult. 1991, 43, 63–67. [Google Scholar]
- EMAE—Fundação de Energia e Saneamento. PCH Corubataí, Rio claro—SP.; Fundação de Energia e Saneamento: Sao Paulo, Brazil, 2014. [Google Scholar]
- Ricardi, A.; Meneguello, C.; Bueno, E.; Santos, G.; Magalhães, G.; Limnos, G.; Bizello, M.L.; Xavier, M.; Diniz, R.; Furlan, S.; et al. Relaório Técnico da 8° Expedição: Usinas Lobo, São Joaquim, Buritis; Esmeril: São Paulo, Brazil, 2015; Volume 8.
- Mortati, D.; Bueno, E.; Dizzio, F.; Limnios, G.; Magalhães, G.; Midori, M.; Lima, N.; Gazoni, P.; Diniz, R.; Carvalho, T. Relatório Técnico da 2° Expedição: Usinas de Porto Goes, Lavras, Salto Grande, Cariobinha, Carioba, America, Jaguari; Esmeril: São Paulo, Brazil, 2014; Volume 2.
- Capeleti, A.R.; Petrere Jr, M. Migration of the curimbatá Prochilodus lineatus (Valenciennes, 1836) (Pisces, Prochilodontidae) at the waterfall “Cachoeira de Emas” of the Mogi-Guaçu river—São Paulo, Brazil. Brazilian J. Biol. 2006, 66, 651–659. [Google Scholar] [CrossRef]
- Peressin, A.; Gonçalves, S.; Manoel, F.; Braga, D.S. Reproductive strategies of two Curimatidae species in a Mogi Guaçu impoundment, upper Paraná River basin, São Paulo, Brazil. Neotrop. Ichthyol. 2012, 10, 847–854. [Google Scholar] [CrossRef]
- Migliorini, P.C.P. Repotenciação da Pequena Central Hidrelétrica de Emas “Nova”—Pirassununga—SP: Aspectos Técnicos, Socioambientais e Economicos; Universidade de São Paulo: Sao Paulo, Brazil, 2011. [Google Scholar]
- Gonçalves, C.D.S.; Braga, F.M.D.S. Diversidade e ocorrência de peixes na área de influência da UHE Mogi Guaçu e lagoas marginais, bacia do alto rio Paraná, São Paulo, Brasil. Biota Neotrop. 2008, 8, 103–114. [Google Scholar] [CrossRef]
- Esguícero, A.L.H.; Arcifa, M.S. Fragmentation of a Neotropical migratory fish population by a century-old dam. Hydrobiologia 2010, 638, 41–53. [Google Scholar] [CrossRef]
- Ricardi, A.; Argollo, A.; Freitas, C.; Barbanti, C.; Mortati, D.; Geribello, D.; Bueno, E.; Drizzo, F.; Santos, G.; Magalhães, G.; et al. Relatório técnico 5° Expedição: Usinas de Corumbataí, Capão Pretro, Monjolinho, Lia-Marmelos I, II, III e Isabl. 2014. Available online: http://eletromemoria.fflch.usp.br/sites/eletromemoria.fflch.usp.br/files/relatorio_da_5a_expedicao.pdf (accessed on 5 July 2019).
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwall, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latrubesse, E.M.; Arima, E.Y.; Dunne, T.; Park, E.; Baker, V.R.; D’Horta, F.M.; Wight, C.; Wittmann, F.; Zuanon, J.; Baker, P.A.; et al. Damming the rivers of the Amazon basin. Nature 2017, 546, 363–369. [Google Scholar] [CrossRef]
- ALESP. LEI N° 9.798, de 07 de Outubro de 1997; Assembleia Legislativa do Estado de São Paulo: Sao Paulo, Brazil, 1997.
- Minas Gerais. Lei N° 12.488, de 9 de Abril de 1997. 1997. Available online: http://www.ctpeixes.ufmg.br/html/conteudo/lei_12488.htm (accessed on 5 July 2019).
- Agostinho, A.A.; Marques, E.E.; Agostinho, C.S.; Almeida, D.A.; Oliveira, R.J.; Rodrigues, J.; Melo, B. Fish ladder of Lajeado Dam: Migrations on one-way routes? Neotrop. Ichthyol. 2007, 5, 121–130. [Google Scholar] [CrossRef]
- Agostinho, C.S.; Pelicice, F.M.; Marques, E.E.; Soares, A.B.; de Almeida, D.A.A. All that goes up must come down? Absence of downstream passage through a fish ladder in a large Amazonian river. Hydrobiologia 2011, 675, 1–12. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Agostinho, C.S. Deficient downstream passage through fish ladders: The case of Peixe Angical Dam, Tocantins River, Brazil. Neotrop. Ichthyol. 2012, 10, 705–713. [Google Scholar] [CrossRef]
- Baigún, C.R.M.; Nestler, J.M.; Minotti, P.; Oldani, N.O. Fish passage system in an irrigation dam (Pilcomayo River basin): When engineering designs do not match ecohydraulic criteria. Neotrop. Ichthyol. 2012, 10, 741–750. [Google Scholar]
- Oldani, N.O.; Baigún, C.R.M.; Nestler, J.M.; Goodwin, R.A. Is fish passage technology saving fish resources in the lower La Plata River basin? Neotrop. Ichthyol. 2007, 5, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Croze, O.; Bau, F.; Delmouly, L. Efficiency of a fish lift for returning Atlantic salmon at a large-scale hydroelectric complex in France. Fish. Manag. Ecol. 2008, 15, 467–476. [Google Scholar] [CrossRef]
- Larinier, M.; Marmulla, G. Fish passes: Types, principles and geographical distribution—An overview. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries; Welcomme, R.L., Petr, T., Eds.; FAO Fisheries: Bangkok, Thailand, 2004; pp. 183–206, ISBN 974-7946-65-3. [Google Scholar]
- Makrakis, S.; Dias, J.H.P.; Lopes, J.D.M.; Fontes-Junior, H.M.; Godinho, A.L.; Martinez, C.B.; Makrakis, M.C. Premissas e Critérios Mínimos para Implantação, Avaliação e Monitoramento de Sistemas de Transposição para Peixes. Bol. Soc. Bras. Ictiol. 2015, 114. [Google Scholar]
- Pelicice, F.M.; Azevedo-Santos, V.M.; Esguícero, A.L.H.; Agostinho, A.A.; Arcifa, M.S. Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotrop. Ichthyol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Da Silva, P.S.; Miranda, L.E.; Makrakis, S.; Assumpção, L.; Dias, J.H.P.; Makrakis, M.C. Tributaries as biodiversity preserves: An ichthyoplankton perspective from the severely impounded Upper Paraná River. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 258–269. [Google Scholar] [CrossRef]
- Marques, H.; Dias, J.H.P.; Perbiche-Neves, G.; Kashiwaqui, E.A.L.; Ramos, I.P. Importance of dam-free tributaries for conserving fish biodiversity in Neotropical reservoirs. Biol. Conserv. 2018, 224, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Assumpção, L.; Makrakis, S.; Sarai, P.; Makrakis, M.C. Espécies de peixes ameaçadas de extinção no Parque Nacional do Iguaçu. Biodiversidade Bras. 2017, 7, 4–17. [Google Scholar]
- Ferreira, D.G.; Souza-Shibatta, L.; Shibatta, O.A.; Sofia, S.H.; Carlsson, J.; Dias, J.H.P.; Makrakis, S.; Makrakis, M.C. Genetic structure and diversity of migratory freshwater fish in a fragmented Neotropical river system. Rev. Fish Biol. Fish. 2017, 27, 209–231. [Google Scholar] [CrossRef]




| Hydropower Plant Classification | Planned | Under Construction | In Operation | Total |
|---|---|---|---|---|
| Large-sized: UHE | 61 | 2 | 86 | 149 |
| Medium-sized: PCH | 516 | 6 | 102 | 623 |
| Small-sized: CGH | - | - | 201 | 201 |
| Total | 577 | 8 | 389 | 974 |
| N | Dam ID | Dam Name | Coordinates X, Y | River | Fishway Type | Year | Fishway Design | Baffle Type/Material | References |
|---|---|---|---|---|---|---|---|---|---|
| Large-Sized—UHE | |||||||||
| 1 | 05 | Igarapava | −19.989255° −47.755567° | Grande | FL• | 1999 | Vertical Slot | -/Concrete | [64,65] |
| 2 | 24 | Canoas I | −22.939658° −50.517983° | Paranapanema | FL* • | 2000 | Weir and Orifice | Bottom orifice and notch/Concrete | [48,62,66,67] |
| 3 | 25 | Canoas II | −22.939505° −50.251990° | Paranapanema | FL* • | 2000 | Weir and Orifice | Bottom orifice and notch/Concrete | [48,62,66,67] |
| 4 | 26 | Ourinhos | −23.070170° −49.838412° | Paranapanema | FL • | 2005 | Pool and Weir | -/Concrete | [68] |
| 5 | 28 | Piraju I | −23.154237° −49.379971° | Paranapanema | FL | 1971 | Weir and Orifice | Bottom orifice/Concrete | [62,69,70,71] |
| 6 | 29 | Piraju II | −23.187694° −49.384466° | Paranapanema | FL | NO | Weir and Orifice | Bottom orifice/Concrete | [69] |
| 7 | 06 | São Domingos | −20.083344° −53.174786° | Verde | FL | 2015 | Weir and Orifice | Bottom orifice and notch/Metal | [72] |
| 8 | 22 | Engenheiro Sérgio Motta (Porto Primavera) | −22.483899° −52.957212° | Paraná | FL • | 2001 | Weir and Orifice | Bottom orifice and notch/Concrete | [22,34,38,39,73,74,75] |
| 9 | 22 | Engenheiro Sérgio Motta (Porto Primavera) | −22.483899° −52.957212° | Paraná | EL • | 1999 | Pipe and Gravity | -/Metal | [76,77,78,79] |
| 10 | 35 | Itaipu Binacional | −25.430486° −54.581765° | Paraná | LS • | 2002 | Semi-natural Vertical Slot Pool and weir | -/Concrete, rocks and excavated | [41,80,81,82] |
| 11 | 01 | Rochedo | −17.388624° −49.216373° | Meia Ponte | FL | NO | NO | NO/NO | [83] |
| 12 | 12 | Funil | −21.144011° −45.036538° | Grande | EL | 2004 | Flume channel | -/Metal | [44,84,85] |
| Medium-sized—PCH | |||||||||
| 13 | 34 | Salto Mauá | −24.058585° −50.712232° | Tibagi | FL | 1943 | Pool and Weir | -/Concrete | [62,69,71,86] |
| 14 | 04 | Salto Moraes | −18.950109° −49.382568° | Tijuco | FL • | 1951 | Weir and Orifice | Bottom orifice/Concrete | [62,71,87] |
| 15 | 14 | João Baptista de Lima Figueiredo | −21.584954° −46.746961° | Pardo | FL | NO | NO | NO/NO | [83] |
| 16 | 21 | Corumbataí | −22.480472° −47.592333° | Corumbataí | FL | NO | Pool and Weir | NO/Concrete | [88] |
| 17 | 07 | Retiro | −20.436132° −47.889945° | Sapucaí | FL | NO | NO | NO/NO | [83] |
| 18 | 08 | Anhanguera | −20.493856° −47.858010° | Sapucaí | FL | NO | NO | NO/NO | [83] |
| 19 | 09 | Palmeiras | −20.550638° −47.813264° | Sapucaí | FL | NO | NO | NO/NO | [83] |
| 20 | 10 | São Joaquim | −20.581831° −47.780019° | Sapucaí | FL | NO | NO | NO/NO | [89] |
| 21 | 10 | São Joaquim | −20.581831° −47.780019° | Sapucaí | FL | 1911 | Pool and Weir | -/Concrete | [69,89] |
| 22 | 11 | Dourados | −20.666837° −47.654310° | Sapucaí | FL | 1926 | Pool and Weir | -/Concrete | [69] |
| 23 | 13 | Itaipava | −21.413491° −47.334915° | Pardo | FL | 1911 | Pool and Weir | -/Concrete | [69,71] |
| 24 | 27 | San Juan | −23.149462° −47.793913° | Sorocaba | FL | NO | NO | NO/NO | [83] |
| 25 | 23 | Salto Grande | −22.933744° −46.896052° | Atibaia | FL | 1911 | Pool and Weir | -/Concrete | [35,83,90] |
| 26 | 18 | Cachoeira de Emas | −21.926500º −47.366357º | Mogi-Guaçu | FL • | 1922 1943 | Pool and Weir | -/Concrete | [71,91,92,93] |
| 27 | 20 | Mogi-Guaçu | −22.379299° −46.900892° | Mogi-Guaçu | FL | NO | Pool and Weir | -/Concrete | [83,94] |
| 28 | 19 | São José | −21.938628° −46.816218° | Jaguari Mirim | FL | NO | NO | NO | [83] |
| 29 | 33 | Cachoeira Poço Preto II | −24.047850° −49.457784° | Itararé | FL | NO | NO | NO | [83] |
| 30 | 32 | Cachoeira Poço Preto I | −24.036877° −49.462776° | Itararé | FL | NO | NO | NO | [83] |
| 31 | 03 | Jataí | −17.943613° −51.726355° | Claro | FL | NO | NO | NO | [83] |
| 32 | 02 | Ypê | −17.725705° −50.451810° | Verdão | FL | NO | NO | NO | [83] |
| 33 | 15 | Gavião-Peixoto | −21.847628° −48.489485° | Jacaré-Guaçu | FL | 1913 1987 1995 2007 | Mixed system: Pool and weir and excavated rock | -/Concrete and rocks | [43,95] |
| 34 | 16 | Capão Preto | −21.895113° −47.814506° | Quilombo | FL | NO | NO | NO/NO | [83,96] |
| Small-sized—CGH | |||||||||
| 35 | 17 | Quatiara | −21.951352° −50.929426° | Do Peixe | FL | 1949 | Weir and Orifice | Notch/concrete | [69] |
| 36 | 30 | Santa Adélia | −23.327529° −47.768980° | Sorocaba | FL | NO | NO | NO/NO | [83] |
| 37 | 31 | Do Túnel | −23.414886° −50.452437° | Laranjinha | FL | NO | NO | NO/NO | [83] |
| N | River | River Length (km) | River Slope (%) | Number of Dams per River | Number of Dams per River with Fishway | % of Dams with Fishway per River | Dam Location (State) |
|---|---|---|---|---|---|---|---|
| 1 | Atibaia | 181.5 | 0.23 | 2 | 1 | 50.0 | São Paulo |
| 2 | Claro | 353.8 | 0.14 | 4 | 1 | 25.0 | Goiás |
| 3 | Corumbataí | 84.7 | 0.29 | 1 | 1 | 100.0 | São Paulo |
| 4 | Peixe | 92.1 | 1.01 | 1 | 1 | 100.0 | São Paulo |
| 5 | Grande | 1156.9 | 0.08 | 12 | 2 | 16.7 | Minas Gerais * |
| 6 | Itararé | 197.8 | 0.21 | 2 | 2 | 100.0 | São Paulo |
| 7 | Jacaré-Guaçu | 171.7 | 0.21 | 2 | 1 | 50.0 | São Paulo |
| 8 | Jaguari-mirim | 98.3 | 0.21 | 2 | 1 | 50.0 | São Paulo |
| 9 | Laranjinha | 257.8 | 0.26 | 1 | 1 | 100.0 | Paraná |
| 10 | Meia Ponte | 434.9 | 0.16 | 1 | 1 | 100.0 | Goiás |
| 11 | Mogi-Guaçu | 406.1 | 0.24 | 6 | 2 | 33.3 | São Paulo |
| 12 | Paraná | 802.4 | 0.02 | 4 | 2 | 50.0 | São Paulo and Paraná# |
| 13 | Paranapanema | 718.3 | 0.08 | 11 | 5 | 45.5 | São Paulo * |
| 14 | Pardo | 481.0 | 0.18 | 7 | 2 | 28.6 | São Paulo |
| 15 | Quilombo | 35.7 | 0.56 | 1 | 1 | 100.0 | São Paulo |
| 16 | Sapucaí | 305.8 | 0.17 | 5 | 5 | 100.0 | São Paulo |
| 17 | Sorocaba | 204.1 | 0.19 | 5 | 2 | 40.0 | São Paulo |
| 18 | Tibagi | 336.8 | 0.11 | 2 | 1 | 50.0 | Paraná |
| 19 | Tijuco | 274.8 | 0.15 | 2 | 1 | 50.0 | Minas Gerais |
| 20 | Verdão | 384.3 | 0.15 | 1 | 1 | 100.0 | Goiás |
| 21 | Verde | 386.3 | 0.07 | 3 | 1 | 33.3 | Mato Grosso do Sul |
| Total | 7365.1 | - | 75 | 35 | 46.7 | - |
| Family and Species | Common Name | Status of Conservation | SL (cm) | Feeding | Spawning Season | Habitat | Migratory Movements |
|---|---|---|---|---|---|---|---|
| CHARACIFORMES | |||||||
| Anostomidae | |||||||
| Megaleporinus obtusidens | Piapara | 51.7 | Omnivorous | Dec-Jan | FFRS | LON-UP | |
| Megaleporinus piavussu | Piapara | 40.0 | Omnivorous | Nov-Jan | FFRS | ||
| Megaleporinus macrocephalus | Piavuçu | 50.0 | Omnivorous | - | FFRS | ||
| Bryconidae | |||||||
| Brycon orbignyanus | Piracanjuba | EN1, CR2 | 62.5 | Insectivorous | Oct-Jan | FFRS | LONG-UP |
| Salminus brasiliensis | Dourado | VU3 | 85.9 | Piscivorous | Oct-Jan | FFRS | LON-UPDO, LAT |
| Salminus hilarii* | Tabarana | 34.0 | Piscivorous | Nov-Jan | FFRS | ||
| Cynodontidae | |||||||
| Rhaphiodon vulpinus | Dourado-cachorro | 78.0 | Piscivorous | Out-Jan | FFRS, RES | - | |
| Prochilodontidae | |||||||
| Prochilodus lineatus | Curimba | 54.2 | Iliophagous | Oct-Jan | FFRS, RES | LON-UPDO, LAT | |
| Serrasalmidae | |||||||
| Piaractus mesopotamicus | Pacu | 52.6 | Omnivorous | Oct-Jan | FFRS | LON-UP, LAT | |
| SILURIFORMES | |||||||
| Doradidae | |||||||
| Pterodoras granulosus | Armado | 63.5 | Omnivorous | Jan-Mar | FFRS, RES | LON-UPDO, LAT | |
| Rhinelepis aspera | Cascudo-preto | VU3 | 49.0 | Iliophagous | Oct-Jan | FFRS | |
| Pimelodidae | |||||||
| Hemisorubim platyrhynchos | Jurupoca | 51.4 | Piscivorous | Dec-Jan | FFRS | LON-UP | |
| Pimelodus maculatus | Mandi amarelo | 36.0 | Omnivorous | Nov-Jan | FFRS, RES | LON-UPDO, LAT | |
| Pinirampus pirinampu | Barbado | 68.0 | Piscivorous | Dec-Jan | FFRS, RES | LON-UPDO, LAT | |
| Pseudoplatystoma corruscans | Pintado | NT3 | 140.0 | Piscivorous | Nov-Feb | FFRS | LON-UP, LAT |
| Sorubim lima | Jurupensem | 60.5 | Piscivorous | Nov-Dec | FFRS | ||
| Steindachneridion scriptum* | Surubim | EN1 | 64.0 | Piscivorous | Dec-Jan | FFRS | |
| Zungaro jahu* | Jaú | EN2 | 83.0 | Piscivorous | Dec-Feb | FFRS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makrakis, S.; Bertão, A.P.S.; Silva, J.F.M.; Makrakis, M.C.; Sanz-Ronda, F.J.; Celestino, L.F. Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin. Sustainability 2019, 11, 3749. https://doi.org/10.3390/su11133749
Makrakis S, Bertão APS, Silva JFM, Makrakis MC, Sanz-Ronda FJ, Celestino LF. Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin. Sustainability. 2019; 11(13):3749. https://doi.org/10.3390/su11133749
Chicago/Turabian StyleMakrakis, Sergio, Ana P. S. Bertão, Jhony F. M. Silva, Maristela C. Makrakis, Fco. Javier Sanz-Ronda, and Leandro F. Celestino. 2019. "Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin" Sustainability 11, no. 13: 3749. https://doi.org/10.3390/su11133749
APA StyleMakrakis, S., Bertão, A. P. S., Silva, J. F. M., Makrakis, M. C., Sanz-Ronda, F. J., & Celestino, L. F. (2019). Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin. Sustainability, 11(13), 3749. https://doi.org/10.3390/su11133749






