Etch Characteristics and Morphology of Al2O3/TiO2 Stacks for Silicon Surface Passivation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
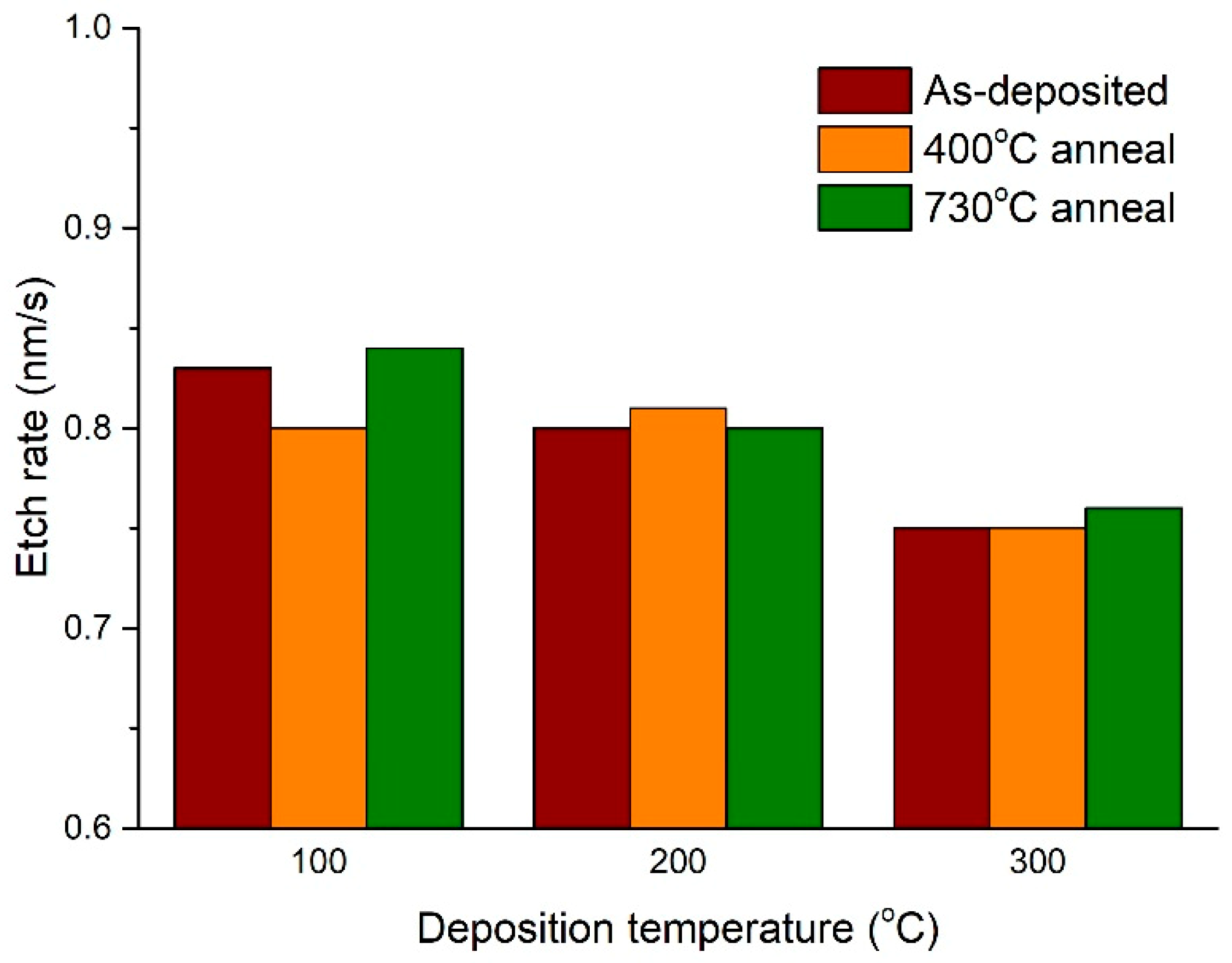
3.1. Dry Etching
3.2. Wet Etching
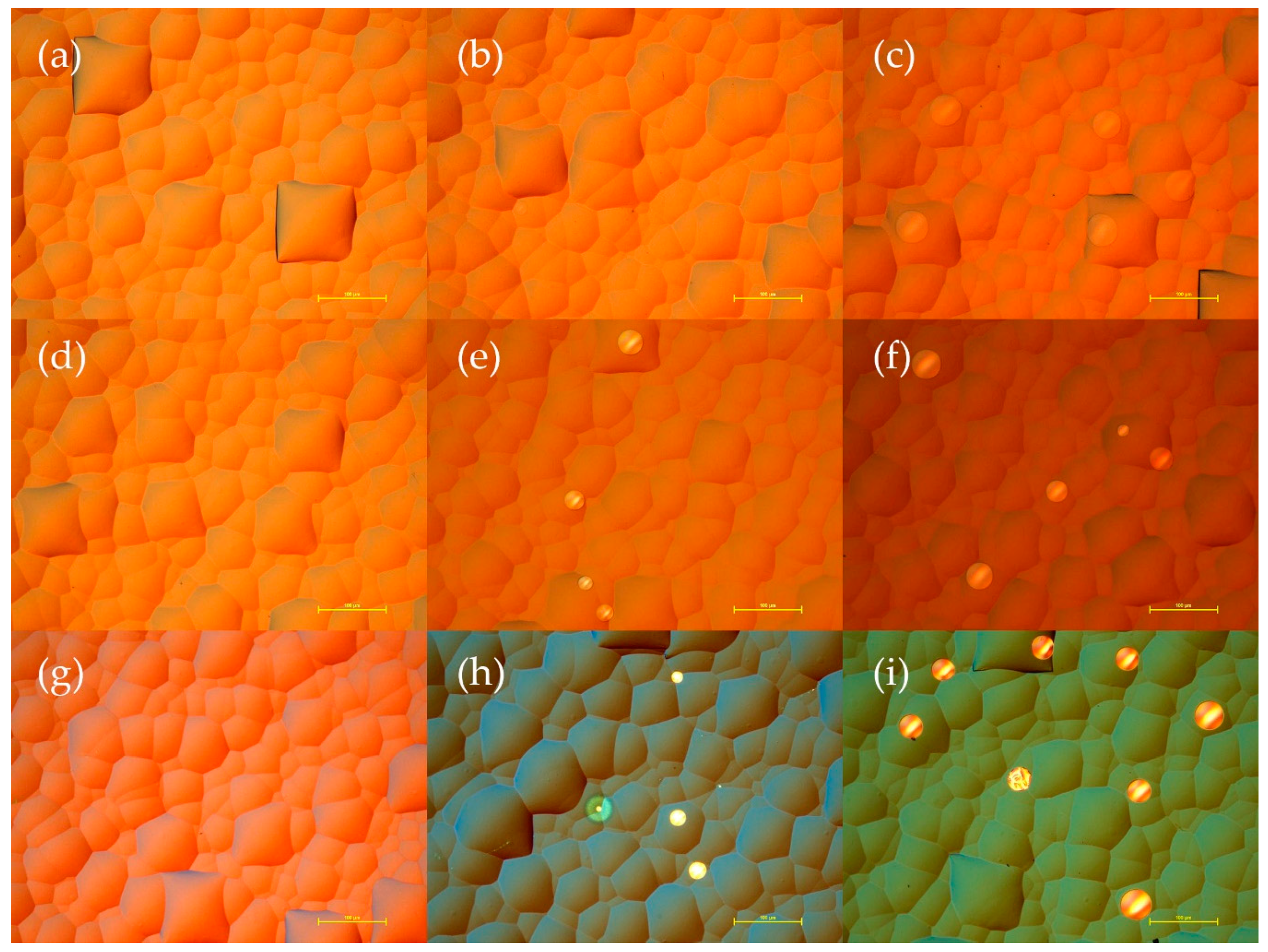
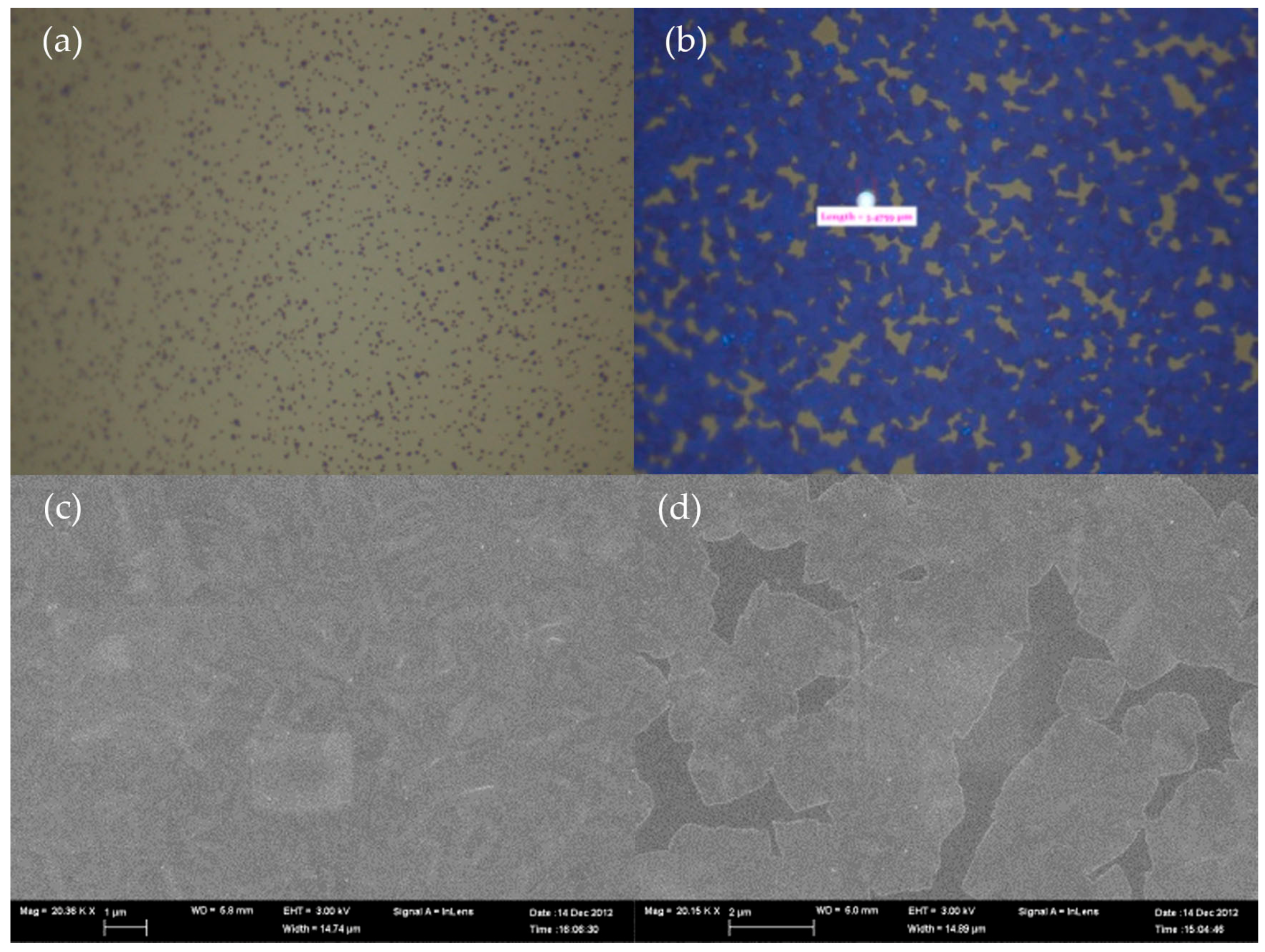
3.3. Morphology
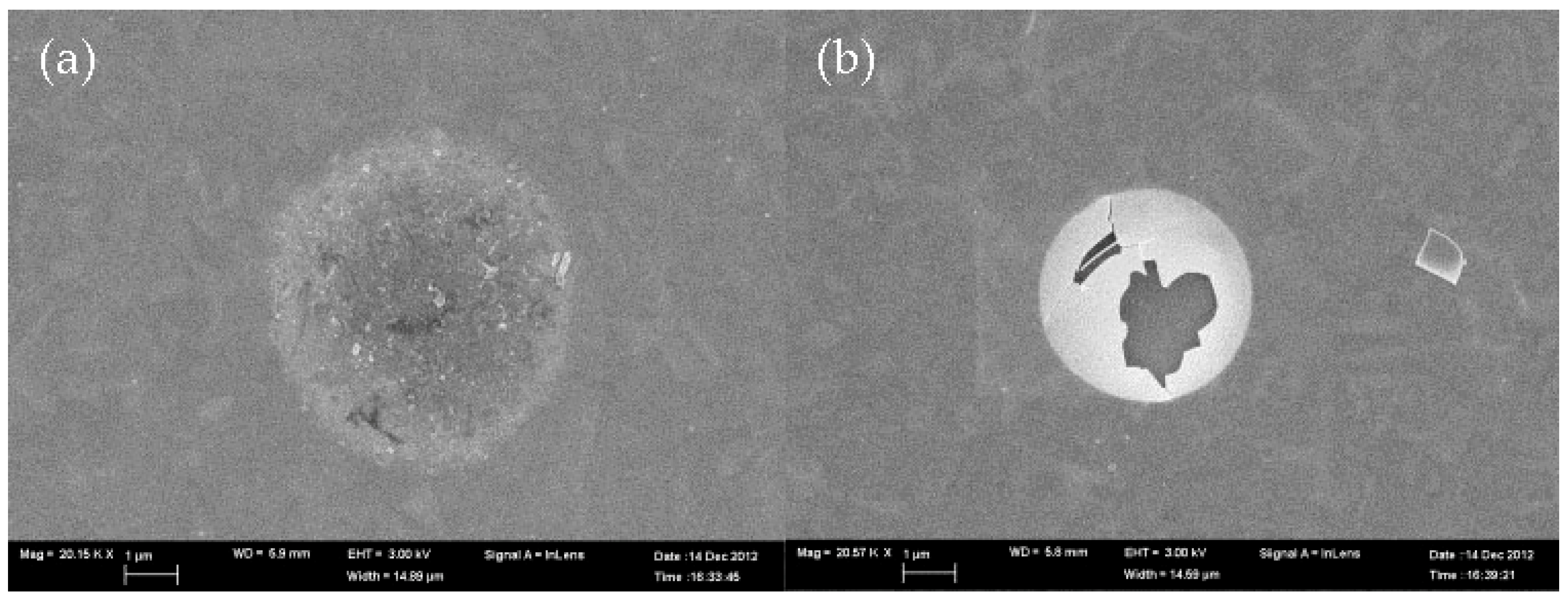
3.4. Blistering
3.5. Passivation
4. Conclusions
Funding
Conflicts of Interest
References
- Lee, J.; Michaelson, L.; Munoz, K.; Tyson, T.; Gallegos, A.; Sullivan, J.; Buonassisi, T. In-situ photoluminescence imaging for passivation-layer etching process control for photovoltaics. Appl. Phys. Lett. 2014, 105, 043901. [Google Scholar] [CrossRef]
- Deligiannis, D.; Marioleas, V.; Vasudevan, R.; Visser, C.C.; van Swaaij, R.A.; Zeman, M. Understanding the thickness-dependent effective lifetime of crystalline silicon passivated with a thin layer of intrinsic hydrogenated amorphous silicon using a nanometer-accurate wet-etching method. J. Appl. Phys. 2016, 119, 235307. [Google Scholar] [CrossRef]
- Rahman, T.; To, A.; Pollard, M.E.; Grant, N.E.; Colwell, J.; Payne, D.N.; Murphy, J.D.; Bagnall, D.M.; Hoex, B.; Boden, S.A. Minimising bulk lifetime degradation during the processing of interdigitated back contact silicon solar cells. Prog. Photovolt. Res. Appl. 2018, 26, 38–47. [Google Scholar] [CrossRef]
- Dullweber, T.; Schmidt, J. Industrial silicon solar cells applying the passivated emitter and rear cell (PERC) concept—A review. IEEE J. Photovolt. 2016, 6, 1366–1381. [Google Scholar] [CrossRef]
- Cornagliotti, E.; Uruena, A.; Horzel, J. How much rear side polishing is required? A study on the impact of rear side polishing in PERC solar cells. In Proceedings of the 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, Germany, 24–28 September 2012; pp. 561–566. [Google Scholar]
- Huang, H.; Lv, J.; Bao, Y.; Xuan, R.; Sun, S.; Sneck, S.; Li, S.; Modanese, C.; Savin, H.; Wang, A. 20.8% industrial PERC solar cell: ALD Al2O3 rear surface passivation, efficiency loss mechanisms analysis and roadmap to 24%. Sol. Energy Mater. Sol. Cells 2017, 161, 14–30. [Google Scholar] [CrossRef]
- Suh, D.; Weber, K. Effective silicon surface passivation by atomic layer deposited Al2O3/TiO2 stacks. Phys. Status Solidi (RRL) Rapid Res. Lett. 2014, 8, 40–43. [Google Scholar] [CrossRef]
- Suh, D.; Choi, D.-Y.; Weber, K.J. Al2O3/TiO2 stack layers for effective surface passivation of crystalline silicon. J. Appl. Phys. 2013, 114, 154107. [Google Scholar] [CrossRef]
- Vermang, B.; Goverde, H.; Uruena, A.; Lorenz, A.; Cornagliotti, E.; Rothschild, A.; John, J.; Poortmans, J.; Mertens, R. Blistering in ALD Al2O3 passivation layers as rear contacting for local Al BSF Si solar cells. Sol. Energy Mater. Sol. Cells 2012, 101, 204–209. [Google Scholar] [CrossRef]
- Vermang, B.; Goverde, H.; Lorenz, A.; Uruena, A.; Vereecke, G.; Meersschaut, J.; Cornagliotti, E.; Rothschild, A.; John, J.; Poortmans, J. On the blistering of atomic layer deposited Al2O3 as Si surface passivation. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 13 June 2011; pp. 003562–003567. [Google Scholar]
- Richter, A.; Henneck, S.; Benick, J.; Hörteis, M.; Hermle, M.; Glunz, S. Firing stable Al2O3/SiNx layer stack passivation for the front side boron emitter of n-type silicon solar cells. In Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition, Valencia, Spain, 6–9 September 2010; pp. 1453–1456. [Google Scholar]
- Kania, D.; Saint-Cast, P.; Hofmann, M.; Rentsch, J.; Preu, R. High temperature stability of PECVD aluminium oxide layers applied as negatively charged passivation on c-Si surfaces. In Proceedings of the 25th European Photovoltaic Solar Energy Conference, Valencia, Spain, 6–9 September 2010; pp. 2292–2296. [Google Scholar]
- Sperlich, H.; Decker, D.; Saint-Cast, P.; Erben, E.; Peters, L. High productive Solar Cell Passivation on Roth&Rau MAiA® MW-PECVD inline machine—A comparison of Al2O3, SiO2 and SiNx-H process conditions and performance. In Proceedings of the 25th European Photovoltaic Solar Energy Conference and Exhibition, Valencia, Spain, 6–9 September 2010; pp. 1352–1357. [Google Scholar]
- Gardner, D.S.; Flinn, P.A. Mechanical stress as a function of temperature in aluminum films. IEEE Trans. Electron Devices 1988, 35, 2160–2169. [Google Scholar] [CrossRef]
- Dingemans, G.; Van de Sanden, M.; Kessels, W. Influence of the deposition temperature on the c-Si surface passivation by Al2O3 films synthesized by ALD and PECVD. Electrochem. Solid-State Lett. 2010, 13, H76–H79. [Google Scholar] [CrossRef]
- Kern, W. Handbook of Semiconductor Wafer Cleaning Technology; Noyes Publication: Saddle River, NJ, USA, 1993; pp. 111–196. [Google Scholar]
- Sinton, R.; Macdonald, D. WCT-120 Photoconductance Lifetime Tester: User Manual; Sinton Consulting: Boulder, CO, USA, 2006. [Google Scholar]
- Chen, D.; Edwards, M.; Wenham, S.; Abbott, M.; Hallam, B. Laser enhanced gettering of silicon substrates. Front. Energy 2017, 11, 23–31. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Kiisler, A.-A.; Uustare, T.; Sammelselg, V. Effect of crystal structure on optical properties of TiO2 films grown by atomic layer deposition. Thin Solid Films 1997, 305, 270–273. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, G.J.; Kim, J.H.; Hwang, C.S. Growth behavior of Al-doped TiO2 thin films by atomic layer deposition. Chem. Mater. 2008, 20, 3723–3727. [Google Scholar] [CrossRef]
- Vazsonyi, E.; De Clercq, K.; Einhaus, R.; Van Kerschaver, E.; Said, K.; Poortmans, J.; Szlufcik, J.; Nijs, J. Improved anisotropic etching process for industrial texturing of silicon solar cells. Sol. Energy Mater. Sol. Cells 1999, 57, 179–188. [Google Scholar] [CrossRef]
- Hauser, A.; Hahn, G.; Spiegel, M.; Fath, P.; Bucher, E.; Feist, H.; Breitenstein, O.; Rakotoniaina, J.P. Comparison of different techniques for edge isolation. In Proceedings of the 17th European photovoltaic solar energy conference, Munich, Germany, 22–26 October 2001; pp. 1739–1742. [Google Scholar]
- De Wolf, S.; Agostinelli, G.; Beaucarne, G.; Vitanov, P. Influence of stoichiometry of direct plasma-enhanced chemical vapor deposited SiNx films and silicon substrate surface roughness on surface passivation. J. Appl. Phys. 2005, 97, 063303. [Google Scholar] [CrossRef]
- Souriau, L.; Terzieva, V.; Meuris, M.; Caymax, M. A Wet Etching Technique to Reveal Threading Dislocations in Thin Germanium Layers. Solid State Phenomena 2008, 134, 83–86. [Google Scholar] [CrossRef]
- Hennen, L.; Granneman, E.; Kessels, W. Analysis of blister formation in spatial ALD Al2O3 for silicon surface passivation. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 001049–001054. [Google Scholar]
- Granneman, E.H.; Kuznetsov, V.I.; Vermont, P. Spatial ALD, Deposition of Al2O3 Films at Throughputs Exceeding 3000 Wafers per Hour. ECS Trans. 2014, 61, 3–16. [Google Scholar] [CrossRef]
- Matero, R.; Rahtu, A.; Ritala, M. In situ quadrupole mass spectrometry and quartz crystal microbalance studies on the atomic layer deposition of titanium dioxide from titanium tetrachloride and water. Chem. Mater. 2001, 13, 4506–4511. [Google Scholar] [CrossRef]
- Ferguson, J.; Yoder, A.; Weimer, A.; George, S. TiO2 atomic layer deposition on ZrO2 particles using alternating exposures of TiCl4 and H2O. Appl. Surf. Sci. 2004, 226, 393–404. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 9. [Google Scholar] [CrossRef]
- Keller, P.; Zuschlag, A.; Karzel, P.; De Moro, F.; Hahn, G. Defect characterization and investigation of RST-silicon ribbon wafers. In Proceedings of the 28th European Photovoltaic Solar Energy Conference and Exhibition, Paris, France, 30 September–4 October 2013; pp. 1512–1517. [Google Scholar]




| Tdep (°C) | Tann (°C) | Etch Rate (nm/min) | |||||
|---|---|---|---|---|---|---|---|
| HF (25 °C) | SC1 | SC2 | |||||
| 1% | 10% | 25 °C | 65 °C | 25 °C | 65 °C | ||
| 100 | - | 1.1 ± 0.17 | 90.0 ± 13 | 0.29 ± 0.09 | 15.0 ± 1.4 | 0.56 ± 0.04 | 3.0 ± 1.4 |
| 400 | no etch | scratched | no etch | no etch | no etch | no etch | |
| 730 | no etch | no etch | no etch | no etch | no etch | no etch | |
| 200 | - | 1.0 ± 0.07 | 87.0 ± 7.8 | 0.1 ± 0.01 | 13.0 ± 1.0 | 0.52 ± 0.13 | 2.8 ± 0.8 |
| 400 | no etch | no etch | no etch | no etch | no etch | no etch | |
| 730 | |||||||
| 300 | - | no etch | no etch | no etch | no etch | no etch | no etch |
| 400 | |||||||
| 730 | |||||||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, D. Etch Characteristics and Morphology of Al2O3/TiO2 Stacks for Silicon Surface Passivation. Sustainability 2019, 11, 3857. https://doi.org/10.3390/su11143857
Suh D. Etch Characteristics and Morphology of Al2O3/TiO2 Stacks for Silicon Surface Passivation. Sustainability. 2019; 11(14):3857. https://doi.org/10.3390/su11143857
Chicago/Turabian StyleSuh, Dongchul. 2019. "Etch Characteristics and Morphology of Al2O3/TiO2 Stacks for Silicon Surface Passivation" Sustainability 11, no. 14: 3857. https://doi.org/10.3390/su11143857
APA StyleSuh, D. (2019). Etch Characteristics and Morphology of Al2O3/TiO2 Stacks for Silicon Surface Passivation. Sustainability, 11(14), 3857. https://doi.org/10.3390/su11143857





