Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Waste and Co-Substrate Characterization
2.2. Inoculum and Substrates
2.3. Reactor Start-Up
2.4. Semi-Continuous Anaerobic Digestion Experiments
3. Results
3.1. Food Waste Characteristics
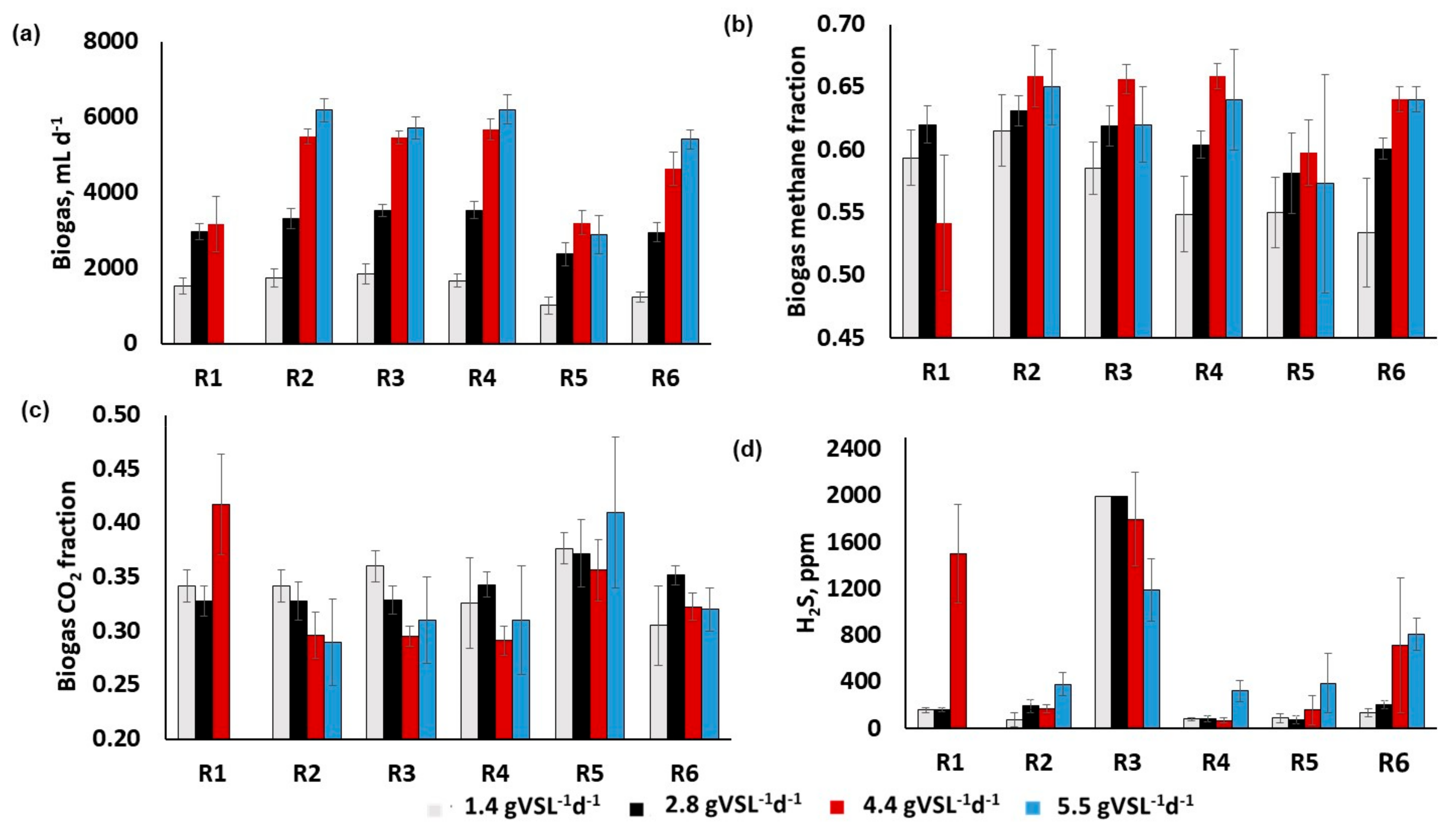
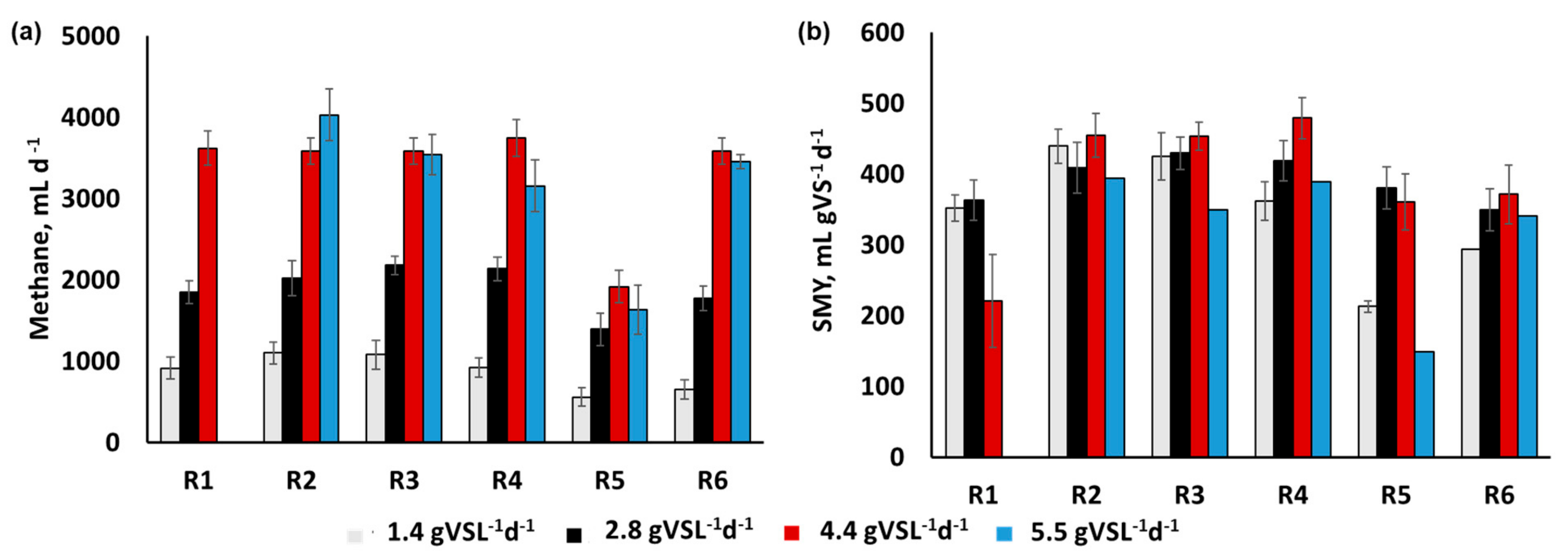
3.2. Process Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Liu, J.; Shi, F.; Wang, S.; Gao, Y.; Zhang, D. High-solid Anaerobic Co-digestion of Food Waste and Rice Straw for Biogas Production. J. Northeast. Agric. Univ. 2014, 21, 61–66. [Google Scholar] [CrossRef]
- Stan, C.; Collaguazo, G.; Streche, C.; Apostol, T.; Cocarta, D.M. Pilot-scale anaerobic co-digestion of the OFMSW: Improving biogas production and startup. Sustainability 2018, 10, 1939. [Google Scholar] [CrossRef]
- Martín-González, L.; Colturato, L.F.; Font, X.; Vicent, T. Anaerobic co-digestion of the organic fraction of municipal solid waste with FOG waste from a sewage treatment plant: Recovering a wasted methane potential and enhancing the biogas yield. Waste Manag. 2010, 30, 1854–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsyurbenko, O.R.; Chin, K.-J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- WRAP. Operators Briefing Note. Defra Research Project, U.K. 2012. Available online: http://www.wrap.org.uk/sites/files/wrap/Operators%20Briefing%20Note.pdf (accessed on 12 November 2016).
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014, 152, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. In situ biogas stripping of ammonia from a digester using a gas mixing system. Environ. Technol. 2017, 38, 3216–3224. [Google Scholar] [CrossRef]
- Cho, S.-K.; Lee, M.-K.; Kim, D.-H.; Yun, Y.-M.; Jung, K.-W.; Shin, H.-S.; Oh, S.E. Enhanced anaerobic digestion of livestock waste by ultrasonication: A tool for ammonia removal and solubilization. Korean J. Chem. Eng. 2014, 31, 619–623. [Google Scholar] [CrossRef]
- Lin, L.; Yuan, S.; Chen, J.; Xu, Z.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation. J. Hazard. Mater. 2009, 161, 1063–1068. [Google Scholar] [CrossRef]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Esposito, G.; Yeh, D.H.; Lens, P.N.L. Enhanced Anaerobic Digestion of Food Waste by Supplementing Trace Elements: Role of Selenium (VI) and Iron (II). Front. Environ. Sci. 2016, 4, 8. [Google Scholar] [CrossRef]
- Facchin, V.; Cavinato, C.; Fatonea, F.; Pavan, P.; Bolzonell, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of foodwaste in batch trials: The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Peng, X.; Wang, X.; Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 248, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Li, F.; Yang, G.; Banks, C.; Humphreys, P. Effects of Temperature and Carbon-Nitrogen (C/N) Ratio on the Performance of Anaerobic Co-Digestion of Dairy Manure, Chicken Manure and Rice Straw: Focusing on Ammonia Inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C.J.; Heaven, S. Co-digestion of source segregated domestic food waste to improve process stability. Bioresour. Technol. 2012, 114, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Nayono, S.E.; Gallert, C.; Winter, J. Co-digestion of press water and food waste in a biowaste digester for improvement of biogas production. Bioresour. Technol. 2010, 101, 6987–6993. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Mutnuri, S. Anaerobic co-digestion of sewage sludge and food waste. Waste Manag. Res. 2016, 34, 307–315. [Google Scholar] [CrossRef]
- Babaee, A.; Shayegan, J. Effect of Organic Loading Rates (OLR) on Production of Methane from Anaerobic Digestion of Vegetables Waste. In World Renewable Energy Congress—Sweden; Linköping University Electronic Press: Linköping, Sweden, 2011; pp. 411–417. [Google Scholar] [CrossRef] [Green Version]
- Zahan, Z.; Othman, M.Z.; Rajendram, W. Anaerobic Codigestion of Municipal Wastewater Treatment Plant Sludge with Food Waste: A Case Study. BioMed Res. Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- EPA. METHOD 1684: Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; Environmental Protection Agency: Columbia, WA, USA, 2001.
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, S.; Deepanraj, B.V.S. Study on the effect of ph on biogas production from food waste by anaerobic digestion. In Proceedings of the 9th Annual Green Energy Conference, Tianjin, China, 25–28 May 2014. [Google Scholar]
- Shah, A.A.; Nawaz, A.; Kanwal, L.; Hasan, F.; Khan, S.; Badshah, M. Degradation of poly(ε-caprolactone) by a thermophilic bacterium Ralstonia sp. Strain MRL-TL isolated from hot spring. Int. Biodeterior. Biodegrad. 2015, 98, 35–42. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V. Dry-grind processing using amylase corn and superior yeast to reduce the exogenous enzyme requirements in bioethanol production. Biotechnol. Biofuels 2016, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Zheng, P.; Wang, Y. Anaerobic digestion of food waste stabilized by lime mud from papermaking process. Bioresour. Technol. 2014, 170, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, A.J.; Pereira, M.A.; Alves, M. Enhancement of methane production from long chain fatty acid based effluents. Bioresour. Technol. 2008, 99, 4086–4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labatut, R.A.; Gooch, C.A. Monitoring of Anaerobic Digestion Process to Optimize Performance and Prevent System Failure. Proc Got Manure? Enhancing Environ. Econ. Sustain. 2012, 1, 209–225. Available online: https://ecommons.cornell.edu/handle/1813/36531 (accessed on 30 January 2017).
- Liu, X.; Xiang, L.; Song, Y.; Qian, F.; Meng, X. The effects and mechanism of alkalinity on the phosphate recovery from anaerobic digester effluent using dolomite lime. Environ. Earth Sci. 2015, 73, 5067–5073. [Google Scholar] [CrossRef]
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501. [Google Scholar] [CrossRef]
- Liu, A.; Ren, F.; Lin, W.Y.; Wang, J.Y. A review of municipal solid waste environmental standards with a focus on incinerator residues. Int. J. Sustain. Built Environ. 2015, 4, 165–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.Y.; Sun, Z.; Leung, C.C.J.; Han, W.; Lau, K.Y.; Li, M.; Lin, C.S.K. Valorisation of bakery waste for succinic acid production. Green Chem. 2013, 15, 690. [Google Scholar] [CrossRef]
- Cook, A.M.; Denger, K. Metabolism of taurine in microorganisms: A primer in molecular biodiversity? Adv. Exp. Med. Biol. 2006, 583, 3–13. [Google Scholar] [CrossRef]
- Prabhudessai, V.; Ganguly, A.; Mutnuri, S. Effect of caffeine and saponin on anaerobic digestion of food waste. Ann. Microbiol. 2009, 59, 643–648. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of organic loading rate on anaerobic digestion of food waste under mesophilic and thermophilic conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Guo, Y.; Yuan, Z.; Wang, Y.; Zhen, F. Continuous anaerobic digestion of food waste and design of digester with lipid removal. Environ. Technol. 2013, 34, 2135–2143. [Google Scholar] [CrossRef] [PubMed]


| Food Waste Type | Co-Substrate | Reactor Volume [L] | OLR [gVSL−1d−1] | Operation Mode | Ratio FW/Co-Sub | SMY [mL gVS−1d−1] | HRT [d] | Reference |
|---|---|---|---|---|---|---|---|---|
| Cafeteria food waste (CFW) | Chicken manure (ChM) | 5 | 2.5 | Semi-continuous with only FW fed on day 1 and 2, ChM fed on day 3; the sequence repeated | NA | 508 | 50 | [17] |
| CFW | ChM | 1 | 15 | Semi-continuous with once a day feeding and discharge | 2 | 317 | NR | [18] |
| De-fibered kitchen waste | Biowaste | NR | 10.9 | Semi-continuous with two times a day feeding, five days a week | NR | 420 | 7 | [19] |
| CFW | Sewage sludge | 5 | 1 | Batch | 0.5 | 494 | 21 | [20] |
| CFW | Rice straw | 1 | 5 | Batch | 5 | 392 | NR | [2] |
| Vegetable waste | none | 75 | 1.4 | Semi-continuous with once a day feeding and discharge | NA | 250 | 25 | [21] |
| Greasy food processing waste | Municipal sludge | 0.5 | 2 | Semi-continuous with once a day feeding and discharge | 1 | 633 | 20 | [22] |
| Organic fraction of MSW | Fats, oils, and grease | 5 | 4 | Semi-continuous with once a day feeding and discharge | 6.7 | 318 | 16 | [4] |
| Reactor | Co-Substrate | Abbreviation |
|---|---|---|
| R1 | None | - |
| R2 | Acid whey | AW |
| R3 | Energy drink | ED |
| R4 | Waste bread | WB |
| R5 | Paper napkins | PN |
| R6 | Cow manure | CM |
| Characteristics of CFW | |
|---|---|
| Physical and Chemical Properties, n = 6 | |
| pH | 4.2 ± 0.3 |
| TS % | 23.8 ± 2.9 |
| VS/TS % | 90.9 ± 2.4 |
| VS % | 22.9 ± 1.2 |
| COD g/L | 197 ± 42 |
| Ash % | 1.8 ± 0.8 |
| Calorific value, kJ/kg (n = 2) | 23,098 |
| Macronutrients, n = 4 | |
| Crude protein (CP) % | 13.3 ± 10 |
| Available protein % | 13 ± 9.9 |
| Soluble protein % of CP | 53 ± 4.2 |
| Lignin % | 0.7 ± 0.9 |
| Starch % | 10.5 ± 8.5 |
| Simple Sugars % | 7.5 ± 4 |
| Crude fat % | 13.4 ± 11 |
| Minerals, n = 4 | |
| Calcium, ppm | 1225 ± 1014 |
| Potassium, ppm | 5950 ± 4088 |
| Magnesium, ppm | 425 ± 263 |
| Phosphorous, ppm | 1900 ± 1449 |
| Sodium, ppm | 4013 ± 2984 |
| Iron, ppm | 23 ± 17 |
| Zinc, ppm | 12.8 ± 8.4 |
| Copper, ppm | 2.7 ± 1.5 |
| Manganese, ppm | 6.5 ± 4.7 |
| Molybdenum, ppm | ND |
| Sulfur, ppm | 1525 ± 1187 |
| Chlorine ion, ppm | 7125 ± 5227 |
| Elemental composition, n = 2 | |
| Carbon % | 52.4 |
| Hydrogen % | 7.4 |
| Nitrogen % | 3.3 |
| Oxygen % | 30.6 |
| Physical and Chemical Properties, n = 3 | AW | ED | WB | PN | CM |
|---|---|---|---|---|---|
| pH | 4.2 ± 0.2 | 3.3 ± 0 | NM | NM | 6.8 ± 0.5 |
| TS % | 2.9 ± 0.1 | 0.7 ± 0.1 | 95.6 ± 0.7 | 94.5 ± 3.5 | 10.3 ± 1.4 |
| VS/TS % | 73.3 ± 0.2 | 80.2 ± 5.0 | 89.4 ± 2.9 | 86 ± 6.9 | 83.5 ± 0.8 |
| VS % | 2.1 ± 0.1 | 0.6 ± 0.1 | 85.4 ± 2.3 | 81.4 ± 9.4 | 8.6 ± 1.2 |
| COD g/L | 43 ± 4.0 | 11 ± 0.1 | 1167 ± 97 | 1176 ± 142 | 97 ± 6.0 |
| Ash % | 4.2 ± 0.2 | 0.2 ± 0 | 10.2 ± 2.8 | 13.1 ± 5.9 | 1.7 ± 0.3 |
| Elemental composition (single measurements) | |||||
| Carbon % | 1.5 | 0.5 | 45.2 | 44.8 | 54.8 |
| Hydrogen % | 11.2 | 10.8 | 6.6 | 6.1 | NM |
| Nitrogen % | 0.5 | 0.5 | 2.2 | 0.3 | 3.6 |
| Oxygen % | NM | NM | 42.6 | 47 | NM |
| Sulfur, ppm | 30 | 1000 | 1700 | 300 | 2600 |
| Phosphorus, ppm | 875 | 1 | 1854 | 29 | 3000 |
| Mineral Composition of Selected Co-Substrates | |||
|---|---|---|---|
| Minerals | AW | WB | CM |
| Calcium, ppm | 1000 ± 282 | 1100 | 2340 |
| Potassium, ppm | 125 ± 35 | 2300 | 2190 |
| Magnesium, ppm | 100 ± 0 | 400 | 670 |
| Sodium, ppm | 290 ± 85 | 7300 | 395 |
| Iron, ppm | ND | 76 | 163 |
| Zinc, ppm | 3.5 | 10 | 188 |
| Copper, ppm | ND | 2 | 72 |
| Manganese, ppm | ND | 7 | 205 |
| Molybdenum, ppm | ND | ND | 2.6 |
| Chlorine ion, ppm | ND | ND | 620 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegde, S.; Trabold, T.A. Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates. Sustainability 2019, 11, 3875. https://doi.org/10.3390/su11143875
Hegde S, Trabold TA. Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates. Sustainability. 2019; 11(14):3875. https://doi.org/10.3390/su11143875
Chicago/Turabian StyleHegde, Swati, and Thomas A. Trabold. 2019. "Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates" Sustainability 11, no. 14: 3875. https://doi.org/10.3390/su11143875
APA StyleHegde, S., & Trabold, T. A. (2019). Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates. Sustainability, 11(14), 3875. https://doi.org/10.3390/su11143875






