Soil Organic Carbon Accumulation in Post-Agricultural Soils under the Influence Birch Stands
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Report 2018. Report on the Condition of Forests in Poland; CILP: Warszawa, Poland, 2018. [Google Scholar]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators offorest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Van-Camp, L.; Bujarrabal, B.; Gentile, A.R.; Jones, R.J.A.; Montanarella, L.; Olazabal, C.; Selvaradjou, S.K. Reports of the Technical Working Groups Established under the Thematic Strategy for Soil Protection; EUR 21319EN/3; Office for official Publications of the European Communities: Luxembourg, 2004; p. 872. [Google Scholar]
- Steiner, C.; Teixeira, W.G.; Lehman, J.; Nehls, T.; de Macêdo, J.L.U.; Blum, W.E.M.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Yang, L.; Luo, P.; Wen, L.; Li, D. Soil organic carbon accumulation during post-agricultural succession in a karst area, southwest China. Sci. Rep. 2016, 6, 37118. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gifford, R. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Mao, D.M.; Min, Y.W.; Yu, L.L.; Martens, R.; Insam, H. Effect of afforestation on microbial biomass and activity in soils of tropical China. Soil Biol. Biochem. 1992, 24, 865–872. [Google Scholar]
- Podrázský, V.; Holubík, O.; Vopravil, J.; Khel, T.; Moser, W.K.; Prknová, H. Effects of afforestation on soil structure formation in two climatic regions of the Czech Republic. J. For. Sci. 2015, 61, 225–234. [Google Scholar] [CrossRef]
- Bolat, I.; Kara, Ö.; Sensoy, H.; Yüksel, K. Influences of Black Locust (Robinia pseudoacacia L.) afforestation on soil microbial biomass and activity. iFor. Biogeosci. For. 2017, 9, 171–177. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Kucharski, J. Relacje między aktywnością enzymów a żyznością gleby. In Drobnoustroje w Środowisku, Występowanie, Aktywność i Znaczenie; Barabasz, W., Ed.; AR: Kraków, Poland, 1997; pp. 327–347. [Google Scholar]
- Wolińska, A.; Stępniewska, Z.; Pytlak, A. The effect of environmental factors on total soil DNA content and dehydrogenase activity. Arch. Biol. Sci. 2015, 67, 493–501. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Gruba, P. Enzymatic activity and stabilization of organic matter in soil with different detritus inputs. J. Soil Sci. Plant Nutr. 2017, 63, 242–247. [Google Scholar]
- Kaczynski, P.; Lozowicka, B.; Hrynko, I.; Wolejko, E. Behaviour of mesotrione in maize and soil system and its influence on soil dehydrogenase activity. Sci. Total Environ. 2016, 71, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Lasota, J.; Zwydak, M.; Klamerus-Iwan, A.; Gołąb, J. Restoration of forest soil and vegetation 15 years after landslides in a lower zone of mountains in temperate climates. Ecol. Eng. 2016, 97, 503–515. [Google Scholar] [CrossRef]
- Pająk, M.; Błońska, E.; Frąc, M.; Oszust, K. Functional diversity and microbial activity of forest soils that are heavily contaminated by lead and zinc. Water Air Soil Pollut. 2016, 227, 348. [Google Scholar] [CrossRef]
- Józefowska, A.; Pietrzykowski, M.; Woś, B. Tree species and soil substrate effects on soil biota during early soil forming stages at afforested mine sites. Appl. Soil Ecol. 2016, 102, 70–79. [Google Scholar] [CrossRef]
- Van der Wal, A.; van Veen, J.A.; Smant, W.; Boschker, H.; Bloem, J.; Kardol, P.; van der Putten, W.H.; de Boer, W. Fungalbiomass development in a chronosequence of land abandon-ment. Soil Biol. Biochem 2006, 38, 51–60. [Google Scholar] [CrossRef]
- Gunina, A.; Smith, A.R.; Godbold, D.L.; Jones, D.L.; Kuzyakov, Y. Response of soil microbial community to afforestation with pure and mixed species. Plant Soil 2016, 412, 357–368. [Google Scholar] [CrossRef]
- WRB (World Reference Base for Soil Resource); FAO: Rome, Italy, 2014.
- Ostrowska, A.; Porębska, G.; Kanafa, M. Carbon accumulation and Distribution in Profiles of Forest Soils. Pol. J. Environ. Stud. 2010, 19, 1307–1315. [Google Scholar]
- Alef, K.; Nannipieri, P. Enzyme activities. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995. [Google Scholar]
- Richter, D.D.; Markewitz, D.; Trumbore, S.E.; Wells, C.G. Rapid accumulation and turnover of soil carbon in a reestablishing forest. Nature 1999, 400, 56–58. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.H.; Hu, Y.L.; Li, L.J.; Yyng, D. Soil organic carbon and nitrogen stocks in an age-sequence of poplar stands planted on marginal agricultural land in Northeast China. Plant Soil 2010, 332, 277–287. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv. Agron. 2005, 88, 35–66. [Google Scholar]
- Kotroczó, Z.; Veres, Z.; Fekete, J.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérisz, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Janssens, J.A.; Sampson, D.A.; Curiel-Yuste, J.; Carrara, A.; Cenlemans, R. The carbon cost of fine root turnover in a Scots pine forest. For. Ecol. Manag. 2002, 168, 231–240. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef]
- Holubík, O.; Podrázský, V.; Vopravil, J.; Khel, T.; Remeš, J. Effect of agricultural lands afforestation and tree species composition on the soil reaction, total organic carbon and nitrogen content in the uppermost mineral soil profile. Soil Water Res. 2014, 9, 192–200. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Parc, D. Carbon ac-cumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Deng, L.; Shangguang, Z. Afforestation drives soil carbon and nitrogen changes in China. Land Degrad. Dev. 2016, 21, 151–165. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Zhang, W.; Yang, L.; Xiao, K.; Chen, H.; Wang, K. Afforestation effects on soil organic carbon and nitro gen pools modulated by lithology. For. Ecol. Manag. 2017, 400, 85–92. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simple measure of net N mineralization from stabilized soil organic matter in sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C:N ratios in European forest soil. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Riqueiro-Rodríguez, A.; Mosquera-Losada, M.R.; Férnández-Núñez, E. Afforestation of agricultural land with Pinus radiata D. Don and Betula alba L. in NW Spain: Effects on soil pH, understory production and floristic diversity eleven years after establishment. Land Degrad. Dev. 2012, 23, 227–241. [Google Scholar]
- Ritter, E.; Vesterdal, L.; Gundersen, P. Changes in soil properties after afforestation of former intensively manager soil with oak and Norway spruce. Plant Soil 2003, 249, 319–330. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol Res 2016, 31(5), 655–664. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Condron, L.M.; Cavis, M.R.; Sherlock, R.R. Effects of afforestation on phosphorus dynamics and biological propertiesin a New Zealand grassland soil. Plant Soil 2000, 220, 151–163. [Google Scholar] [CrossRef]
- Wanic, T.; Błońska, E. Zastosowanie metody SIG w ocenie przydatności terenów porolnych do hodowli lasu. Rocz. Glebozn. 2011, 62, 173–181. [Google Scholar]
- Ren, C.; Kang, D.; Wu, J.P.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Georgiadis, P.; Vesterdal, L.; Stupak, I.; Raulund-Rasmussen, K. Accumulation of soilorganic carbon after cropland conversion to short-rotation willow and poplar. GCB Bioenergy 2017, 9, 1390–1401. [Google Scholar] [CrossRef]
- Kara, O.; Babur, E.; Altun, L.; Seyis, M. Effects of afforestation on microbial biomass C and respiration in eroded soils of Turkey. J. Sustain. For. 2016, 35, 385–396. [Google Scholar] [CrossRef]
- Kang, H.; Gao, H.; Yu, W.; Yi, Y.; Wang, Y.; Ning, M. Changes in soil microbial community structure and function after afforestation depend on species and age: Case study in a subtropical alluvial Island. Sci. Total Environ. 2018, 625, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
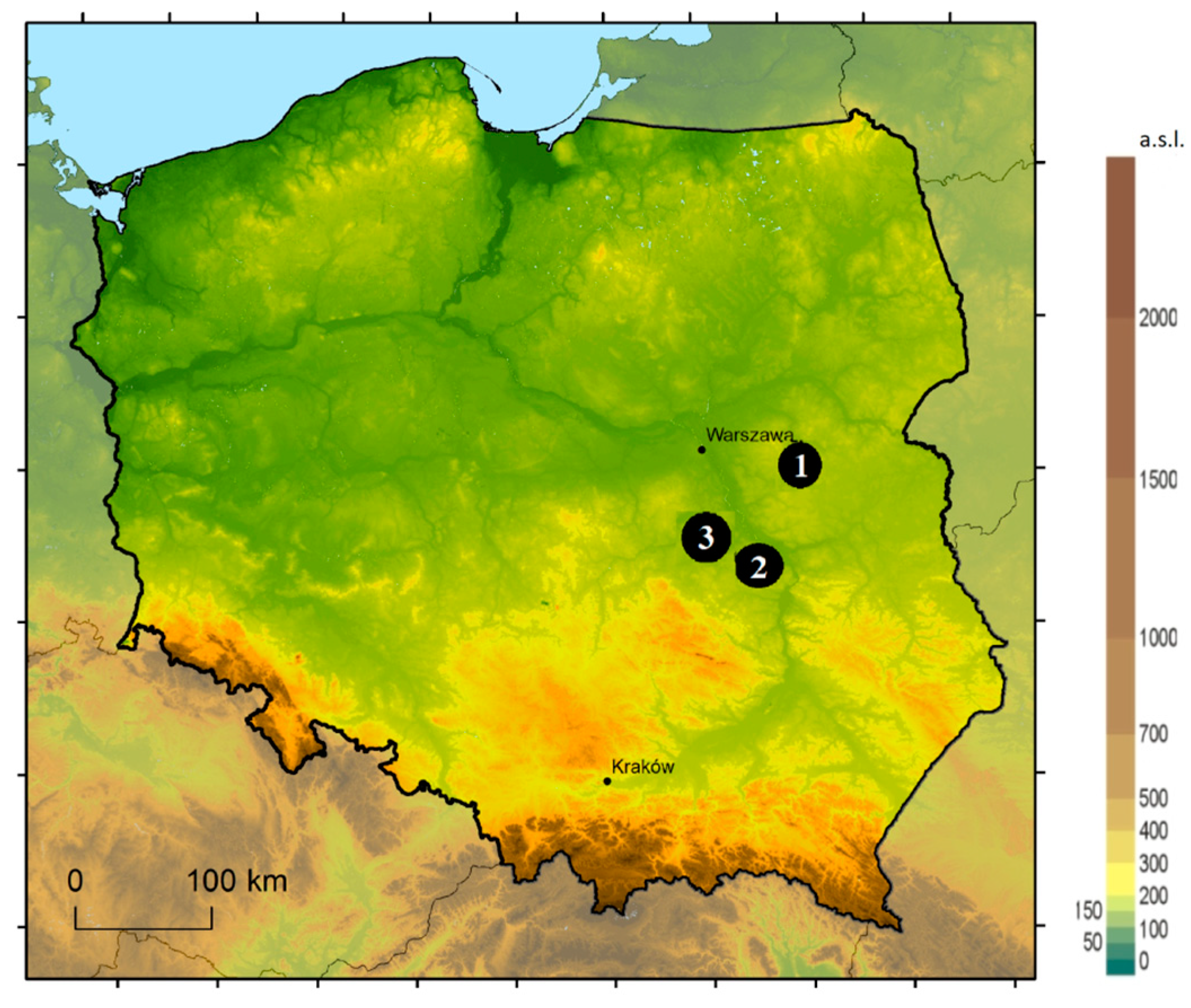

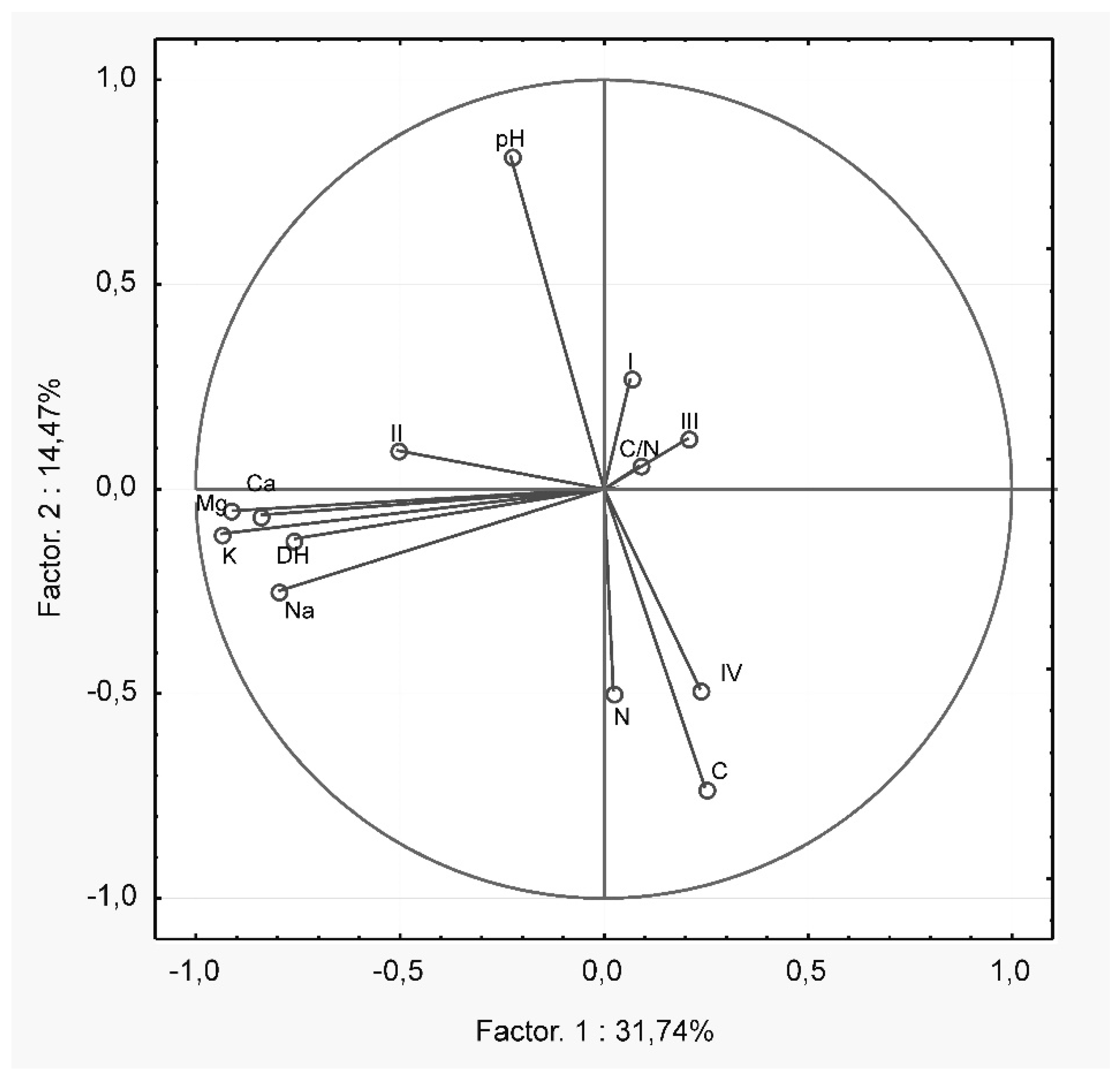

| Study Site | GPS | Soil Type |
|---|---|---|
| Mińsk Maz. | 52°10′ N, 21°40′ E | Brunic arenosol |
| Kozienice | 51°24′ N, 21°26′ E | Brunic arenosol |
| Dobieszyn 1 | 51°35′ N, 21°10′ E | Brunic arenosol |
| Dobieszyn 2 | 51°33′ N, 21°09′ E | Brunic arenosol |
| Chronosequence | Depth | pH in H2O | pH in KCl | C | N | C/N | Na | K | Ca | Mg | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | cmol (+)·kg−1 | % | |||||||||||
| I | 0–5 | 4.52 ± 0.14 a | 3.88 ± 0.12 a | 1.57 ± 0.33 a | 0.07 ± 0.02 a | 22.3 ± 6.5 a | 0.17 ± 0.05 a | 0.95 ± 0.30 a | 1.62 ± 0.21 ab | 1.57 ± 0.57 a | 83 ± 4 a | 14 ± 3 a | 2 ± 1 a |
| 5–15 | 4.73 ± 0.30 ab | 3.97 ± 0.19 a | 1.17 ± 0.71 a | 0.07 ± 0.03 a | 14.1 ± 4.9 a | 0.21 ± 0.07 a | 1.05 ± 0.47 a | 1.60 ± 0.57 ab | 1.60 ± 0.70 a | 84 ± 4 ab | 13 ± 3 a | 2 ± 1 a | |
| 15–50 | 5.03 ± 0.48 a | 4.23 ± 0.16 a | 0.49 ± 0.49 a | 0.03 ± 0.02b | 19.0 ± 16.2 a | 0.25 ± 0.07 a | 1.50 ± 0.40 a | 1.69 ± 0.56 a | 2.60 ± 0.85 a | 79 ± 11 a | 18 ± 7 a | 3 ± 2 a | |
| II | 0–5 | 4.62 ± 0.31 a | 3.98 ± 0.07 a | 1.30 ± 0.31 a | 0.09 ± 0.07 a | 15.2 ± 6.5 a | 0.22 ± 0.10 a | 1.98 ± 0.30 a | 2.13 ± 0.71 a | 2.23 ± 1.39 a | 76 ± 2 b | 19 ± 2 a | 4 ± 1 a |
| 5–15 | 4.87 ± 0.23 a | 4.13 ± 0.26 a | 1.10 ± 0.47 a | 0.32 ± 0.35 a | 11.1 ± 2.7 a | 0.21 ± 0.10 a | 1.39 ± 1.34 a | 2.37 ± 1.11 a | 2.30 ± 1.48 a | 78 ± 5 b | 18 ± 4 a | 4 ± 2 a | |
| 15–50 | 5.29 ± 0.38 a | 4.35 ± 0.39 a | 0.16 ± 0.10 a | 0.04 ± 0.03 ab | 8.4 ± 5.3 a | 0.22 ± 0.07 a | 1.96 ± 0.34 a | 2.25 ± 1.01 a | 3.55 ± 0.31 a | 80 ± 5 a | 17 ± 4 a | 3 ± 1 a | |
| III | 0–5 | 4.58 ± 0.15 a | 3.79 ± 0.12 a | 1.38 ± 0.58 a | 0.11 ± 0.06 a | 15.8 ± 7.2 a | 0.20 ± 0.10 a | 0.80 ± 0.25 a | 1.38 ± 0.14 b | 1.44 ± 0.32 a | 83 ± 7 ab | 14 ± 5 a | 2 ± 2 a |
| 5–15 | 4.60 ± 0.20 ab | 3.94 ± 0.15 a | 1.19 ± 0.63 a | 0.06 ± 0.04 a | 19.7 ± 4.9 a | 0.19 ± 0.06 a | 0.81 ± 0.27 a | 1.41 ± 0.18 a | 1.51 ± 0.37 a | 86 ± 4 ab | 12 ± 3 a | 2 ± 1 a | |
| 15–50 | 4.91 ± 0.36 a | 4.27 ± 0.05 a | 0.68 ± 0.53 a | 0.05 ± 0.05 ab | 18.2 ± 15.1 a | 0.21 ± 0.05 a | 1.07 ± 0.55 a | 1.35 ± 0.24 ab | 1.92 ± 0.52 a | 87 ± 3 a | 12 ± 2 a | 2 ± 1 a | |
| IV | 0–5 | 4.38 ± 0.13 a | 3.85 ± 0.25 a | 1.39 ± 0.50 a | 0.09 ± 0.06 a | 16.6 ± 6.2 a | 0.21 ± 0.03 a | 1.11 ± 0.18 a | 1.66 ± 0.31 ab | 1.97 ± 0.27 a | 85 ± 7 ab | 13 ± 5 a | 2 ± 1 a |
| 5–15 | 4.35 ± 0.30 b | 3.93 ± 0.09 a | 1.33 ± 0.55 a | 0.15 ± 0.06 a | 11.5 ± 2.7 a | 0.23 ± 0.03 a | 1.10 ± 0.26 a | 1.41 ± 0.19 b | 1.81 ± 0.38 a | 88 ± 1 a | 11 ± 1 a | 1 ± 1 a | |
| 15–50 | 4.65 ± 0.59 a | 4.13 ± 0.29 a | 1.11 ± 0.90 a | 0.19 ± 0.02 a | 12.2 ± 8.9 a | 0.22 ± 0.08 a | 1.19 ± 0.33 a | 1.54 ± 0.09 a | 2.22 ± 0.58 a | 88 ± 2 a | 11 ± 2 a | 2 ± 1 a | |
| Chronosequence | Depth | SOCstock (kg·m−2) | Total SOCstock in All Layers | % Participation SOCstock |
|---|---|---|---|---|
| I | 0–5 | 0.94 | 4.57 | 27.5 |
| 5–15 | 1.43 | 32.2 | ||
| 15–50 | 2.20 | 40.2 | ||
| II | 0–5 | 0.78 | 4.34 | 24.1 |
| 5–15 | 1.37 | 38.4 | ||
| 15–50 | 2.19 | 37.5 | ||
| III | 0–5 | 0.82 | 5.41 | 18.0 |
| 5–15 | 1.49 | 29.1 | ||
| 15–50 | 3.10 | 52.8 | ||
| IV | 0–5 | 0.69 | 6.16 | 13.6 |
| 5–15 | 1.54 | 27.7 | ||
| 15–50 | 3.93 | 58.6 |
| pHH2O | pHKCl | Na | K | Ca | Mg | C | N | Sand | Silt | Clay | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DH | −0.04 | −0.13 | 0.43 | 0.81 * | 0.64 * | 0.60 * | 0.09 | 0.10 | −0.20 | 0.20 | 0.11 |
| Chronosequence | Stem | Branches | Foliage | Bark | Roots |
|---|---|---|---|---|---|
| I | 867.6 b | 548.0 b | 863.5 b | 242.4 b | 1058.7 b |
| II | 7947.7 ab | 1924.9 b | 1745.9 ab | 1931.4 ab | 2866.8 b |
| III | 23907.2 ab | 5147.2 ab | 2299.7 ab | 4581.3 ab | 6485.4 ab |
| IV | 54307.9 a | 11357.8 a | 3242.6 a | 9173.7 a | 13492.7 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęda, T.; Błońska, E.; Małek, S. Soil Organic Carbon Accumulation in Post-Agricultural Soils under the Influence Birch Stands. Sustainability 2019, 11, 4300. https://doi.org/10.3390/su11164300
Gawęda T, Błońska E, Małek S. Soil Organic Carbon Accumulation in Post-Agricultural Soils under the Influence Birch Stands. Sustainability. 2019; 11(16):4300. https://doi.org/10.3390/su11164300
Chicago/Turabian StyleGawęda, Tomasz, Ewa Błońska, and Stanisław Małek. 2019. "Soil Organic Carbon Accumulation in Post-Agricultural Soils under the Influence Birch Stands" Sustainability 11, no. 16: 4300. https://doi.org/10.3390/su11164300
APA StyleGawęda, T., Błońska, E., & Małek, S. (2019). Soil Organic Carbon Accumulation in Post-Agricultural Soils under the Influence Birch Stands. Sustainability, 11(16), 4300. https://doi.org/10.3390/su11164300





