Feed Intake of Small Ruminants on Spring and Summer Pastures in the Mongolian Altai Mountains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.2.1. Determination of Grazing Itineraries
2.2.2. Determination of Herbage Yield and Quality along Grazing Itineraries
2.2.3. Determination of Grazing Behaviour and Feed Intake
2.3. GPS Data Evaluation and Statistical Analyses
3. Results
3.1. Grazing Itineraries
3.2. Quantity and Quality of Pasture Vegetation
3.3. Grazing Behaviour and Forage Intake
4. Discussion
4.1. Grazing Itineraries
4.2. Amount and Quality of Herbage Offer on Spring and Summer Pastures
4.3. Grazing Behaviour and Feed Intake
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Upton, C. Living off the land: Nature and nomadism in Mongolia. Geoforum 2010, 41, 865–874. [Google Scholar] [CrossRef]
- Nergui, D.; Jigjidpurev, S.; Otgoo, T.; Dugersuren, S.; Lkhagvajav, D.; Enkhtsetseg, B. Animal Feed and Feeding Research. Review for the 50th Anniversary of the Mongolian Institute of Animal Husbandry; Munkhiin Useg Group Ltd.: Ulaanbaatar, Mongolia, 2011; p. 664. [Google Scholar]
- FAOSTAT 2018. Food and Agriculture Data, Live Animals, Mongolia. Available online: www.foa.org/faostat/en/#data/QA (accessed on 8 October 2018).
- Otgonjargal, A. Nutritive Value, Methane and Carbon Dioxide Production from Pastures of Mountainous Steppe. Ph.D. Dissertation, Mongolian University of Agriculture, Ulaanbaatar, Mongolia, 2012; p. 124. (In Mongolian). [Google Scholar]
- Murray, A.H.; Daalkhaijav, D.; Wood, C.D. The rumen degradability of Mongolian pastures measured in sacco and by in vitro gas production. Trop. Sci. 1998, 38, 198–205. [Google Scholar]
- Ellis, J.E.; Swift, D.M. Stability of African pastoral ecosystems: Alternate paradigms and implications for development. J. Range Manag. 1988, 41, 450–459. [Google Scholar] [CrossRef]
- Munkhtsetseg, E.; Kimura, R.; Wang, J.; Shinoda, M. Pasture yield response to precipitation and high temperature in Mongolia. J. Arid Environ. 2007, 70, 94–110. [Google Scholar] [CrossRef]
- Batima, P.; Natsagdorj, L.; Gombluudev, P.; Erdenetsetseg, B. Observed Climate Change in Mongolia. Assessment of Impacts and Adaptations to Climate Change; AIACC Working Paper No.12; Assessments of Impacts and Adaptations to Climate Change: Washington, DC, USA, 2005; pp. 1–26. [Google Scholar]
- Dagvadorj, D.; Natsagdorj, L.; Dorjpurev, J.; Namkhainyam, B. Mongolia Assessment Report on Climate Change 2009; Ministry of Environment and Green Development of Mongolia: Ulaanbaatar, Mongolia, 2009; p. 228. [Google Scholar]
- Oyunmunkh, B.; Weijers, S.; Loeffler, J.; Byambagerel, S.; Soninkhishig, N.; Buerkert, A.; Goenster-Jordan, S.; Simmer, C. Climate variations over the southern Altai and Dzungarian Basin region, Central Asia, since 1580 CE. Int. J. Climatol. 2019, 39, 4543–4558. [Google Scholar] [CrossRef]
- Briske, D.D.; Derner, J.D.; Brown, J.R.; Fuhlendorf, S.D.; Teague, W.R.; Havstad, K.M.; Gillen, R.L.; Ash, A.J.; Willms, W.D. Rotational grazing on rangelands: Reconciliation of perception and experimental evidence. Rangel. Ecol. Manag. 2008, 61, 3–17. [Google Scholar] [CrossRef]
- Fernández-Giménez, M.E. The role of Mongolian nomadic pastoralist’s ecological knowledge in rangeland management. Ecol. Appl. 2000, 10, 1318–1326. [Google Scholar] [CrossRef]
- Addison, J.; Brown, C. A multi-scaled analysis of the effect of climate, commodity prices and risk on the livelihoods of Mongolian pastoralists. J. Arid Environ. 2014, 109, 54–64. [Google Scholar] [CrossRef]
- Behnke, R.H.; Fernández-Gimenez, M.E.; Turner, M.D.; Stammler, F. Pastoral migration: Mobile systems of livestock husbandry. In Animal Migration; Milner-Gulland, E.J., Fryxell, J.M., Sinclair, A.R.E., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 144–171. [Google Scholar]
- Lkhagvadorj, D.; Hauck, M.; Dulamsuren, C.; Tsogtbaatar, J. Pastoral nomadism in the forest-steppe of the Mongolian Altai under a changing economy and a warming climate. J. Arid Environ. 2013, 88, 82–89. [Google Scholar] [CrossRef]
- Middleton, N.; Rueff, H.; Sternberg, T.; Batbuyan, B.; Thomas, D. Explaining spatial variations in climate hazard impacts in western Mongolia. Landsc. Ecol. 2015, 30, 91–107. [Google Scholar] [CrossRef]
- Begzsuren, S.; Ojima, J.E.; Coughenour, D.S.; Chuluun, M.B. Livestock responses to droughts and severe winter weather in the Gobi Three Beauty National Park, Mongolia. J. Arid Environ. 2004, 59, 785–796. [Google Scholar] [CrossRef]
- Khishigbayar, J.; Fernández-Giménez, M.E.; Angerer, J.P.; Reid, R.S.; Chantsallkham, J.; Baasandorj, Y.; Zumberelmaa, D. Mongolian rangelands at a tipping point? Biomass and cover are stable but composition shifts and richness declines after 20 years of grazing and increasing temperatures. J. Arid Environ. 2015, 115, 100–112. [Google Scholar] [CrossRef]
- Hambler, C.; Canney, S.; Coe, M.J.; Henderson, P.A.; Illius, A.W. Grazing and “degradation”. Science 2007, 316, 1564–1565. [Google Scholar] [CrossRef] [PubMed]
- Addison, J.; Friedel, M.; Brown, C.; Davies, J.; Waldron, S. A critical review of degradation assumptions applied to Mongolia’s Gobi Desert. Rangel. J. 2012, 34, 125–137. [Google Scholar] [CrossRef]
- Ykhanbai, H. Economics of Environment and Sustainable Development: Pasture Resources Accounting. Ph.D. Dissertation, Mongolian University of Agriculture, Ulaanbaatar, Mongolia, 2000; p. 208. (In Mongolian). [Google Scholar]
- Adler, P.B.; Raff, D.A.; Lauenroth, W.K. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 2001, 128, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Giménez, M.E.; Allen-Diaz, B. Testing a non-equilibrium model of rangeland vegetation dynamics in Mongolia. J. Appl. Ecol. 1999, 36, 871–885. [Google Scholar] [CrossRef]
- Freschi, P.; Musto, M.; Paolino, R.; Cosentino, C. Grazing and biodiversity conservation: Highlights on a Natura 2000 network site. In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer: Cham, Switzerland, 2015; pp. 271–288. [Google Scholar]
- Jordan, G.; Goenster, S.; Munkhnasan, T.; Shabier, A.; Buerkert, A.; Schlecht, E. Spatio-temporal patterns of herbage availability and livestock movements: A cross-border analysis in the Chinese-Mongolian Altay. Pastoralism 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Tsvegemed, M.; Shabier, A.; Schlecht, E.; Jordan, G.; Wiehle, M. Evolution of rural livelihood strategies in a remote Sino-Mongolian border area: A cross-country analysis. Sustainability 2018, 10, 1011. [Google Scholar] [CrossRef]
- Altmann, B.A.; Jordan, G.; Schlecht, E. Participatory mapping as an approach to identify grazing pressure in the Altay Mountains, Mongolia. Sustainability 2018, 10, 1960. [Google Scholar] [CrossRef]
- NSOM (National Statistics Office of Mongolia). Livestock Data 2016. Available online: http://www.en.nso.mn/index.php (accessed on 11 August 2018).
- Zemmrich, A.; Manthey, M.; Zerbe, S.; Oyunchimeg, D. Driving environmental factors and the role of grazing in grassland communities: A comparative study along an altitudinal gradient in Western Mongolia. J. Arid Environ. 2010, 74, 1271–1280. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Khishigjargal, M.; Leuschner, C.; Hauck, M. Response of tree-ring width to climate warming and selective logging in larch forests of the Mongolian Altai. J. Plant Ecol. 2014, 7, 24–38. [Google Scholar] [CrossRef]
- Grunert, J.; Lehmkuhl, F.; Walther, M. Paleoclimatic evolution of the Uvs Nuur basin and adjacent areas (Western Mongolia). Quatern. Int. 2000, 65/66, 171–192. [Google Scholar] [CrossRef]
- VDLUFA. Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III. Die chemische Untersuchung von Futtermitteln; VDLUFA Verlag: Darmstadt, Germany, 2012; p. 2190. [Google Scholar]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Schiborra, A.; Gierus, M.; Wan, H.W.; Glindemann, T.; Wang, C.J.; Susenbeth, A.; Taube, F. Dietary selection of sheep grazing the semi-arid grasslands of Inner Mongolia, China at different grazing intensities. J. Anim. Physiol. Anim. Nutr. 2010, 94, 446–454. [Google Scholar] [CrossRef]
- Reddersen, B.; Fricke, T.; Wachendorf, M. Effects of sample preparation and measurement standardization on the NIRS calibration quality of nitrogen, ash and NDFom content in extensive experimental grassland biomass. Anim. Feed Sci. Tech. 2013, 183, 77–85. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Lin, L.; Dickhoefer, U.; Müller, K.; Susenbeth, A. Grazing behavior of sheep at different stocking rates in the Inner Mongolian steppe, China. Appl. Anim. Behav. Sci. 2011, 129, 36–42. [Google Scholar] [CrossRef]
- Gordon, I.J. Animal-based techniques for grazing ecology research. Small Rumin. Res. 1995, 16, 203–214. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Mahgoub, O.; Schlecht, E. Adjusting homestead feeding to requirements and nutrient intake of grazing goats on semi-arid, subtropical highland pastures. Animal 2011, 5, 471–482. [Google Scholar] [CrossRef]
- Wang, C.J.; Tas, B.M.; Glindemann, T.; Rave, G.; Schmidt, L.; Weißbach, F.; Susenbeth, A. Fecal crude protein content as an estimate for the digestibility of forage in grazing sheep. Anim. Feed Sci. Technol. 2009, 149, 199–208. [Google Scholar] [CrossRef]
- Schlecht, E.; Hiernaux, P.; Kadaouré, I.; Hülsebusch, C.; Mahler, F. A spatio-temporal analysis of forage availability and grazing and excretion behavior of herded and free grazing cattle, sheep and goats in Western Niger. Agric. Ecosyst. Environ. 2006, 113, 226–242. [Google Scholar] [CrossRef]
- Schlecht, E.; Dickhoefer, U.; Gumpertsberger, E.; Buerkert, A. Grazing itineraries and forage selection of goats in the Al Jabal al Akhdar mountain range of northern Oman. J. Arid Environ. 2009, 73, 355–363. [Google Scholar] [CrossRef]
- Kawamura, K.; Akiyama, T.; Yokota, H.; Tsutsumi, M.; Yasuda, T.; Watanabe, O.; Wang, S. Quantifying grazing intensities using geographic information systems and satellite remote sensing in the Xilingol steppe region, Inner Mongolia, China. Agric. Ecosyst. Environ. 2005, 107, 83–93. [Google Scholar] [CrossRef]
- Li, S.G.; Romero, S.H.; Tsujimura, M.; Sugimoto, A.; Sasaki, L.; Davaa, G.; Oyunbaatar, D. Plant water sources in the cold semiarid ecosystem of the upper Kherlen River catchment in Mongolia: A stable isotope approach. J. Hydrol. 2007, 333, 109–117. [Google Scholar] [CrossRef]
- Animut, G.; Goetsch, A.L.; Aiken, G.E.; Puchala, R.; Detweiler, G.; Krehbiel, C.R.; Merkel, R.C.; Sahlu, T.; Dawson, L.J.; Johnson, Z.B.; et al. Grazing behavior and energy expenditure by sheep and goats co-grazing grass/forb pastures at three stocking rates. Small Rumin. Res. 2005, 59, 191–201. [Google Scholar] [CrossRef]
- Joly, F.J.; Samdanjigmed, T.; Cottereau, V.; Feh, C. Ecological constraints on and consequences of land use heterogeneity: A case study of the Mongolian Gobi. J. Arid Environ. 2013, 95, 84–91. [Google Scholar] [CrossRef]
- Gendaram, H. Animal Feed and Feeding; Munkhiin Useg Group Ltd.: Ulaanbaatar, Mongolia, 2009; p. 247. [Google Scholar]
- Schönbach, P.; Wan, H.; Gierus, M.; Loges, R.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Effects of grazing and precipitation on herbage production, herbage nutritive value and performance of sheep in continental steppe. Grass Forage Sci. 2012, 67, 535–545. [Google Scholar] [CrossRef]
- Fernández-Giménez, M.E.; Allen-Diaz, B. Vegetation change along gradients from water sources in three grazed Mongolian ecosystems. Plant Ecol. 2001, 157, 101–118. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohkuro, T.; Jamsran, U.; Takeuchi, K. Changes in the herbage nutritive value and yield associated with threshold responses of vegetation to grazing in Mongolian rangelands. Grass Forage Sci. 2012, 67, 446–455. [Google Scholar] [CrossRef]
- Johnson, D.A.; Sheehy, D.P.; Miller, D.; Damiran, D. Mongolian rangelands in transition. Sécheresse 2006, 17, 133–141. [Google Scholar]
- Miyazaki, S.; Yasunari, T.; Miyamoto, T.; Kaihotsu, I.; Davaa, G.; Oyunbaatar, D.; Natsagdorj, L.; Oki, T. Agrometeorological conditions of grassland vegetation in central Mongolia and their impact for leaf area growth. J. Geophys. Res.-Atmos. 2004, 109, 1–14. [Google Scholar] [CrossRef]
- Tserendash, S. Present conditions and strategies for management of Mongolian rangelands. Erdem 2000, 38, 7–11. (In Mongolian) [Google Scholar]
- Lv, C.; Schlecht, E.; Goenster-Jordan, S.; Buerkert, A.; Zhang, X.; Wesche, K. Vegetation responses to fixed stocking densities in highly variable montane pastures in the Chinese Altay. Rangel. Ecol. Manag. 2019, 72, 812–821. [Google Scholar] [CrossRef]
- Tserendash, S.; Lkhagvajav, N.; Altanzul, T. Pasture Land Research; Review for the 50th Anniversary of the Mongolian Institute of Animal Husbandry; Munkhiin Useg Group Ltd.: Ulaanbaatar, Mongolia, 2011; p. 345. (In Mongolian) [Google Scholar]
- George, M.R.; Bell, M.E. Using Stage of Maturity to Predict the Quality of Annual Range Forage; Rangeland Management Series; University of California, Davis: Davis, CA, USA, 2001; Publication 8019; pp. 1–7. [Google Scholar]
- Dominque, B.F.; Dellow, D.W.; Barry, T.N. Voluntary intake and rumen digestion of a low-quality roughage by goats and sheep. J. Agric. Sci. 1991, 117, 111–120. [Google Scholar] [CrossRef]
- Tserendulam, R. Nutritive Quality of Main Types of Pasture in Mongolia, 1st ed.; Scientific Academy of Mongolia Press: Ulaanbaatar, Mongolia, 1968; p. 143. (In Mongolian) [Google Scholar]
- Togtokhbayar, N. Nutritive Values of Forb Dominated Pasture in Forest Steppe and Steppe Zones in Mongolia. Ph.D. Dissertation, Mongolian University of Agriculture, Ulaanbaatar, Mongolia, 1995; p. 116. (In Mongolian). [Google Scholar]
- Daalkhaijav, D.; Lkagvajav, N. Rumen degradability and chemical composition of Mongolian highland’s pasture grass. Mong. J. Agric. 1997, 2, 15–22. [Google Scholar]
- Togtokh, J. Pasture Utilization in Forest Steppe Zone of Mongolia by Semi-Fine Wool Crossbreed Sheep. Ph.D. Dissertation, Mongolian University of Agriculture, Ulaanbaatar, Mongolia, 1971; p. 84. (In Mongolian). [Google Scholar]
- Batmunkh, D. Determination of Pasture Grass Intake by N Alkanes. In Proceedings No 7. of the School of Biological Resources and Management; Mongolian Agricultural University: Ulanbataar, Mongolia, 2005; pp. 34–38. [Google Scholar]
- Hu, H.; Liu, Y.; Li, Y.; Lu, D.; Min, G. Use of the N-alkanes to estimate intake, apparent digestibility and diet composition in sheep grazing on Stipa breviflora desert steppe. J. Integr. Agric. 2014, 13, 1065–1072. [Google Scholar] [CrossRef]
- Osuji, P.O. The physiology of eating and the energy expenditure of the ruminant at pasture. J. Range Manag. 1974, 27, 437–443. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; p. 384. [Google Scholar]
- Underwood, E.J.; Suttle, N. The Mineral Nutrition of Livestock, 3rd ed.; CABI Publishing: Wallingford, UK, 2001; p. 624. [Google Scholar]
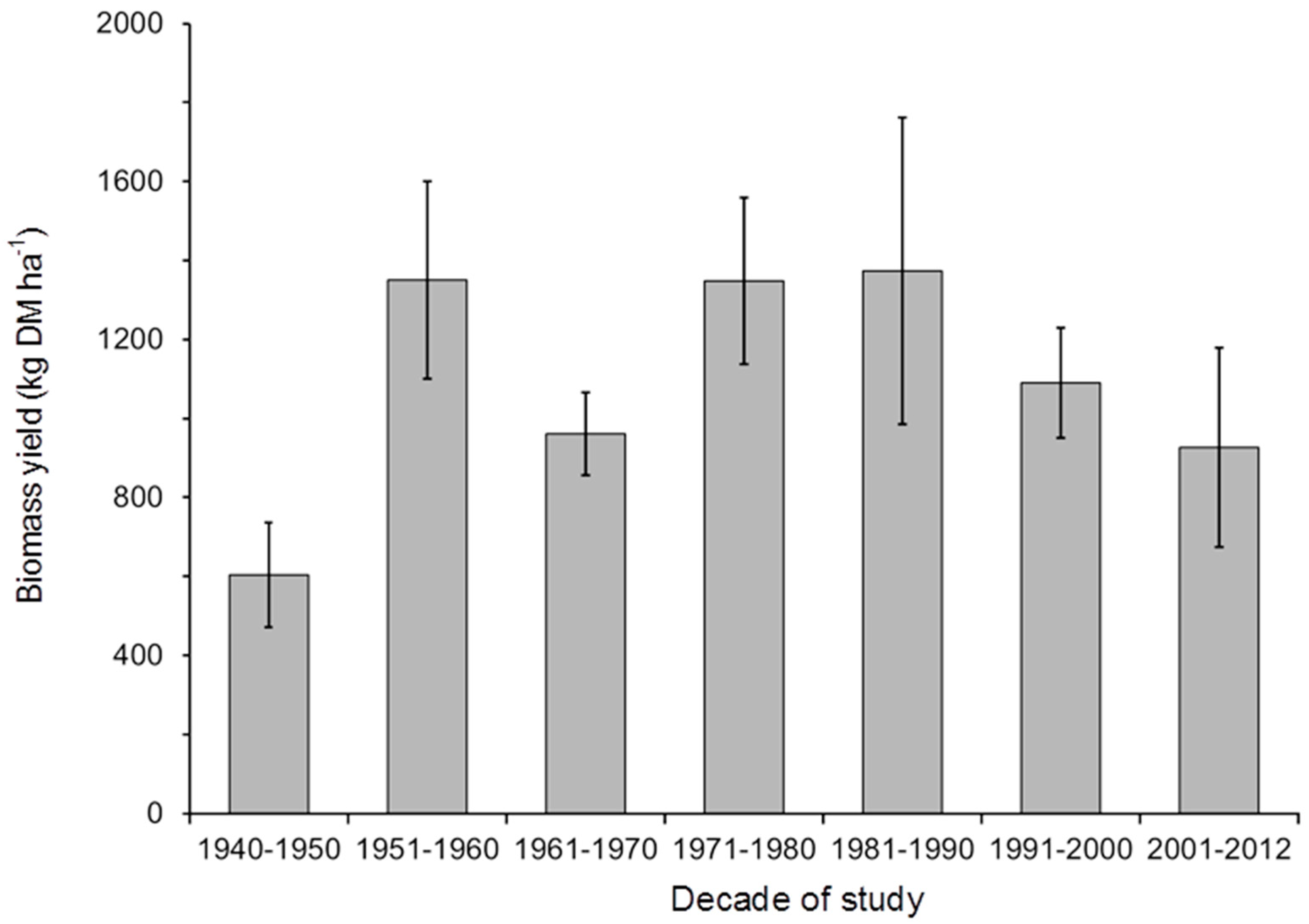
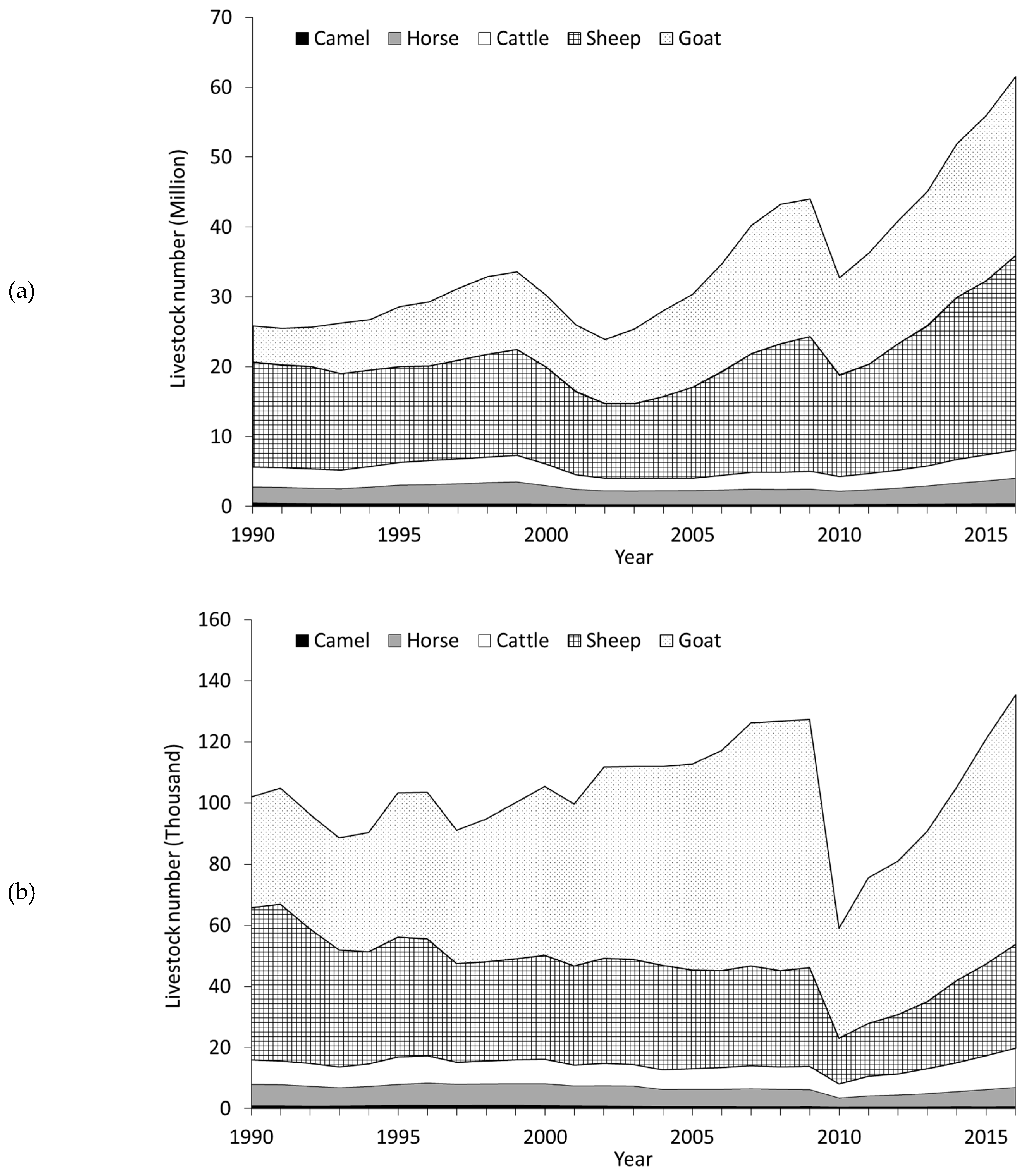
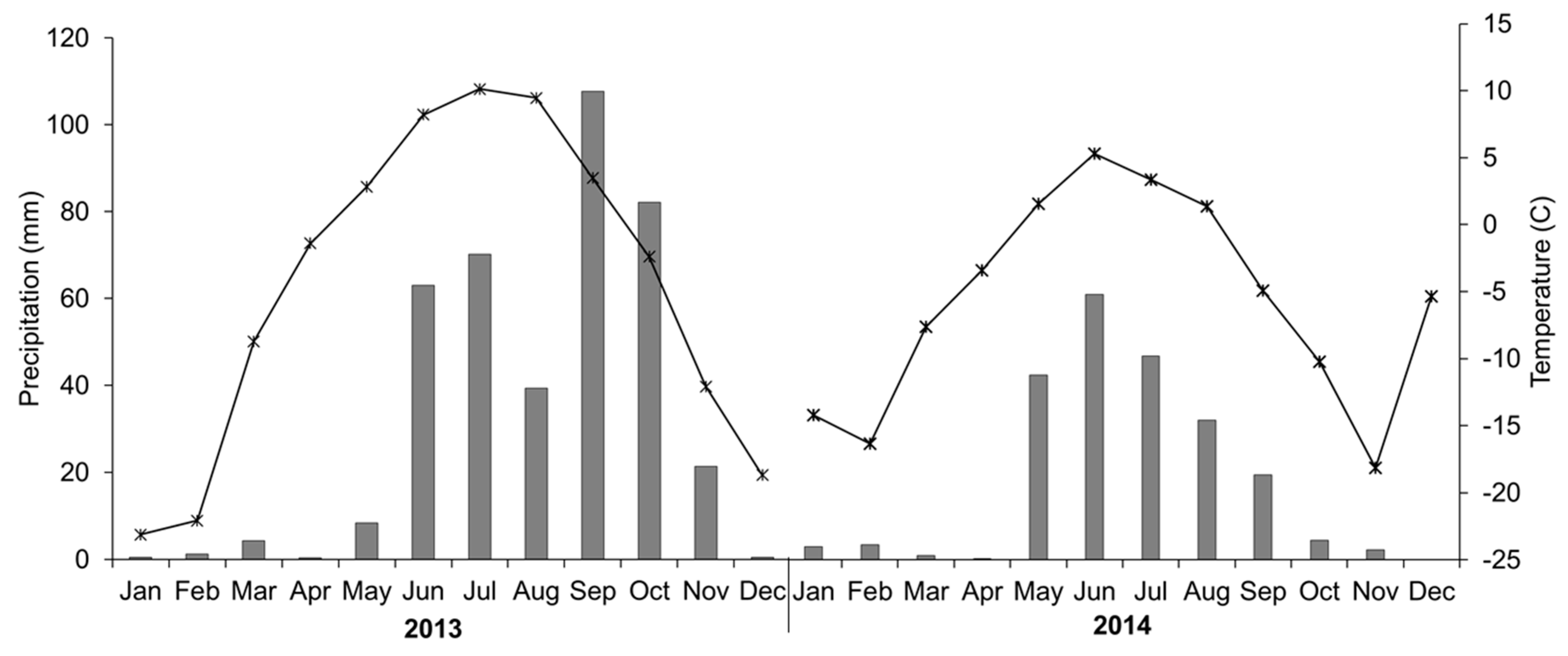


| Year | Month | Bare Soil or Stones | Vegetated Area | Vegetation Height | Herbage Yield | DM | OM | CP | NDF | ADF | Ca | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (cm) | kg DM ha−1 | g kg−1 FM | g kg−1 DM | ||||||||||||||
| 2013 | June | 2.1 b | 43.4 b | 15 A,a | 986 A,a | 473 | B,c | 919 | a | 125 | A,a | 453 | B,c | 279 | b | 9.8 | A,a | 1.8 | A,a |
| July | 6.9 a | 67.8 a | 6 b | 821 ab | 617 | b | 898 | B,b | 129 | a | 520 | b | 290 | A,b | 7.8 | A,b | 1.4 | b | |
| August | 7.2 a | 44.3 b | 9 b | 601 B,b | 767 | a | 902 | b | 82 | b | 617 | a | 332 | A,a | 4.9 | c | 1.1 | c | |
| SEM | 0.84 | 2.91 | 0.6 | 47.3 | 16.8 | 2.3 | 3.0 | 9.8 | 5.0 | 0.4 | 0.04 | ||||||||
| Effect of month | *** | ** | *** | ** | *** | *** | *** | *** | *** | *** | *** | ||||||||
| 2014 | June | 9.2 | 26.7 b | 6 B | 844 B | 742 | A,a | 913 | a | 101 | B,b | 509 | A,b | 291 | a | 5.1 | B | 1.4 | B,a |
| July | 12.7 | 76.3 a | 8 | 904 | 592 | B,b | 923 | A,a | 127 | a | 523 | b | 248 | B,b | 5.5 | B | 1.3 | ab | |
| August | 8.2 | 58.1 a | 9 | 777 A | 749 | a | 898 | b | 79 | c | 579 | a | 298 | B,a | 4.1 | 1.1 | b | ||
| SEM | 2.34 | 3.89 | 0.4 | 66.5 | 18.3 | 2.5 | 3.8 | 9.2 | 6.3 | 0.3 | 0.04 | ||||||||
| Effect of month | n.s. | *** | n.s. | n.s. | *** | *** | *** | ** | ** | n.s. | ** | ||||||||
| Effect of year | June | n.s. | n.s. | * | ** | ** | n.s. | * | ** | n.s. | ** | * | |||||||
| July | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | * | * | n.s. | ||||||||
| August | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. | ** | n.s. | n.s. | ||||||||
| Animal Species and Month | Grazing | Resting | Walking | Other | |
|---|---|---|---|---|---|
| Sheep | June | 56 | 26 | 16 | 2 |
| July | 51 | 27 | 20 | 2 | |
| August | 54 | 33 | 11 | 2 | |
| SEM | 3.7 | 3.5 | 1.7 | 0.5 | |
| Effect of month | n.s. | n.s. | n.s. | n.s. | |
| Goats | June | 56 | 29 | 13 | 2 |
| July | 52 | 34 | 11 | 3 | |
| August | 61 | 28 | 10 | 1 | |
| SEM | 3.7 | 3.5 | 1.7 | 0.5 | |
| Effect of month | n.s. | n.s. | n.s. | n.s. | |
| Fixed effects | |||||
| Species | n.s. | n.s. | n.s. | n.s. | |
| Month | * | n.s. | n.s. | n.s. | |
| Species*Month | n.s. | n.s. | n.s. | n.s. | |
| Species | Year | Month | LW# | ADG## | OMD | Daily Intake (g kg−0.75 LW) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | OM | CP | NDF | ADF | P | Ca | |||||||||||||||
| Sheep | 2013 | June | 44 | A,b | 723 | A,a | 77 | b | 72 | b | 9 | b | 31 | b | 19 | b | 0.13 | a | 0.74 | A,a | |
| July | 48 | ab | 115 | 703 | A,b | 99 | A,b | 92 | A,b | 12 | A,a | 48 | A,a | 28 | A,a | 0.13 | A,a | 0.73 | A,a | ||
| August | 51 | A,a | 68 | 622 | c | 63 | b | 58 | b | 4 | c | 36 | b | 20 | b | 0.06 | b | 0.30 | b | ||
| SEM | 1.2 | 19.5 | 11.9 | 4.9 | 4.5 | 0.9 | 2.4 | 1.3 | 0.010 | 0.060 | |||||||||||
| Effect of month | * | n.s. | *** | *** | *** | *** | *** | ** | *** | * | |||||||||||
| 2014 | June | 34 | B,b | 669 | B,a | 81 | 76 | 8 | 39 | 22 | a | 0.10 | a | 0.38 | B | ||||||
| July | 40 | B,a | 158 | 678 | B,a | 57 | B | 53 | B | 7 | B | 28 | B | 13 | B,b | 0.07 | B,ab | 0.29 | B | ||
| August | 42 | B,a | 107 | 623 | b | 64 | 60 | 5 | 35 | 18 | ab | 0.07 | b | 0.25 | |||||||
| SEM | 1.5 | 19.7 | 7.6 | 5.1 | 4.8 | 0.6 | 2.5 | 1.5 | 0.007 | 0.025 | |||||||||||
| Effect of month | ** | n.s. | *** | n.s. | n.s. | n.s. | n.s. | * | * | n.s. | |||||||||||
| Effect of year | June | * | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | |||||||||||
| July | ** | n.s. | * | ** | ** | * | * | ** | * | * | |||||||||||
| August | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||
| Goats | 2013 | June | 38 | b | 724 | a | 86 | 81 | 10 | ab | 38 | 23 | 0.14 | A,a | 0.73 | A,a | |||||
| July | 40 | b | 65 | 684 | b | 92 | A | 86 | A | 11 | A,a | 44 | A | 24 | A | 0.13 | A,a | 0.66 | A,a | ||
| August | 46 | a | 218 | 599 | c | 64 | 59 | 5 | b | 36 | 19 | 0.07 | b | 0.28 | A,b | ||||||
| SEM | 1.5 | 53.8 | 14.2 | 6.6 | 6.2 | 1.0 | 2.9 | 1.6 | 0.011 | 0.068 | |||||||||||
| Effect of month | ** | n.s. | *** | n.s. | n.s. | * | n.s. | n.s. | * | * | |||||||||||
| 2014 | June | 34 | b | 684 | a | 66 | a | 62 | a | 6 | a | 32 | a | 18 | a | 0.09 | B,a | 0.33 | B,a | ||
| July | 37 | a | 100 | 656 | b | 47 | B,b | 44 | B,b | 6 | B,a | 23 | B,b | 11 | B,b | 0.06 | B,b | 0.25 | B,b | ||
| August | 41 | a | 138 | 595 | c | 52 | ab | 49 | ab | 4 | b | 28 | ab | 14 | ab | 0.05 | b | 0.20 | B,b | ||
| SEM | 1.3 | 36.0 | 11.7 | 3.4 | 3.2 | 0.4 | 1.6 | 1.0 | 0.005 | 0.018 | |||||||||||
| Effect of month | ** | n.s. | *** | * | * | ** | * | ** | * | ** | |||||||||||
| Effect of year | June | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ** | * | |||||||||||
| July | n.s. | n.s. | n.s. | ** | * | * | * | ** | * | * | |||||||||||
| August | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | |||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsevegemed, M.; Norovsambuu, T.; Jordan, G.; Schlecht, E. Feed Intake of Small Ruminants on Spring and Summer Pastures in the Mongolian Altai Mountains. Sustainability 2019, 11, 5759. https://doi.org/10.3390/su11205759
Tsevegemed M, Norovsambuu T, Jordan G, Schlecht E. Feed Intake of Small Ruminants on Spring and Summer Pastures in the Mongolian Altai Mountains. Sustainability. 2019; 11(20):5759. https://doi.org/10.3390/su11205759
Chicago/Turabian StyleTsevegemed, Munkhnasan, Togtokhbayar Norovsambuu, Greta Jordan, and Eva Schlecht. 2019. "Feed Intake of Small Ruminants on Spring and Summer Pastures in the Mongolian Altai Mountains" Sustainability 11, no. 20: 5759. https://doi.org/10.3390/su11205759
APA StyleTsevegemed, M., Norovsambuu, T., Jordan, G., & Schlecht, E. (2019). Feed Intake of Small Ruminants on Spring and Summer Pastures in the Mongolian Altai Mountains. Sustainability, 11(20), 5759. https://doi.org/10.3390/su11205759





