Kinetic Determination of Urease Activity in Fresh Pig Feces and Slurry and the Effect on Ammonia Production at Different Conditions
Abstract
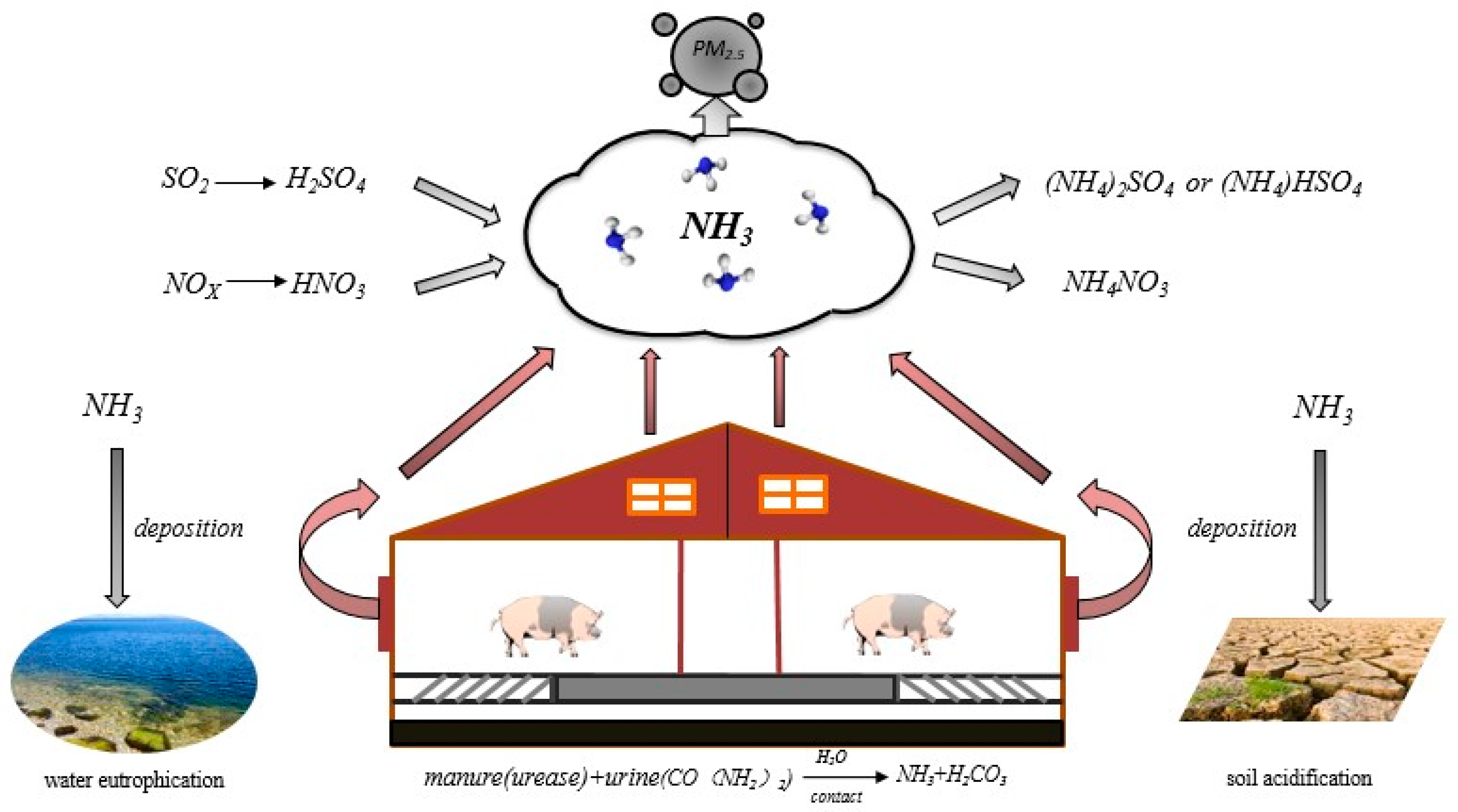
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis Methods
2.3. Experimental Setup
2.3.1. Kinetic Characterization of Urease in Fresh Pig Feces
2.3.2. Effect of Temperature, pH, and Mixing Rate on Urease Activity of Fresh Pig Feces
2.3.3. Determination of Urease Activity in Fresh Slurry
2.4. Statistical Data Treatment
3. Results and Discussion
3.1. Chemical and Physical Properties of Fresh Pig Feces, Urine and Mixed Slurry
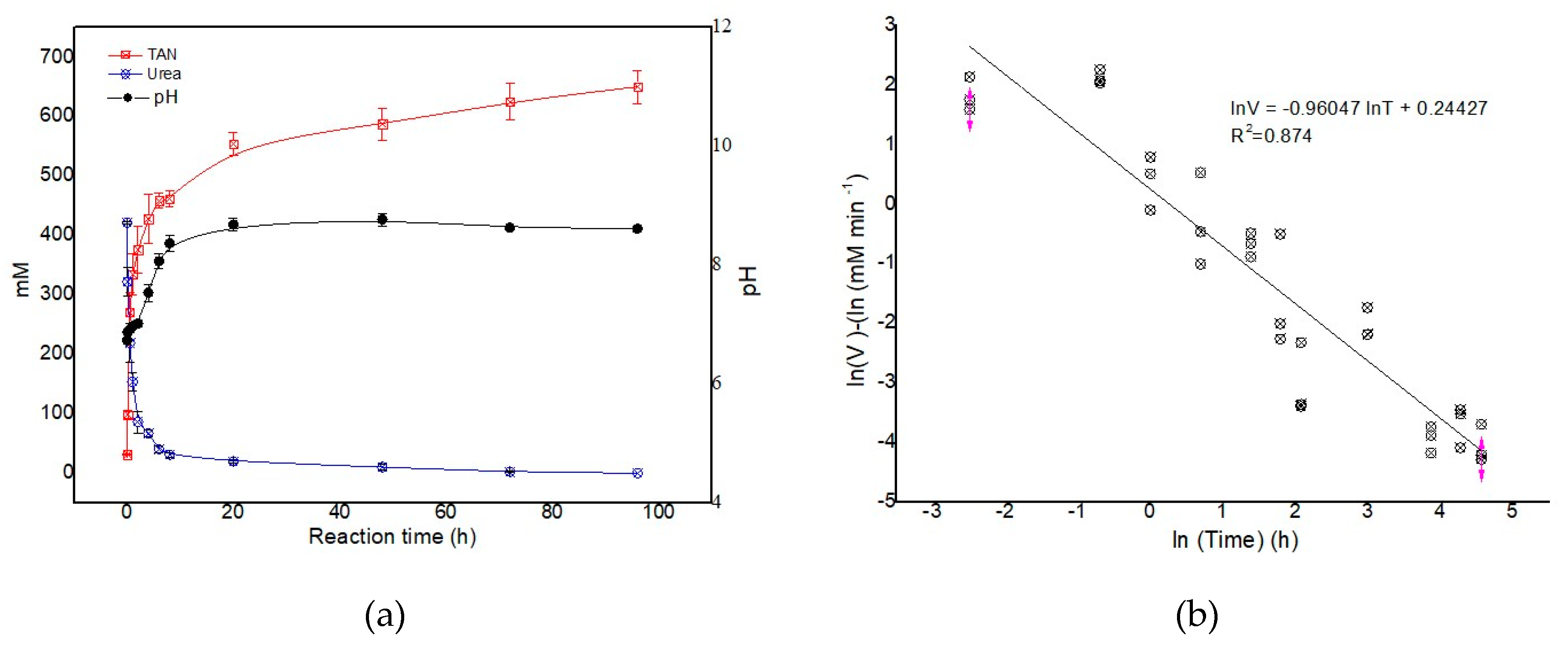
3.2. Urease Activity in Fresh Feces from Fattening Pigs
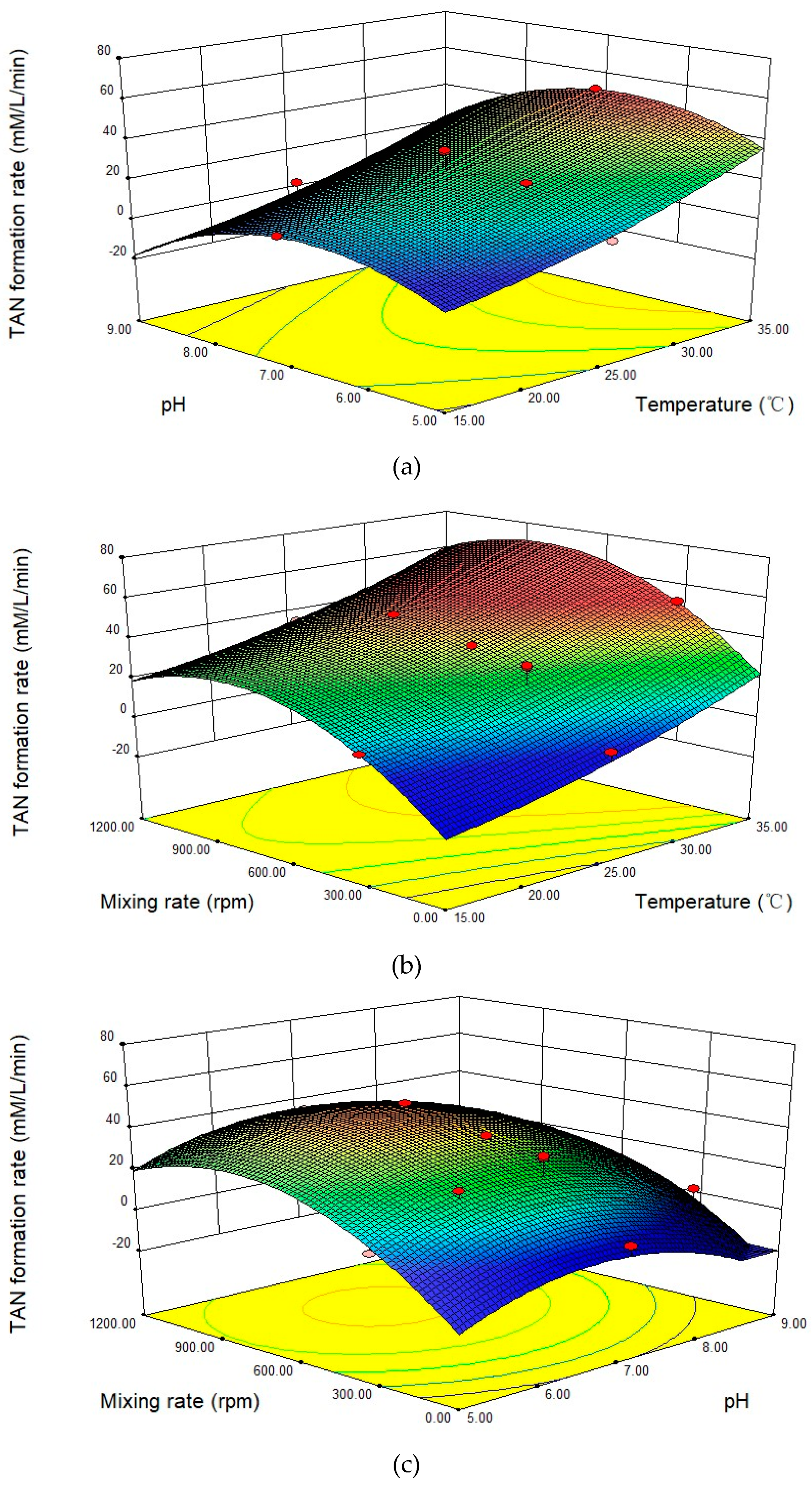
3.3. TheEffects of Temperature, pH, and Mixing Rate on Urease Activity in Fresh Pig Feces
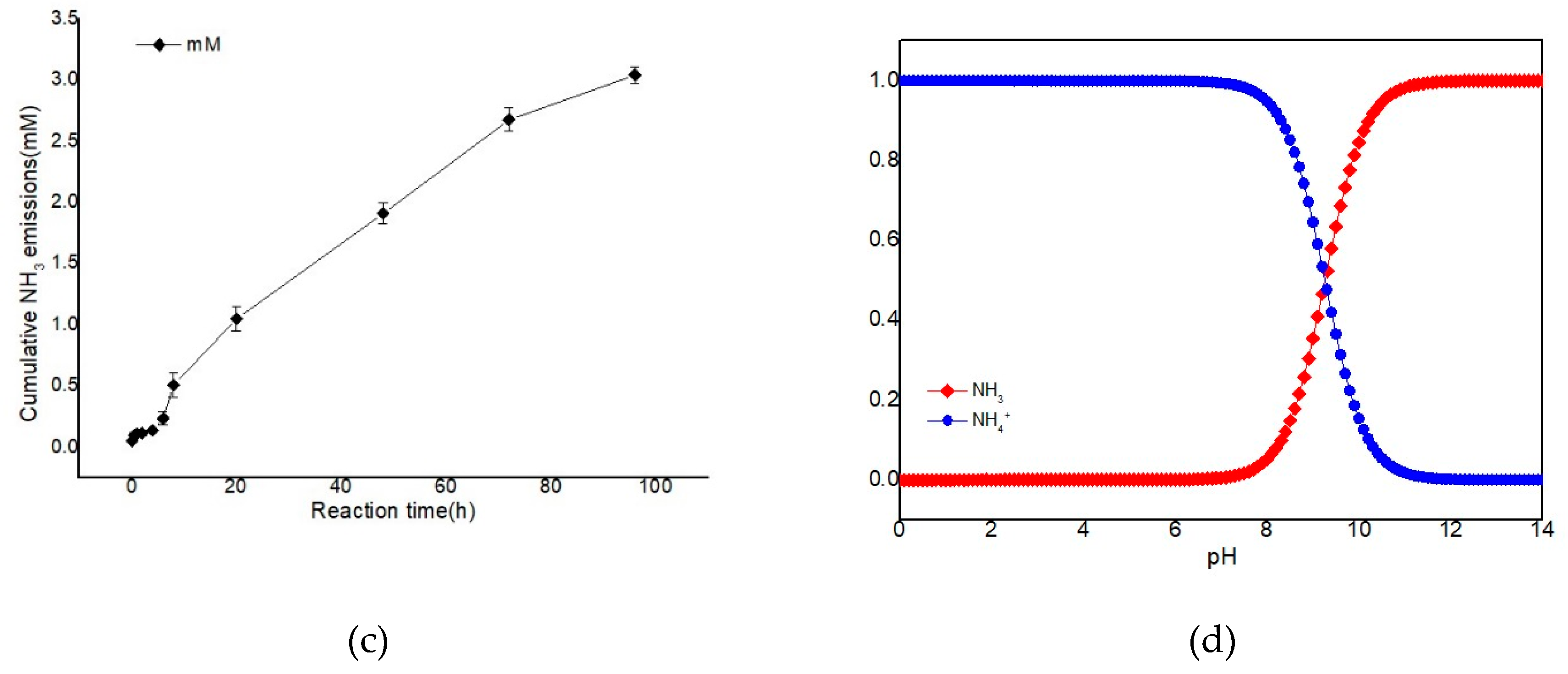
3.4. The Hydrolysis of Urea and Production of TAN in Fresh Pig Slurry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouwman, A.F.; Lee, D.S.; Asman, W.A.H.; Dentener, F.J.; Van Der Hoek, K.W.; Olivier, J.G.J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar] [CrossRef]
- Emission Database for Global Atmospheric Research (EDGAR), Release Version 4.3. Available online: https://edgar.jrc.ec.europa.eu/overview.php?v=432_AP (accessed on 28 June 2019).
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef] [PubMed]
- Asman, W.A.H.; Sutton, M.A.; Schjoerring, J.K. Ammonia: Emission, atmospheric transport and deposition. New Phytol. 1998, 139, 27–48. [Google Scholar] [CrossRef]
- Battye, W. Evaluation and improvement of ammonia emissions inventories. Atmos. Environ. 2003, 37, 3873–3883. [Google Scholar] [CrossRef]
- Jens, J.S.; Simon, S.; Henrik, K. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. [Google Scholar]
- Squizzato, S.; Masiol, M.; Innocente, E.; Pecorari, E.; Rampazzo, G.; Pavoni, B. A procedure to assess local and long-range transport contributions to PM2.5 and secondary inorganic aerosol. J. Aerosol. Sci. 2012, 46, 64–76. [Google Scholar] [CrossRef]
- Pearson, J.; Stewart, G.R. Tansley Review No. 56. The Deposition of Atmospheric Ammonia and Its Effects on Plants. New Phytol. 1993, 125, 283–305. [Google Scholar] [CrossRef]
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Dore, A.J.; Ma, L.; Liu, X.J.; Ma, W.Q.; Cape, J.N.; Zhang, F.S. Agricultural ammonia emissions inventory and spatial distribution in the North China Plain. Environ. Pollut. 2010, 158, 490–501. [Google Scholar] [CrossRef]
- Philippe, F.; Cabaraux, J.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- FAOSTAT. Agriculture Statistics. 2014. Available online: http://faostat3.fao.org/browse/G1/GM/E (accessed on 25 May 2015).
- Mobley, H.L.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar]
- Callahan, B.P.; Yuan, Y.; Wolfenden, R. The burden borne by urease. J. Am. Chem. Soc. 2005, 127, 10828–10829. [Google Scholar] [CrossRef]
- Laidler, K.J.; Hoare, J.P. The molecular kinetics of the urea-urease system. III. Heats and entropies of complex formation and reaction. J. Am. Chem. Soc. 1950, 72, 2489–2494. [Google Scholar] [CrossRef]
- Estiu, G.; Merz, K.M. The Hydrolysis of Urea and the Proficiency of Urease. J. Am. Chem. Soc. 2004, 126, 6932–6944. [Google Scholar] [CrossRef]
- Portejoie, S.; Dourmad, J.Y.; Martinez, J.; Lebreton, Y. Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest. Prod. Sci. 2004, 91, 45–55. [Google Scholar] [CrossRef]
- Balsari, P.; Dinuccio, E.; Gioelli, F. A low cost solution for ammonia emission abatement from slurry storage. Int. Congr. Ser. 2006, 1293, 323–326. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Verstegen, M.W.A. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci. 2007, 109, 194–203. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Kupper, T.; Bonjour, C.; Menzi, H. Evolution of farm and manure management and their influence on ammonia emissions from agriculture in Switzerland between 1990 and 2010. Atmos. Environ. 2015, 103, 215–221. [Google Scholar] [CrossRef]
- Wood, D.; Cowherd, S.; Van Heyst, B. A summary of ammonia emission factors and quality criteria for commercial poultry production in North America. Atmos. Environ. 2015, 115, 236–245. [Google Scholar] [CrossRef]
- Bougouin, A.; Leytem, A.; Dijkstra, J.; Dungan, R.S.; Kebreab, E. Nutritional and Environmental Effects on Ammonia Emissions from Dairy Cattle Housing: A Meta-Analysis. J. Environ. Qual. 2016, 45, 1123. [Google Scholar] [CrossRef] [PubMed]
- Mack, E.; Villars, D.S. Synthesis of urea with the enzyme urease. J. Am. Chem. Soc. 1923, 45, 505–510. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Varel, V.H.; Nienaber, J.A.; Freetly, H.C. Conservation of nitrogen in cattle feedlot waste with urease inhibitors. J. Anim. Sci. 1999, 77, 1162–1168. [Google Scholar] [CrossRef]
- Gac, A.; Béline, F.; Bioteau, T.; Maguet, K. French inventory of gaseous emissions (CH4, N2O, NH3) from livestock manure management using a mass-flow approach. Livest. Sci. 2007, 112, 252–260. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Zhu, Z.; Gerber, P.J.; Xin, H.; Smith, P.; Opio, C.; Steinfeld, H.; Chadwick, D. Mitigating Greenhouse Gas and Ammonia Emissions from Swine Manure Management: A System Analysis. Environ. Sci. Technol. 2017, 51, 4503–4511. [Google Scholar] [CrossRef]
- May, P.B.; Douglas, L.A. Assay for soil urease activity. Plant. Soil. 1976, 45, 301–305. [Google Scholar] [CrossRef]
- Dai, X.; Karring, H. A determination and comparison of urease activity in feces and fresh manure from pig and cattle in relation to ammonia production and pH changes. PLoS ONE 2014, 9, e110402. [Google Scholar] [CrossRef]
- Canh, T.T.; Aarnink, A.J.A.; Schutte, J.B.; Sutton, A.; Langhout, D.J.; Verstegen, M.W.A. Dietary protein affects nitrogen excretion and ammonia emission from slurry of growing–finishing pigs. Livest. Prod. Sci. 1998, 56, 181–191. [Google Scholar] [CrossRef]
- Canh, T.T.; Verstegen, M.W.; Aarnink, A.J.; Schrama, J.W. Influence of dietary factors on nitrogen partitioning and composition of urine and feces of fattening pigs. J. Anim. Sci. 1997, 75, 700–706. [Google Scholar] [CrossRef]
- Canh, T.T.; Sutton, A.L.; Aarnink, A.J.; Verstegen, M.W.; Schrama, J.W.; Bakker, G.C. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J. Anim. Sci. 1998, 76, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, L.; Menten, M.L. The kinetics of the inversion effect. Biochem. Z. 1913, 49, 333–369. [Google Scholar]
- Muck, R.E. Urease Activity in Bovine Feces. J. Dairy. Sci. 1982, 65, 2157–2163. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Hernández, F.; Martínez, S.; López, C.; Megías, M.D.; López, M.; Madrid, J. Effect of dietary crude protein levels in a commercial range, on the nitrogen balance, ammonia emission and pollutant characteristics of slurry in fattening pigs. Animal 2011, 5, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B.; Ureases, I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Dewes, T. Effect of pH, temperature, amount of litter and storage density on ammonia emissions from stable manure. J. Agric. Sci. 1996, 127, 501–509. [Google Scholar] [CrossRef]
- Van der Stelt, B.; Temminghoff, E.J.M.; Van Vliet, P.C.J.; Van Riemsdijk, W.H. Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour. Technol. 2007, 98, 3449–3455. [Google Scholar] [CrossRef]
- Sumner, J.B.; Hand, D.B.; Holloway, R.G. Studies of the intermediate products formed during the hydrolysis of urea by urease. J. Biol. Chem. 1931, 91, 333–341. [Google Scholar]
- Blakeley, R.L.; Hinds, J.A.; Kunze, H.E.; Webb, E.C.; Zerner, B. Jack bean urease (EC 3.5.1.5). Demonstration of a carbamoyl-transfer reaction and inhibition by hydroxamic acids. Biochemistry 1969, 8, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Siddiqui, R.; Choudhary, M. Chemistry and Mechanism of Urease Inhibition. Curr. Med. Chem. 2002, 9, 1323–1348. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Portejoie, S.; Martinez, J.; Guiziou, F.; Coste, C.M. Effect of covering pig slurry stores on the ammonia emission processes. Bioresour. Technol. 2003, 87, 199–207. [Google Scholar] [CrossRef]
- Panetta, D.M.; Powers, W.J.; Lorimor, J.C. Management Strategy Impacts on Ammonia Volatilization from Swine Manure. J. Environ. Qual. 2005, 34, 1119. [Google Scholar] [CrossRef]



| Sample | TAN | Urea | DM | pH | OM 1 | ||
|---|---|---|---|---|---|---|---|
| mmol∙kg−1 | mM | mmol∙kg−1 | mM | % | % | ||
| Pig Feces (PF) | 69.0 ± 8.1 | n.a 2 | 81.3 ± 5.2 | n.a | 29.63 ± 0.26 a | 6.93 ± 0.0 b | 83.89 ± 0.80 a |
| Pig Urine (PU) | n.a | 31.6 ± 1.3 b | n.a | 421.7 ± 1.1 | n.a | 6.73 ± 0.02 c | n.a |
| Mixed Slurry 3 (MS) | n.a | 650.2 ± 22.9 a | n.a | n.d 4 | 7.76 ± 0.18 b | 8.61 ± 0.04 a | 74.56 ± 1.29 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, C.; Pan, Y.; Zhang, Z.; Zeng, Y. Kinetic Determination of Urease Activity in Fresh Pig Feces and Slurry and the Effect on Ammonia Production at Different Conditions. Sustainability 2019, 11, 6396. https://doi.org/10.3390/su11226396
Hao C, Pan Y, Zhang Z, Zeng Y. Kinetic Determination of Urease Activity in Fresh Pig Feces and Slurry and the Effect on Ammonia Production at Different Conditions. Sustainability. 2019; 11(22):6396. https://doi.org/10.3390/su11226396
Chicago/Turabian StyleHao, Chaozhi, Yuepeng Pan, Zhongyi Zhang, and Yang Zeng. 2019. "Kinetic Determination of Urease Activity in Fresh Pig Feces and Slurry and the Effect on Ammonia Production at Different Conditions" Sustainability 11, no. 22: 6396. https://doi.org/10.3390/su11226396
APA StyleHao, C., Pan, Y., Zhang, Z., & Zeng, Y. (2019). Kinetic Determination of Urease Activity in Fresh Pig Feces and Slurry and the Effect on Ammonia Production at Different Conditions. Sustainability, 11(22), 6396. https://doi.org/10.3390/su11226396






