Grassland Productivity Response to Climate Change in the Hulunbuir Steppes of China
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Data Aquisition
2.3. Model Simulation
2.3.1. Biome-BGC Model Description
2.3.2. Model Calibration
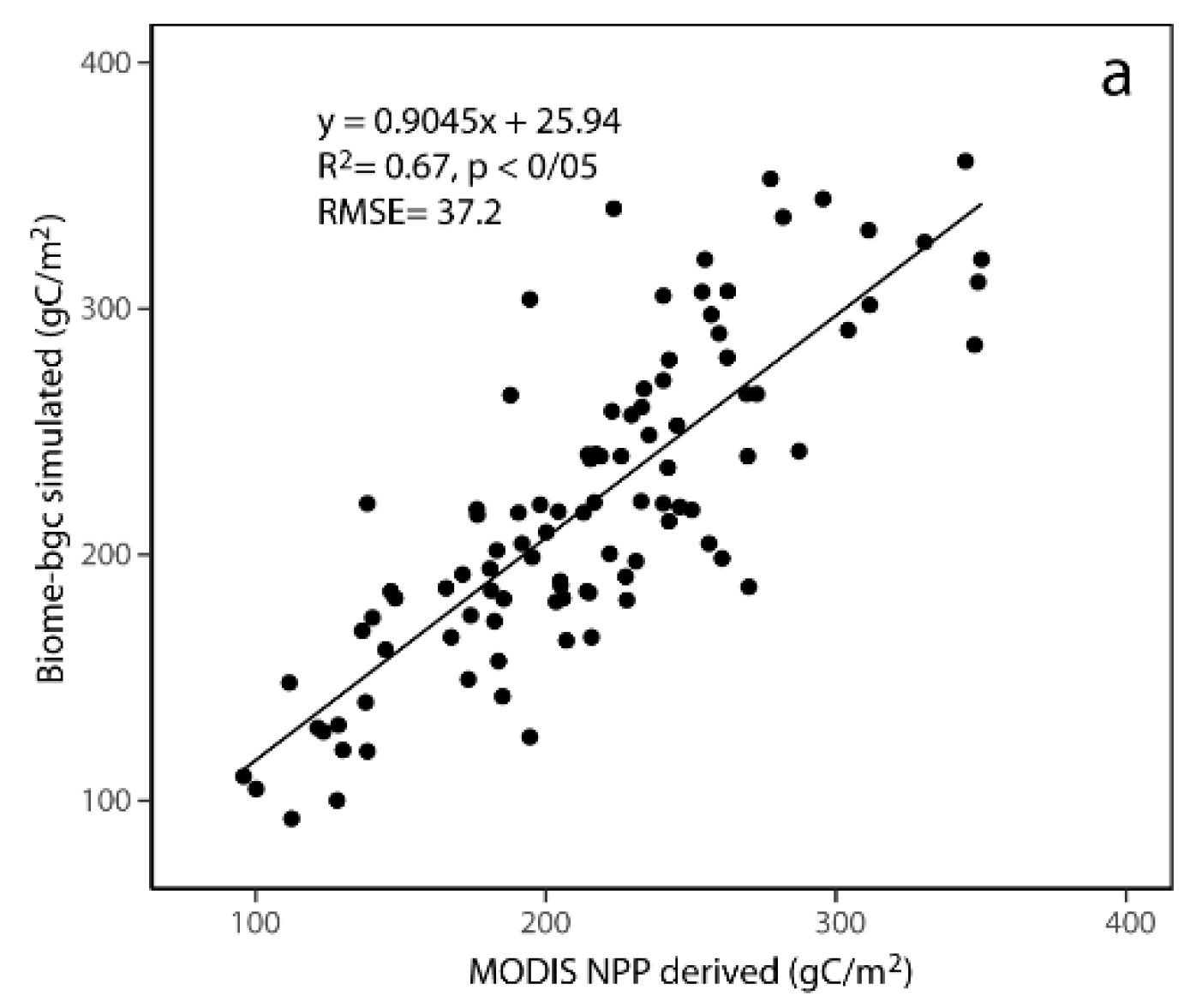
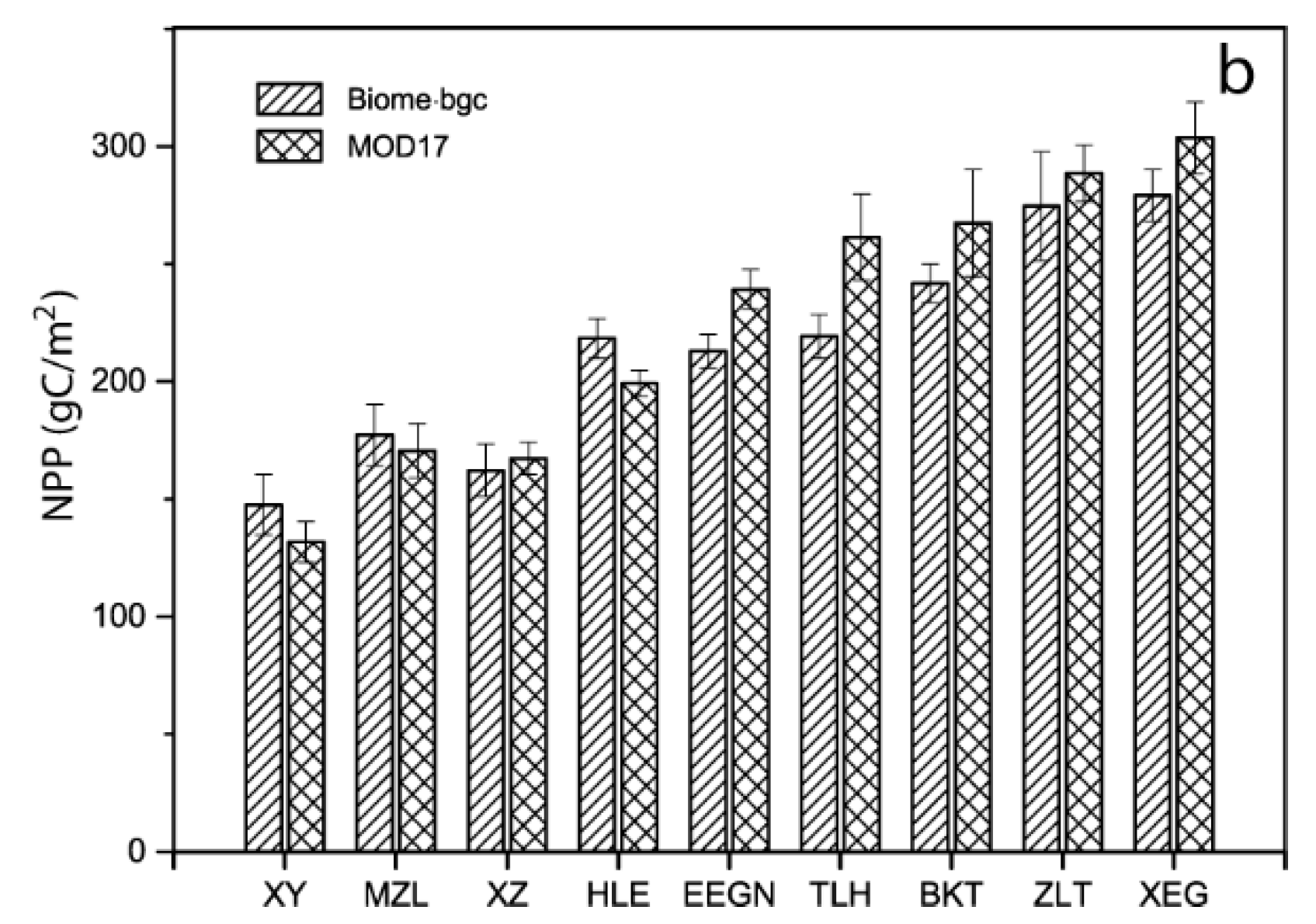
2.4. Model Validation of Other Sites
2.5. Lag of Climate Effect on Grass NPP
2.6. Statistics
3. Result
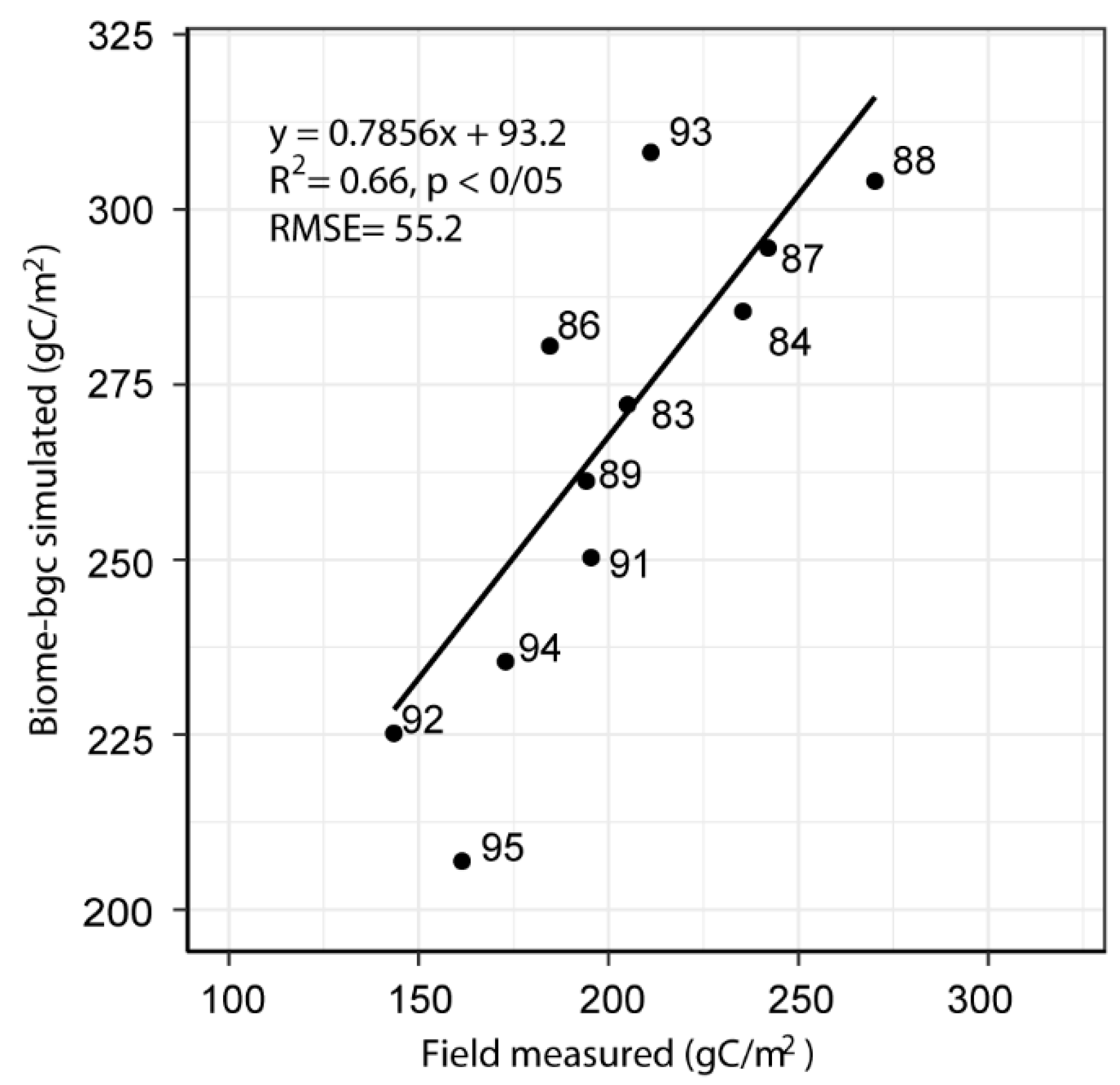
3.1. Model Validation
3.2. Trend of Climate Change
3.3. Dynamic Changes of NPP
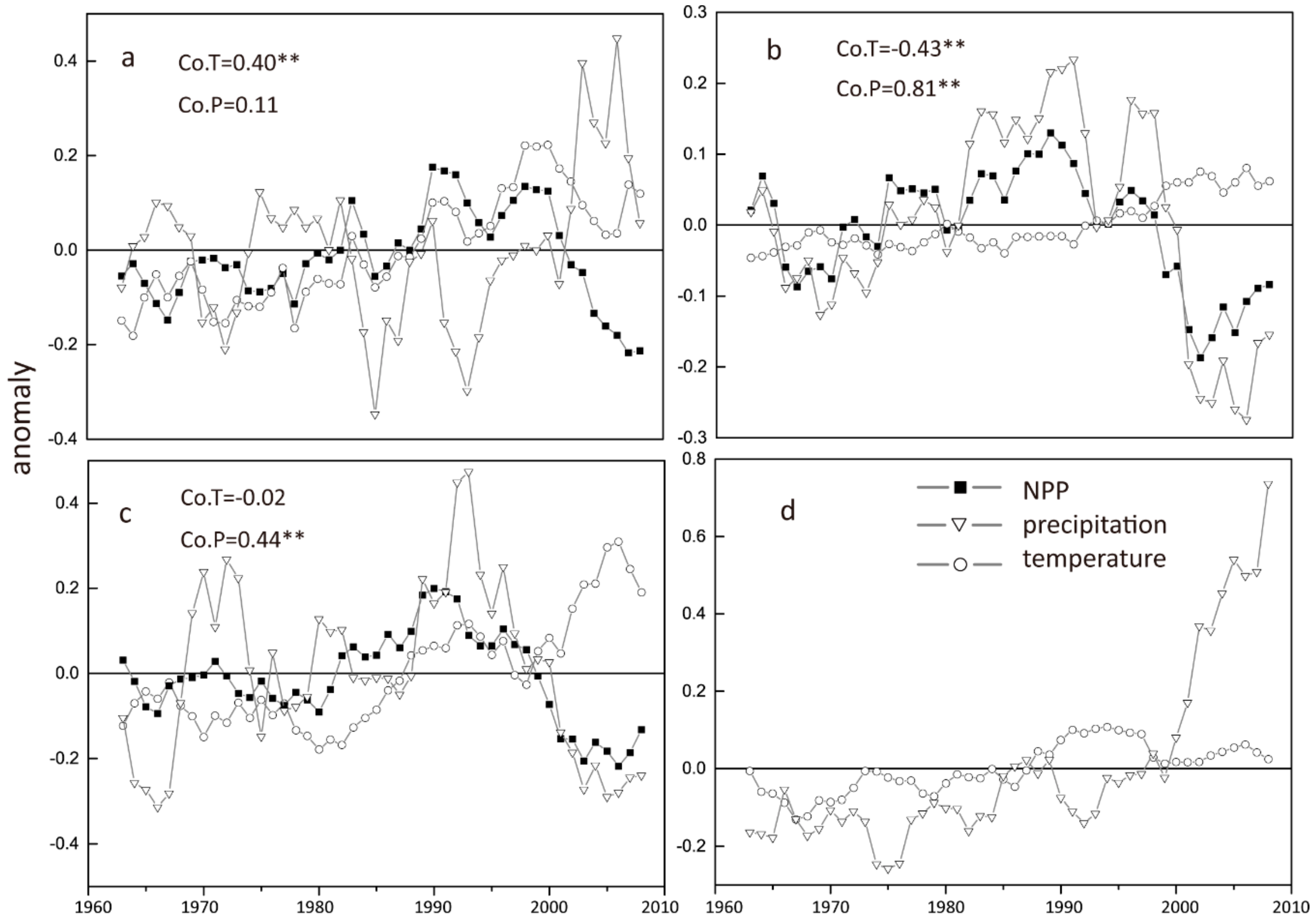
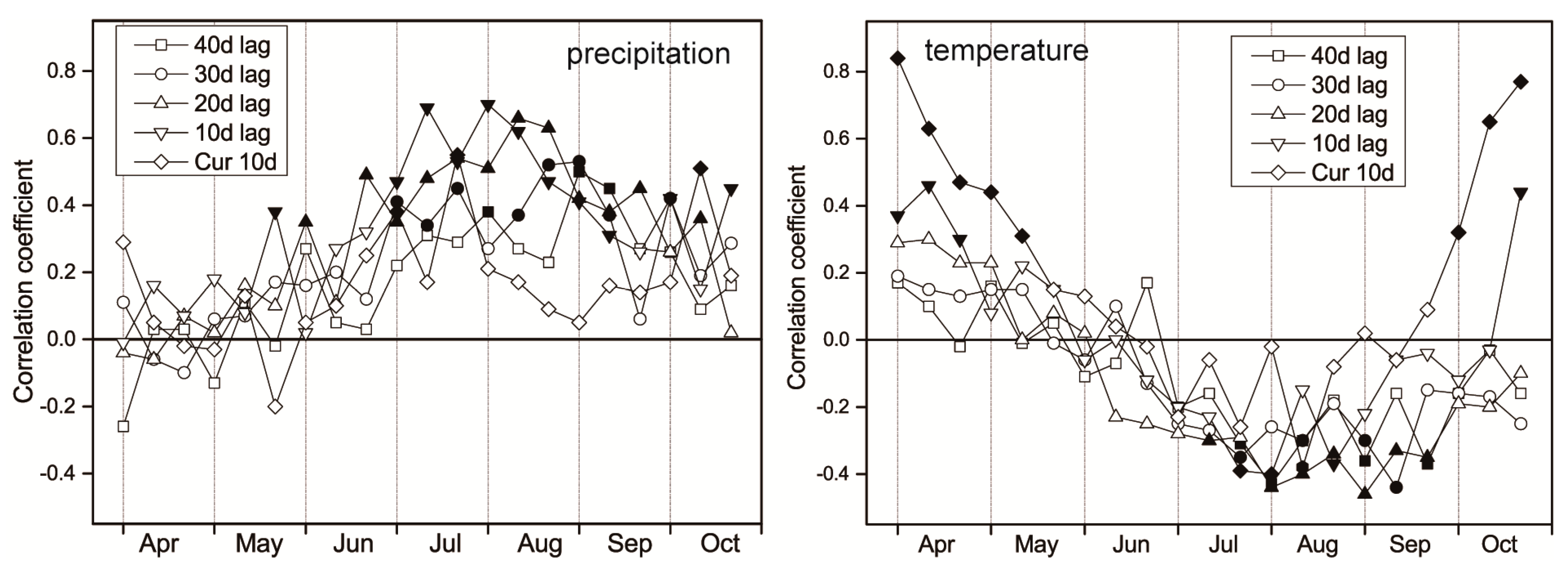
3.4. Lag Effect of Climate Factors
4. Discussion
4.1. Model Validation
4.2. Response of Grassland NPP to Climate Change
4.3. Lag Effects
4.4. Uncertainty
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. The Physical Science Basis. Contribution of Working Group Ι to the Fourth Assessment Report of the Intergrovernmental Panel on Climate Change; Cambrudge University Press: Cambridge, UK, 2007.
- IPCC. IPCC Fifth Assessment Synthesis Report; IPCC: Geneva, Switzerland, 2014.
- Trenberth, K.E. Change in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Ni, J. Estimating net primary productivity of grasslands from field biomass measurements in temperate northern China. Plant Ecol. 2004, 174, 217–234. [Google Scholar] [CrossRef]
- Zhang, G.; Kang, Y.; Han, G.; Sakurai, K. Effect of climate change over the past half century on the distribution, extent and NPP of ecosystems of Inner Mongolia. Glob. Chang. Biol. 2011, 17, 377–389. [Google Scholar] [CrossRef]
- Lieth, H. Patterns of Primary Productivity in the Biosphere. In Patterns of Primary Productivity in the Biosphere; Dowden, Hutchinson and Ross Inc.: Stroudburg, PA, USA, 1978. [Google Scholar]
- Sun, H. Ecosystems of China; Science Press: Beijing, China, 2005. [Google Scholar]
- Guisan, A.; Theurillat, J.P. Assessing alpine plant vulnerability to climate change: A modeling perspective. Integr. Assess. 2000, 1, 307–320. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S. Typical Steppe Ecosystems of China; Science Press: Beijing, China, 2000. [Google Scholar]
- Cramer, W.; Kicklighter, D.W.; Bondeau, A.; Iii, B.M.; Churkina, G.; Nemry, B.; Ruimy, A.; Schloss, A.L.; The Participants of The Potsdam NPP Model Intercomparison. Comparing global models of terrestrial net primary productivity (NPP): Overview and key results. Glob. Chang. Biol. 1999, 5, 1–15. [Google Scholar] [CrossRef]
- Nemani, R.R. Climate–Driven Increases in Global Terrestrial Net Primary Production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef]
- Running, S.W.; Baldocchi, D.D.; Turner, D.P.; Gower, S.T.; Bakwin, P.S.; Hibbard, K.A. A Global Terrestrial Monitoring Network Integrating Tower Fluxes, Flask Sampling, Ecosystem Modeling and EOS Satellite Data. Remote Sens. Environ. 1999, 70, 108–127. [Google Scholar] [CrossRef]
- Williams, J.W.; Seabloom, E.W.; Slayback, D.; Stoms, D.M.; Viers, J.H. Anthropogenic impacts upon plant species richness and net primary productivity in California. Ecol. Lett. 2005, 8, 127–137. [Google Scholar] [CrossRef]
- Lobell, D.B.; Hicke, J.A.; Asner, G.P.; Field, C.B.; Tucker, C.J.; Los, S.O. Satellite estimates of productivity and light use efficiency in United States agriculture, 1982–1998. Glob. Chang. Biol. 2002, 8, 722–735. [Google Scholar] [CrossRef]
- Roy, J.; Saugier, B.; Mooney, H.A. Terrestrial Global Productivity. Austral Ecol. 2002, 27, 584–585. [Google Scholar]
- Donmez, C.; Berberoglu, S.; Forrest, M.; Cilek, A.; Hickler, T. Comparing Process–Based Net Primary Productivity Models in a Mediterranean Watershed. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2013, XL–7/W2, 67–74. [Google Scholar] [CrossRef]
- Hidy, D.; Barcza, Z.; Haszpra, L.; Churkina, G.; Pintér, K.; Nagy, Z. Development of the Biome–BGC model for simulation of managed herbaceous ecosystems. Ecol. Model. 2012, 226, 99–119. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, S.; Zhou, G.; Zhou, G.; Tieszen, L.L.; Baldocchi, D.; Bernhofer, C.; Gholz, H.; Goldstein, A.H.; Goulden, M.L.; et al. Deriving a light use efficiency model from eddy covariance flux data for predicting daily gross primary production across biomes. Agric. For. Meteorol. 2007, 143, 189–207. [Google Scholar] [CrossRef]
- Zhang, M.; Lal, R.; Zhao, Y.; Jiang, W.; Chen, Q. Estimating net primary production of natural grassland and its spatio–temporal distribution in China. Sci. Total Environ. 2016, 553, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wan, Y.; Li, Y.; Guo, Y.; Ganjurjav; Qin, X.; Jiangcun, W.; Wang, B. Effects of topography and human activity on the net primary productivity (NPP) of alpine grassland in northern Tibet from 1981 to 2004. Int. J. Remote Sens. 2013, 34, 2057–2069. [Google Scholar] [CrossRef]
- Feng, X.; Liu, G.; Chen, J.M.; Chen, M.; Liu, J.; Ju, W.M.; Sun, R.; Zhou, W. Net primary productivity of China’s terrestrial ecosystems from a process model driven by remote sensing. J. Environ. Manag. 2007, 85, 563–573. [Google Scholar] [CrossRef]
- Liu, Y.; Ju, W.; He, H.; Wang, S.; Sun, R.; Zhang, Y. Changes of net primary productivity in China during recent 11 years detected using an ecological model driven by MODIS data. Front. Earth Sci. 2013, 7, 112–127. [Google Scholar] [CrossRef]
- Wang, H.; Prentice, I.C.; Ni, J. Primary production in forests and grasslands of China: Contrasting environmental responses of light– and water–use efficiency models. Biogeosciences 2012, 9, 4689–4705. [Google Scholar] [CrossRef]
- Vetter, M.; Churkina, G.; Jung, M.; Reichstein, M.; Zaehle, S.; Bondeau, A.; Chen, Y.; Ciais, P.; Feser, F.; Freibauer, A.; et al. Analyzing the causes and spatial pattern of the European 2003 carbon flux anomaly using seven models. Biogeosciences 2008, 5, 561–583. [Google Scholar] [CrossRef]
- Barcza, Z.; Haszpra, L.; Somogyi, Z.; Hidy, D.; Lovas, K.; Churkina, G.; Horvath, L. Estimation of the biospheric carbon dioxide balance of Hungary using the BIOME–BGC model. Idojárás 2009, 113, 203–219. [Google Scholar]
- Maselli, F.; Argenti, G.; Chiesi, M.; Angeli, L.; Papale, D. Simulation of grassland productivity by the combination of ground and satellite data. Agric. Ecosyst. Environ. 2013, 165, 163–172. [Google Scholar] [CrossRef]
- Sun, Q.; Li, B.; Zhang, T.; Yuan, Y.; Gao, X.; Ge, J.; Li, F.; Zhang, Z. An improved Biome–BGC model for estimating net primary productivity of alpine meadow on the Qinghai–Tibet Plateau. Ecol. Model. 2017, 350, 55–68. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Zheng, Z.; Ye, S.; Chang, X. Effects of the Annual Precipitation Fluctuation on Primary Productivity in Hulunbeier. Res. Environ. Sci. 2015, 4, 550–558. [Google Scholar]
- Nachtergaele, F.; van Velthuizen, H.; Verelst, L. Harmonized World Soil Database, Food and Agriculture Organization of the United Nations; FAO: Roma, Italy, 2009. [Google Scholar]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef]
- Running, S.W.; Hunt, E.R. Generalization of a Forest Ecosystem Process Model for Other Biomes, BIOME–BGC, and an Application for Global–Scale Models. In Scaling Physiological Processes; Roy, J., Ehleringer, J.R., Field, C.B., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 141–158. ISBN 978-0-12-233440-5. [Google Scholar]
- Running, S.W.; Gower, S.T. FOREST–BGC, A general model of forest ecosystem processes for regional applications. II. Dynamic carbon allocation and nitrogen budgets. Tree Physiol. 1991, 9, 147–160. [Google Scholar] [CrossRef]
- White, M.A.; Thornton, P.E.; Running, S.W.; Nemani, R.R. Parameterization and Sensitivity Analysis of the BIOME–BGC Terrestrial Ecosystem Model: Net Primary Production Controls. Earth Interact. 2000, 4, 1–85. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Thornton, P.E.; Running, S.W. Theoretical Framework of Biome–BGC Version4.2; Technical Documentation: Missoula, MT, USA, 2010. [Google Scholar]
- Running, S.W.; Coughlan, J.C. A General Model of Forest Ecosystem Processes for Regional Applications I. Hydrologic Balance, Canopy GAS Exchange and Primary Production Processes. Ecol. Model. 1988, 42, 125–154. [Google Scholar] [CrossRef]
- Bohn, T.J.; Livneh, B.; Oyler, J.W.; Running, S.W.; Nijssen, B.; Lettenmaier, D.P. Global evaluation of MTCLIM and related algorithms for forcing of ecological and hydrological models. Agric. For. Meteorol. 2013, 176, 38–49. [Google Scholar] [CrossRef]
- Potter, C.S.; Randerson, J.T.; Field, C.B.; Matson, P.A.; Vitousek, P.M.; Mooney, H.A.; Klooster, S.A. Terrestrial ecosystem production: A process model based on global satellite and surface data. Glob. Biogeochem. Cycles 1993, 7, 811–841. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Simulations show decreasing carbon stocks and potential for carbon emissions in Rocky Mountain forests over the next century. Ecol. Appl. 2010, 20, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yu, M. Simulation analysis on net primary productivity of grassland communities along a water gradient and their responses to climate change. J. Plant Ecol. 2008, 32, 531–543. [Google Scholar]
- Zhang, L.; Wylie, B.K.; Ji, L.; Gilmanov, T.G.; Tieszen, L.L. Climate-Driven Interannual Variability in Net Ecosystem Exchange in the Northern Great Plains Grasslands. Rangel. Ecol. Manag. 2010, 63, 40–50. [Google Scholar] [CrossRef]
- Reeves, M.C.; Moreno, A.L.; Bagne, K.E.; Running, S.W. Estimating climate change effects on net primary production of rangelands in the United States. Clim. Chang. 2014, 126, 429–442. [Google Scholar] [CrossRef]
- Heinsch, F.A.; Zhao, M.; Running, S.W.; Kimball, J.S.; Nemani, R.R.; Davis, K.J.; Bolstad, P.V.; Cook, B.D.; Desai, A.R.; Ricciuto, D.M.; et al. Evaluation of remote sensing based terrestrial productivity from MODIS using regional tower eddy flux network observations. IEEE Trans. Geosci. Remote. Sens. 2006, 44, 1908–1925. [Google Scholar] [CrossRef]
- Maselli, F.; Chiesi, M.; Moriondo, M.; Fibbi, L.; Bindi, M.; Running, S.W. Modelling the forest carbon budget of a Mediterranean region through the integration of ground and satellite data. Ecol. Model. 2009, 220, 330–342. [Google Scholar] [CrossRef]
- Han, Q.; Luo, G.; Li, C.; Ye, H.; Chen, Y. Modeling grassland net primary productivity and water–use efficiency along an elevational gradient of the Northern Tianshan Mountains. J. Arid Land 2013, 5, 354–365. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Xiang, Q.; Pan, Q.; Huang, J.; Yang, D.; Han, X. Primary production and rain use efficiency across a precipitation gradient on the mongolia plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef]
- Tatarinov, F.A.; Cienciala, E. Long–term simulation of the effect of climate changes on the growth of main Central–European forest tree species. Ecol. Model. 2009, 220, 3081–3088. [Google Scholar] [CrossRef]
- Dai, L.; Ke, X.; Du, Y.; Zhang, F.; Li, Y.; Li, Q.; Lin, L.; Peng, C.; Shu, K.; Cao, G.; et al. Nitrogen controls the net primary production of an alpine Kobresia meadow in the northern Qinghai–Tibet Plateau. Ecol. Evol. 2019, 9, 8865–8875. [Google Scholar] [CrossRef]
- Gao, W.; Yan, D. Warming suppresses microbial biomass but enhances N recycling. Soil Biol. Biochem. 2019, 131, 111–118. [Google Scholar] [CrossRef]
- Ren, J.Z.; Hu, Z.Z.; Zhao, J.; Zhang, D.G.; Hou, F.J.; Lin, H.L.; Mu, X.D. A grassland classification system and its application in China. Rangeland J. 2008, 30, 199–209. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Wen, Y.; Ou, J. Quantitative analysis of the contributions of climatic and human factors to grassland productivity in northern China. Ecol. Indic. 2019, 103, 542–553. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Zhang, A.; Cui, X.; Zhao, A. Spatiotemporal variations and its influencing factors of grassland net primary productivity in Inner Mongolia, China during the period 2000–2014. J. Arid. Environ. 2019, 165, 106–118. [Google Scholar] [CrossRef]
- Xu, X.; Sherry, R.A.; Niu, S.; Li, D.; Luo, Y. Net primary productivity and rain–use efficiency as affected by warming, altered precipitation, and clipping in a mixed–grass prairie. Glob. Chang. Biol. 2013, 19, 2753–2764. [Google Scholar] [CrossRef]
- Tucker, C.J.; Slayback, D.A.; Pinzon, J.E.; Los, S.O.; Myneni, R.B.; Taylor, M.G. Higher northern latitude normalized difference vegetation index and growing season trends from 1982 to 1999. Int. J. Biometeorol. 2001, 45, 184–190. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Y.; Sivakumar, B.; Long, A.; Qiu, L.; Chen, J.; Wang, L.; Liu, S.; Hu, H. Climatic and hydrologic controls on net primary production in a semiarid loess watershed. J. Hydrol. 2019, 568, 803–815. [Google Scholar] [CrossRef]
- Sun, J.; Du, W. Effects of precipitation and temperature on net primary productivity and precipitation use efficiency across China’s grasslands. GISci. Remote Sens. 2017, 54, 881–897. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, M.D. Variation Among Biomes in Temporal Dynamics of Primary Aboveground Production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef]
- Huxman, T.E.; Cable, J.M.; Ignace, D.D.; Eilts, J.A.; English, N.B.; Weltzin, J.; Williams, D.G. Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi–arid grassland: The role of native versus non–native grasses and soil texture. Oecologia 2004, 141, 295–305. [Google Scholar] [CrossRef]
- Bao, G.; Chen, J.; Chopping, M.; Bao, Y.; Bayarsaikhan, S.; Dorjsuren, A.; Tuya, A.; Jirigala, B.; Qin, Z. Dynamics of net primary productivity on the Mongolian Plateau: Joint regulations of phenology and drought. Int. J. Appl. Earth Observ. Geoinf. 2019, 81, 85–97. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Sala, O.E.; Paruelo, J.M. Patterns and Controls of Primary Production in the Patagonian Steppe: A Remote Sensing Approach. Ecol. Soc. Am. 2009, 83, 307–319. [Google Scholar]
- Shen, M.; Tang, Y.; Chen, J.; Zhu, X.; Zheng, Y. Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai–Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1711–1722. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G. Exploring the effects of water on vegetation change and net primary productivity along the IGBP Northeast China Transect. Environ. Earth Sci. 2011, 62, 1481–1490. [Google Scholar] [CrossRef]
- Epstein, H.E.; Burke, I.C.; Lauenroth, W.K. Regional Patterns of Decomposition and Primary Production Rates in the U.S. Great Plains. Ecology 2002, 83, 320–327. [Google Scholar]
- Munkhtsetseg, E.; Kimura, R.; Wang, J.; Shinoda, M. Pasture yield response to precipitation and high temperature in Mongolia. J. Arid Environ. 2007, 70, 94–110. [Google Scholar] [CrossRef]
- Mu, S.; Zhou, S.; Chen, Y.; Li, J.; Ju, W.; Odeh, I.O.A. Assessing the impact of restoration–induced land conversion and management alternatives on net primary productivity in Inner Mongolian grassland, China. Glob. Planet. Chang. 2013, 108, 29–41. [Google Scholar] [CrossRef]
- Shen, J.; Kuo, A.Y. Eutrophication Model Calibration as a Coupled Inverse Problem. Estuar. Coast. Model. 2001, 2002, 585–599. [Google Scholar]
- Gobeyn, S.; Mouton, A.M.; Cord, A.F.; Kaim, A.; Volk, M.; Goethals, P.L.M. Evolutionary algorithms for species distribution modelling: A review in the context of machine learning. Ecol. Model. 2019, 392, 179–195. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef]
- Tian, H.; Melillo, J.; Lu, C.; Kicklighter, D.; Liu, M.; Ren, W.; Xu, X.; Chen, G.; Zhang, C.; Pan, S.; et al. China’s terrestrial carbon balance: Contributions from multiple global change factors. Glob. Biogeochem. Cycles 2011, 25, 1007. [Google Scholar] [CrossRef]




| Eco-Physiological Parameters. | Values |
|---|---|
| Soil depth (m) | 1 [40] |
| Annual leaf and fine root turnover fraction (1/a) * | 1 |
| New fine root C:new leaf C (ration) * | 1.5 |
| C:N of leaves (kgC/kgN) * | 16.82 |
| C:N of leaf litter (kgC/kgN) * | 26 |
| C:N of fine roots (kgC/kgN) * | 30 |
| Leaf litter labile proportion (DIM) | 0.563 |
| Leaf litter cellulose proportion (DIM) | 0.369 |
| Leaf litter lignin proportion (DIM) | 0.068 |
| Fine root labile proportion (DIM) | 0.34 |
| Fine root cellulose proportion (DIM) | 0.44 |
| Fine root lignin proportion (DIM) | 0.22 |
| Dead wood cellulose proportion (DIM) | 0.75 |
| Canopy water interception coefficient (1/d/LAI) | 0.01 |
| Canopy light extinction coefficient (DIM) | 0.48 |
| Canopy average specific leaf area (m2/kgC) | 17.86 |
| Maximum stomatal conductance (m/s) | 0.006 |
| Cuticular conductance (m/s) | 0.00006 |
| Boundary layer conductance (m/s) | 0.04 |
| Leaf water potential: start of conductance reduction (MPa) | −0.73 |
| Leaf water potential: complete conductance reduction (MPa) | −2.7 |
| VPD: start of conductance reduction (Pa) | 1250.0 |
| VPD: complete conductance reduction (Pa) | 5000.0 |
| Site. | Elevation | AT | AP | NPP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Trend | CV | p | mean | range | CV | Trend | p | mean | range | CV | Trend | p | ||
| BKT | 740 | 2253 | 5.2 | 0.05 | <0.01 | 463 | −52%–62% | 0.19 | −0.13 | 0.89 | 275 | –37.1–39.0% | 0.19 | –0.26 | 0.60 |
| EEG | 581 | 2360 | 10.6 | 0.08 | <0.01 | 356 | −42%–52% | 0.20 | −0.33 | 0.67 | 246 | –32.3–45.7% | 0.20 | –0.19 | 0.70 |
| HLE | 610 | 2549 | 8.8 | 0.06 | <0.01 | 347 | −64%–56% | 0.22 | 0.93 | 0.24 | 235 | –54.5–49.8% | 0.21 | 0.67 | 0.14 |
| MZL | 662 | 2492 | 8.9 | 0.07 | <0.01 | 284 | −51%–107% | 0.31 | −1.51 | 0.09 | 209 | –59.3–59.6% | 0.23 | –0.64 | 0.19 |
| TLH | 733 | 1903 | 5.2 | 0.06 | <0.01 | 442 | 36%–47% | 0.17 | −0.81 | 0.30 | 240 | –31.8–12.5% | 0.10 | –0.22 | 0.32 |
| XEG | 286 | 2586 | 9.1 | 0.06 | <0.01 | 496 | −42%–102% | 0.23 | 0.85 | 0.46 | 299 | –38.7–21.3% | 0.13 | –0.04 | 0.91 |
| XY | 554 | 2818 | 8.5 | 0.06 | <0.01 | 241 | −57%–145% | 0.37 | −0.81 | 0.38 | 196 | –48.1–71.9% | 0.27 | –0.46 | 0.40 |
| XZ | 642 | 2745 | 8.0 | 0.06 | <0.01 | 272 | −54%–117% | 0.29 | −0.03 | 0.97 | 200 | –44.2–68.2% | 0.23 | –0.28 | 0.53 |
| ZLT | 307 | 2972 | 8.8 | 0.05 | <0.01 | 487 | −56%–128% | 0.27 | 0.47 | 0.73 | 296 | –56.4–35.9% | 0.19 | –0.04 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, Y.; Li, J. Grassland Productivity Response to Climate Change in the Hulunbuir Steppes of China. Sustainability 2019, 11, 6760. https://doi.org/10.3390/su11236760
Zhang C, Zhang Y, Li J. Grassland Productivity Response to Climate Change in the Hulunbuir Steppes of China. Sustainability. 2019; 11(23):6760. https://doi.org/10.3390/su11236760
Chicago/Turabian StyleZhang, Chaobin, Ying Zhang, and Jianlong Li. 2019. "Grassland Productivity Response to Climate Change in the Hulunbuir Steppes of China" Sustainability 11, no. 23: 6760. https://doi.org/10.3390/su11236760
APA StyleZhang, C., Zhang, Y., & Li, J. (2019). Grassland Productivity Response to Climate Change in the Hulunbuir Steppes of China. Sustainability, 11(23), 6760. https://doi.org/10.3390/su11236760





