Diversity and Pre-Breeding Prospects for Local Adaptation in Oat Genetic Resources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Phenotypic Data
2.2. Statistical Analysis of Phenotypic Data
2.3. Genotypic Data and Population Structure
2.4. Trait Correlations and Response to Selection
3. Results and Discussion
3.1. Quantitative Genetic Parameters and Trait Correlations
3.2. Phenotypic and Genotypic Population Structure
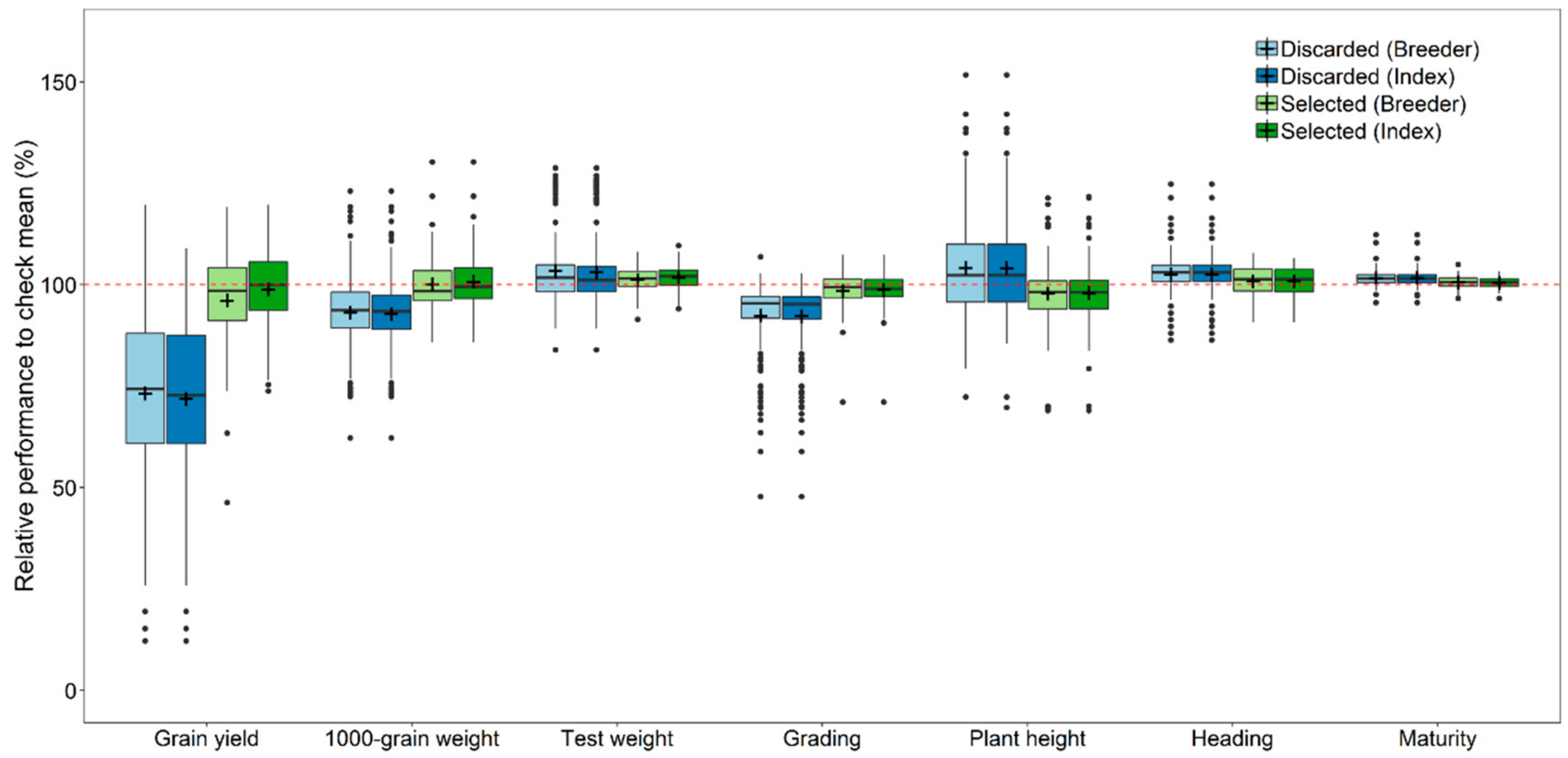
3.3. Breeders’ Decisions and Selection for Local Adaptation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data availability
References
- Vavilov, N.I. Phytogeographic basis of plant breeding. In The Origin, Variation, Immunity and Breeding of Cultivated Plants; Chester, K.S., Translator; Chronica Botanica: Waltham, MA, USA, 1951; Volume 13, pp. 13–54. [Google Scholar]
- Funke, C. Hafer, Avena sativa L.—Bemühungen um das ehemals wichtigste Futtergetreide. Vorträge für Pflanzenzüchtung 2008, 75, 312–320. [Google Scholar]
- Beuch, S. Züchtung auf Ertrag und Qualität bei Hafer (Avena sativa L.)—Entwicklung und Perspektiven. In 61. Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs; LFZ Raumberg-Gumpenstein: Irdning, Austria, 2011; pp. 57–63. [Google Scholar]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat beta-glucan: Its role in health promotion and prevention of diseases. Comp. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Koenig, R.; Dickman, J.R.; Kang, C.; Zhang, T.; Chu, Y.-F.; Ji, L.L. Avenanthramide supplementation attenuates exercise-induced inflammation in postmenopausal women. Nutr. J. 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achleitner, A.; Tinker, N.A.; Zechner, E.; Buerstmayr, H. Genetic diversity among oat varieties of worldwide origin and associations of AFLP markers with quantitative traits. Theor. Appl. Genet. 2008, 117, 1041–1053. [Google Scholar] [CrossRef]
- Newell, M.A.; Cook, D.; Tinker, N.A.; Jannink, J.L. Population structure and linkage disequilibrium in oat (Avena sativa L.): Implications for genome-wide association studies. Theor. Appl. Genet. 2011, 122, 623–632. [Google Scholar] [CrossRef]
- Yan, H.; Martin, S.L.; Bekele, W.A.; Latta, R.G.; Diederichsen, A.; Peng, Y.; Tinker, N.A. Genome size variation in the genus Avena. Genome 2016, 59, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.B.; Peterson, G.W.; Scoles, G.; Rossnagel, B.; Schoen, D.J.; Richards, K.W. Allelic diversity changes in 96 Canadian oat cultivars released from 1886 to 2001. Crop Sci. 2003, 43, 1989–1995. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Kaeppler, H.F. A genetic linkage map for hexaploid, cultivated oat (Avena sativa L.) based on an intraspecific cross ‘Ogle/MAM17-5’. Theor. Appl. Genet. 2003, 107, 26–35. [Google Scholar] [CrossRef]
- Becher, R. EST-derived microsatellites as a rich source of molecular markers for oats. Plant Breed. 2007, 126, 274–278. [Google Scholar] [CrossRef]
- He, X.; Bjørnstad, Å. Diversity of North European oat analysed by SSR, AFLP and DArT markers. Theor. Appl. Genet. 2012, 125, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.R.; Bonman, J.M.; Chao, S.; Yimer, B.A.; Bockelman, H.; Klos, K.E. Population structure and genotype phenotype associations in a collection of oat landraces and historic cultivars. Front. Plant Sci. 2016, 7, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, E.E.; Prescott, J.M. A scale for appraising the foliar intensity of wheat diseases. Plant Dis. Rep. 1975, 59, 377–380. [Google Scholar]
- CPVO. Protocol for Tests on Distinctness, Uniformity and Stability, Avena sativa L., Avena nuda L., Oats, Naked oats, UPOV Code: AVENA_SAT.; AVENA_NUD; Community Plant Variety Office (CPVO): Angers, France, 2015.
- Piepho, H.-P.; Möhring, J. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal locations, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef] [Green Version]
- Li, C.D.; Rossnagel, B.G.; Scoles, G.J. The development of oat microsatellite markers and their use in identifying relationships among Avena species and oat cultivars. Theor. Appl. Genet. 2000, 101, 1259–1268. [Google Scholar] [CrossRef]
- Holland, J.B.; Helland, S.J.; Sharopova, N.; Rhyne, D.C. Polymorphism of PCR-based markers targeting exons, introns, promoter regions, and SSRs in maize and introns and repeat sequences in oat. Genome 2001, 44, 1065–1076. [Google Scholar] [CrossRef]
- Pal, N.; Snaghu, J.S.; Domier, L.L.; Kolb, F.L. Development and characterization of microsatellite and RFLP-derived PCR markers in oat. Crop Sci. 2002, 42, 912–918. [Google Scholar] [CrossRef]
- Oliver, R.E.; Chest, D.E.; Hu, G.; Bonman, J.M.; O’Leary-Jepsen, E.; Jackson, E.W. Development of oat-based markers from barley and wheat microsatellites. Genome 2010, 53, 458–471. [Google Scholar] [CrossRef]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; François, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 2014, 196, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Meth. Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Rogers, J.S. Measures of similarity and genetic distance. In Studies in Genetics VII; University of Texas Publication 7213; University of Texas: Austin, TX, USA, 1972; pp. 145–153. [Google Scholar]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1949, 15, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Butts, C.T. Package ‘SNA’, Vers. 2.4, Tools for Social Network Analysis. Available online: https://cran.r-project.org/web/packages/sna/sna.pdf (accessed on 2 December 2019).
- Butts, C.T. Social network analysis with sna. J. Stat. Softw. 2008, 24, 1–51. [Google Scholar] [CrossRef]
- Johnson, B.; Gardner, C.O.; Wrede, K.C. Application of an optimization model to multi-trait selection programs. Crop Sci. 1988, 28, 723–728. [Google Scholar] [CrossRef]
- Bernardo, R. Retrospective index weights used in multiple trait selection in a maize breeding program. Crop Sci. 1991, 31, 1174–1179. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Krenn, N.; Stephan, U.; Grausgruber, H.; Zechner, E. Agronomic performance and quality of oat (Avena sativa L.) genotypes of worldwide origin produced under Central European growing conditions. Field Crop Res. 2007, 101, 343–351. [Google Scholar] [CrossRef]
- De Koeyer, D.L.; Tinker, N.A.; Wight, C.P.; Deyl, J.; Burrows, V.D.; O’Donoughue, L.S.; Lybaert, A.; Molnar, S.J.; Armstrong, K.C.; Fedak, G.; et al. A molecular linkage map with associated QTLs from a hulless × covered spring oat population. Theor. Appl. Genet. 2004, 108, 1285–1298. [Google Scholar] [CrossRef]
- Ganßmann, W.; Vorwerck, K. Oat milling, processing and storage. In The Oat Crop. Production and Utilization; Welch, R.W., Ed.; Chapman & Hall: London, UK, 1995; pp. 369–408. [Google Scholar]
- Humphreys, D.G.; Mather, D.E. Heritability of β-glucan, groat percentage, and crown rust resistance in two oat crosses. Euphytica 1996, 91, 359–364. [Google Scholar] [CrossRef]
- Luke, H.H.; Barnett, R.D.; Pfahler, P.L. Inheritance of horizontal resistance to crown rust in oats. Phytopathology 1975, 65, 631–632. [Google Scholar] [CrossRef]
- Long, J.; Holland, J.B.; Munkvold, G.P.; Jannink, J.-L. Responses to selection for partial resistance to crown rust in oat. Crop Sci. 2006, 46, 1260–1265. [Google Scholar] [CrossRef]
- Holland, J.B.; Bjørnstad, Å.; Frey, K.J.; Gullord, M.; Wesenberg, D.M. Recurrent selection for broad adaptation affects stability of oat. Euphytica 2002, 126, 265–274. [Google Scholar] [CrossRef]
- Laidig, F.; Drobek, T.; Meyer, U. Genotypic and environmental variability of yield for cultivars from 30 different crops in German official variety trials. Plant Breed. 2008, 127, 541–547. [Google Scholar] [CrossRef]
- Holland, J.B.; Bjørnstad, Å.; Frey, K.J.; Gullord, M.; Wesenberg, D.M.; Buraas, T. Recurrent selection in oat for adaptation to diverse environments. Euphytica 2000, 113, 195–205. [Google Scholar] [CrossRef]
- Panayotova, G. Evaluation of grain yield potential of oat germplasm in Bulgaria. In Proceedings 7th International Oat Conference; Peltonen-Sainio, P., Topi-Hulmi, M., Eds.; Agrifood Research Reports 51; MTT Agrifood Research Finland: Jokioinen, Finland, 2004; p. 159. [Google Scholar]
- Tumino, G.; Voorrips, R.E.; Morcia, C.; Ghizzoni, R.; Germeier, C.U.; Paulo, M.-J.; Terzi, V.; Smulders, M.J.M. Genome-wide association analysis for lodging tolerance and plant height in a diverse European hexaploid oat collection. Euphytica 2017, 213, 163. [Google Scholar] [CrossRef]
- Pinthus, M.J. Lodging in wheat, barley and oats: The phenomenon, its causes, and preventive measures. Adv. Agron. 1973, 25, 209–263. [Google Scholar]
- Peltonen-Sainio, P.; Rajala, A. Duration of vegetative and generative development phases in oat cultivars released since 1921. Field Crop Res. 2007, 101, 72–79. [Google Scholar] [CrossRef]
- Klink, K.; Wiersma, J.J.; Crawford, C.J.; Stuthman, D.D. Impacts of temperature and precipitation variability in the Northern Plains of the United States and Canada on the productivity of spring barley and oat. Int. J. Climatol. 2014, 34, 2805–2818. [Google Scholar] [CrossRef]
- Fu, Y.-B.; Kibite, S.; Richards, K.W. Amplified fragment length polymorphism analysis of 96 Canadian oat cultivars released between 1886 and 2001. Can. J. Plant Sci. 2004, 84, 23–30. [Google Scholar]
- Tinker, N.A.; Kilian, A.; Wight, C.P.; Heller-Uszynska, K.; Wenzl, P.; Rines, H.W.; Bjørnstad, Å.; Howarth, C.J.; Jannink, J.-L.; Anderson, J.M.; et al. New DArT markers for oat provide enhanced map coverage and global germplasm characterization. BMC Genom. 2009, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinker, N.A.; Chao, S.; Lazo, G.R.; Oliver, R.E.; Huang, Y.-F.; Poland, J.A.; Jellen, E.N.; Maughan, P.J.; Kilian, A.; Jackson, E.W. A SNP genotyping array for hexaploid oat. Plant Genome 2014, 7. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Poland, J.A.; Wight, C.P.; Jackson, E.W.; Tinker, N.A. Using genotyping-by-sequencing (GBS) for genomic discovery in cultivated oat. PLoS ONE 2014, 9, e102448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esvelt Klos, K.; Huang, Y.-F.; Bekele, W.A.; Obert, D.E.; Babiker, E.; Beattie, A.D.; Bjørnstad, Å.; Bonman, J.M.; Carson, M.L.; Chao, S.; et al.; et al. Population genomics related to adaptation in elite oat germplasm. Plant Genome 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.-B. Understanding crop genetic diversity under modern plant breeding. Theor. Appl. Genet. 2015, 128, 2131–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffmann, F.A. Oat History, Identification and Classification; USDA-ARS Tech. Bull. 1516; USDA: Washington, DC, USA, 1977.
- Diederichsen, A. Assessment of genetic diversity within a world collection of cultivated hexaploid oat (Avena sativa L.) based on qualitative morphological characters. Genet. Resour. Crop Evol. 2008, 55, 419–440. [Google Scholar] [CrossRef]
- Beuch, S. Oat breeding—Where is the journey heading. In Expert Knowledge for Professionals: Oats—Breaking New Ground, New Edition 2016; Saaten-Union GmbH: Isernhagen, Germany, 2016; pp. 61–65. [Google Scholar]
- Savova, T.; Vulchev, D.; Popova, T.; Djulgerova, B.; Vulcheva, D. Main trends in oat (Avena sativa L.) breeding at the Institute of Agriculture in Karnobat, Bulgaria. I. Winter oats. Trakya Univ. J. Nat. Sci. 2014, 15, 75–81. [Google Scholar]
- Gorash, A.; Armonienė, R.; Mitchell Fetch, J.; Liatukas, Ž.; Danytė, V. Aspects in oat breeding: Nutrition quality, nakedness and disease resistance, challenges and perspectives. Ann. Appl. Biol. 2017, 171, 281–302. [Google Scholar] [CrossRef]
- Valentine, J. Naked oats. In The Oat Crop. Production and Utilization; Welch, R.W., Ed.; Chapman & Hall: London, UK, 1995; pp. 504–532. [Google Scholar]
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Chapter One: Oats—From farm to fork. Adv. Food Nutr. Res. 2016, 77, 1–55. [Google Scholar]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaes, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Hwa Ng, E. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Trait | Trials (n) | h² | Min | Mean | Max | ||
|---|---|---|---|---|---|---|---|
| Grain yield (dt ha−1) | 9 | 112.9 | 82.4 | 0.81 | 7.5 | 46.9 | 69.3 |
| 1000 grain weight (g) | 9 | 11.2 | 2.5 | 0.93 | 22.5 | 34.5 | 47.0 |
| Test weight (kg hL-1) | 9 | 10.8 | 3.9 | 0.89 | 41.2 | 50.4 | 63.2 |
| Grading >2 mm (%) | 8 | 50.1 | 54.1 | 0.59 | 43.5 | 86.0 | 97.8 |
| Plant height (cm) | 9 | 109.8 | 35.2 | 0.91 | 67.8 | 100.3 | 149.0 |
| Heading (d after sowing) | 5 | 9.0 | 0.8 | 0.94 | 51.7 | 61.1 | 74.7 |
| Maturity (d after sowing) | 5 | 2.8 | 1.0 | 0.81 | 97.2 | 103.0 | 114.2 |
| Leaf rust (1–9) | 3 | 1.1 | 1.6 | 0.51 | 1.0 | 3.4 | 6.5 |
| Leaf blotch (1–9) | 4 | 0.1 | 0.4 | 0.35 | 1.9 | 3.6 | 4.9 |
| Panicle number (n m−2) | 4 | 1311 | 2175 | 0.44 | 336.8 | 489.9 | 768.0 |
| Panicle length (cm) | 3 | 5.3 | 1.2 | 0.86 | 13.1 | 17.5 | 32.2 |
| Panicle shape (1–9) | 3 | 0.8 | 0.5 | 0.68 | 1.3 | 5.9 | 9.3 |
| Lemma color (1–9) | 3 | 2.2 | 0.4 | 0.88 | 1.0 | 2.3 | 9.0 |
| Glume glaucosity (1–9) | 3 | 2.4 | 1.0 | 0.77 | 1.0 | 4.5 | 8.8 |
| Recurved flag leaves (1–9) | 3 | 2.4 | 0.6 | 0.84 | 1.0 | 3.7 | 8.1 |
| Second leaf width (mm) | 3 | 3.0 | 6.4 | 0.40 | 17.0 | 24.8 | 35.4 |
| Ground coverage (1–9) | 3 | 0.5 | 0.4 | 0.63 | 2.8 | 6.3 | 8.8 |
| Stem hairiness (1–9) | 3 | 3.3 | 0.2 | 0.95 | 1.0 | 1.8 | 9.0 |
| Leaf hairiness (1–9) | 3 | 0.2 | 0.2 | 0.48 | 1.0 | 1.2 | 7.2 |
| Subpopulation I | Subpopulation II | Subpopulation III | |
|---|---|---|---|
| Subpopulation I | 0.612 (9.4) | 0.056 | 0.029 |
| Subpopulation II | 0.230 | 0.488 (7.5) | 0.033 |
| Subpopulation III | 0.169 | 0.150 | 0.505 (8.3) |
| Trait | Retrospective Selection Index | Selection Gain | Accuracy | ||
|---|---|---|---|---|---|
| Index Weight | Relative Importance (%) | ΔGabs1 | ΔGrel | ||
| Grain yield (dt ha−1) | +0.68 | 23.9 | +8.84 | 119 | 0.64 |
| 1000 grain weight (g) | +0.60 | 21.2 | +1.68 | 105 | 0.95 |
| Test weight (kg hL-1) | −0.10 | 3.6 | −0.69 | 99 | 0.69 |
| Grading >2 mm (%) | −0.36 | 12.8 | +3.74 | 104 | 0.28 |
| Plant height (cm) | −0.15 | 5.5 | −4.09 | 96 | 0.87 |
| Heading (d after sowing) | +0.15 | 5.4 | −0.69 | 99 | 0.89 |
| Maturity (d after sowing) | −0.16 | 5.8 | −0.72 | 99 | 0.69 |
| Leaf blotch (1–9) | −0.05 | 1.6 | +0.09 | 102 | 0.11 |
| Panicle number (n m−2) | −0.01 | 0.4 | +8.68 | 102 | 0.55 |
| Panicle length (cm) | −0.06 | 2.0 | −1.01 | 94 | 0.70 |
| Panicle shape (1–9) | −0.07 | 2.6 | −0.04 | 99 | 0.50 |
| Lemma color (1–9) | −0.21 | 7.4 | −0.27 | 88 | 0.95 |
| Glume glaucosity (1–9) | −0.01 | 0.3 | +0.03 | 101 | 0.64 |
| Recurved flag leaves (1–9) | +0.00 | 0.0 | −0.10 | 97 | 0.78 |
| Second leaf width (mm) | −0.12 | 4.2 | −0.63 | 97 | 0.30 |
| Stem hairiness (1–9) | +0.09 | 3.3 | −0.15 | 91 | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leišová-Svobodová, L.; Michel, S.; Tamm, I.; Chourová, M.; Janovska, D.; Grausgruber, H. Diversity and Pre-Breeding Prospects for Local Adaptation in Oat Genetic Resources. Sustainability 2019, 11, 6950. https://doi.org/10.3390/su11246950
Leišová-Svobodová L, Michel S, Tamm I, Chourová M, Janovska D, Grausgruber H. Diversity and Pre-Breeding Prospects for Local Adaptation in Oat Genetic Resources. Sustainability. 2019; 11(24):6950. https://doi.org/10.3390/su11246950
Chicago/Turabian StyleLeišová-Svobodová, Leona, Sebastian Michel, Ilmar Tamm, Marie Chourová, Dagmar Janovska, and Heinrich Grausgruber. 2019. "Diversity and Pre-Breeding Prospects for Local Adaptation in Oat Genetic Resources" Sustainability 11, no. 24: 6950. https://doi.org/10.3390/su11246950
APA StyleLeišová-Svobodová, L., Michel, S., Tamm, I., Chourová, M., Janovska, D., & Grausgruber, H. (2019). Diversity and Pre-Breeding Prospects for Local Adaptation in Oat Genetic Resources. Sustainability, 11(24), 6950. https://doi.org/10.3390/su11246950





