Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Chlorophyll a Fluorescence
2.3. Measurement of the Absorption Spectrum of Leaves
2.4. Plant Biomass Measurement and Estimation of the Input of Electrical Energy Consumed for Lettuce Production
2.5. Quality and Physiological Evaluation of Lettuce under Different Growing Conditions
2.6. Statistical Analysis
3. Results
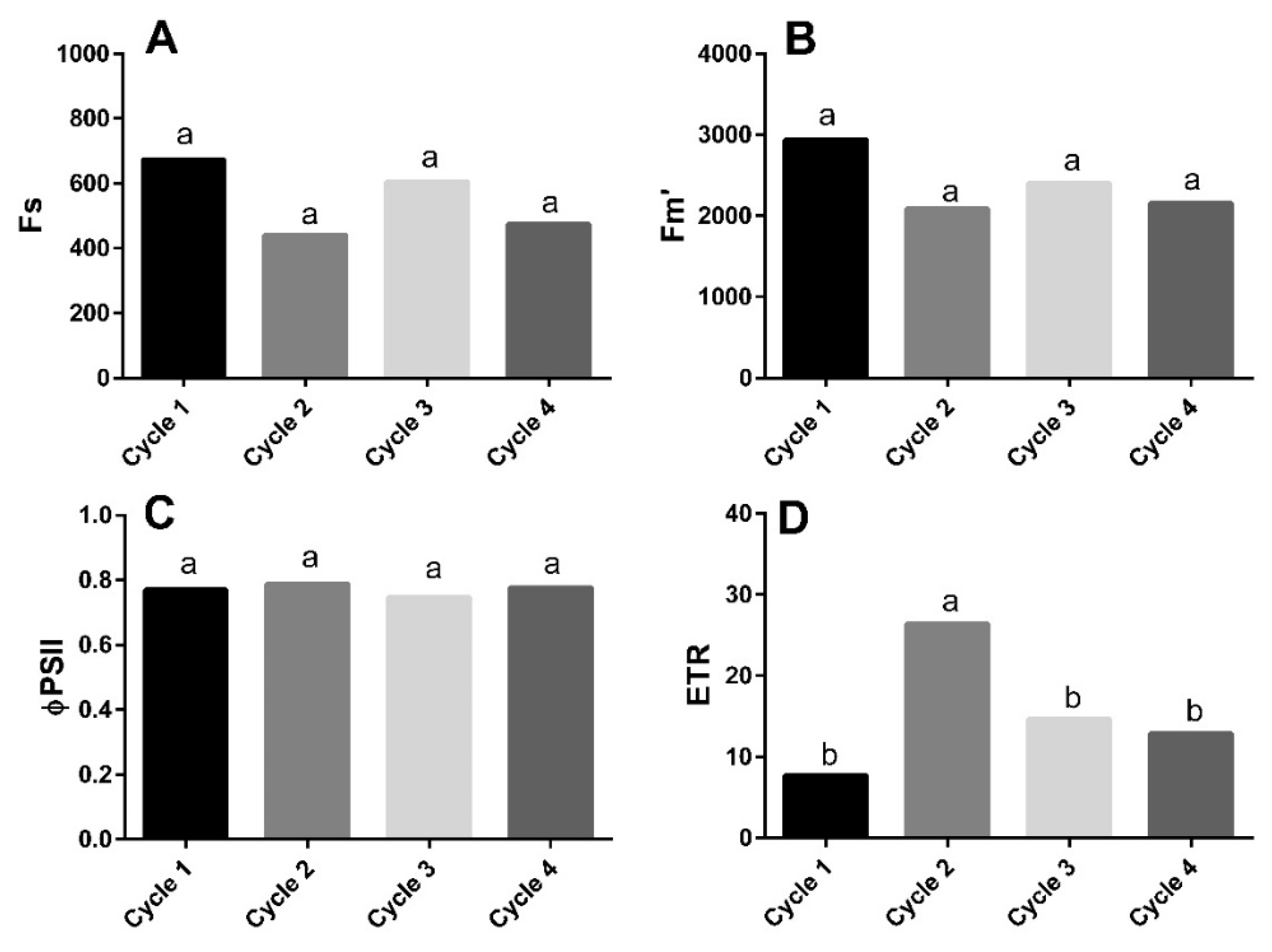
3.1. Chlorophyll a Fluorescence
3.2. Absorbance Spectrum of Leaves
3.3. Plant Biomass Measurement and Estimation of the Input of Electrical Energy Consumed for Lettuce Production
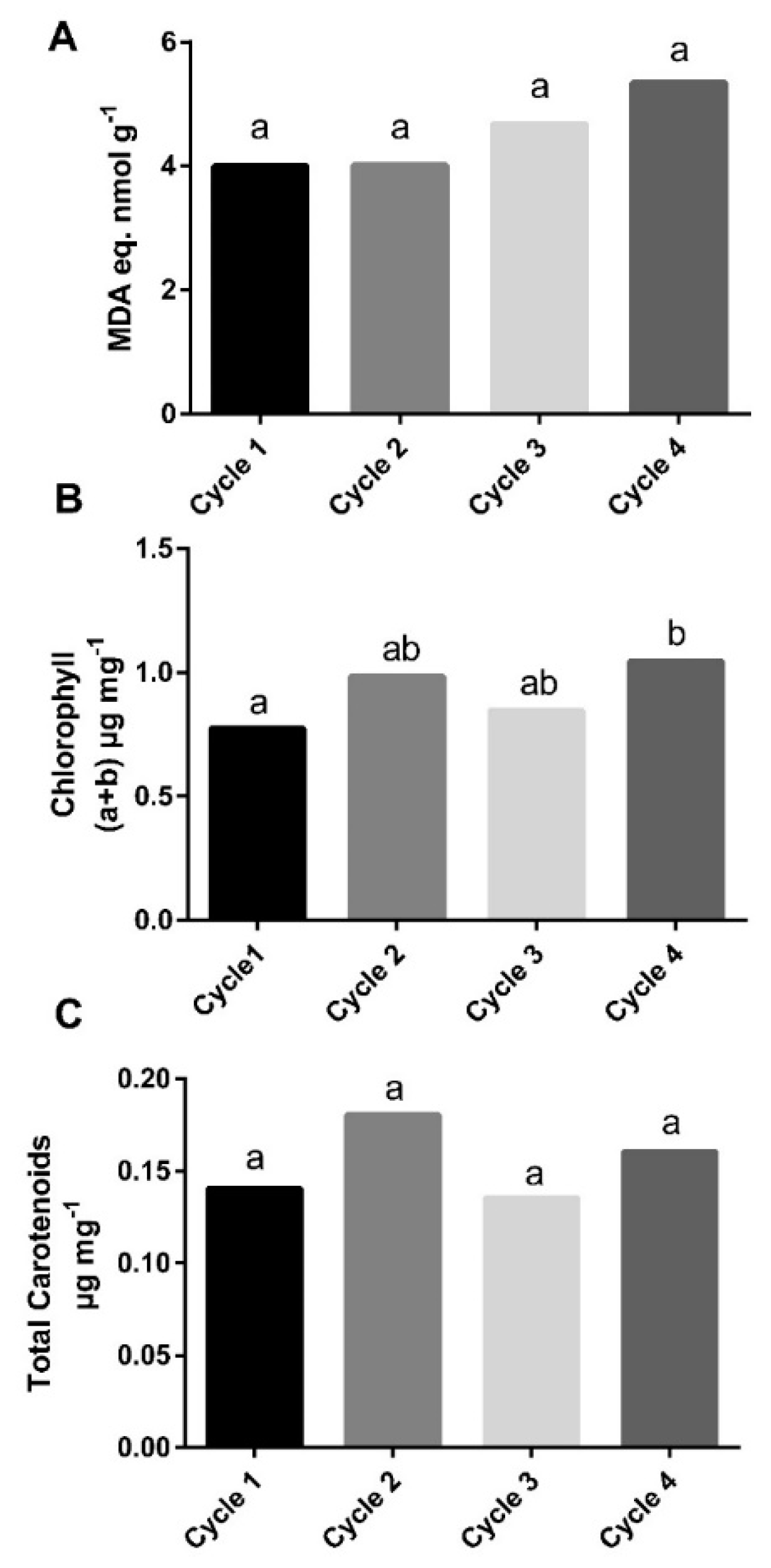
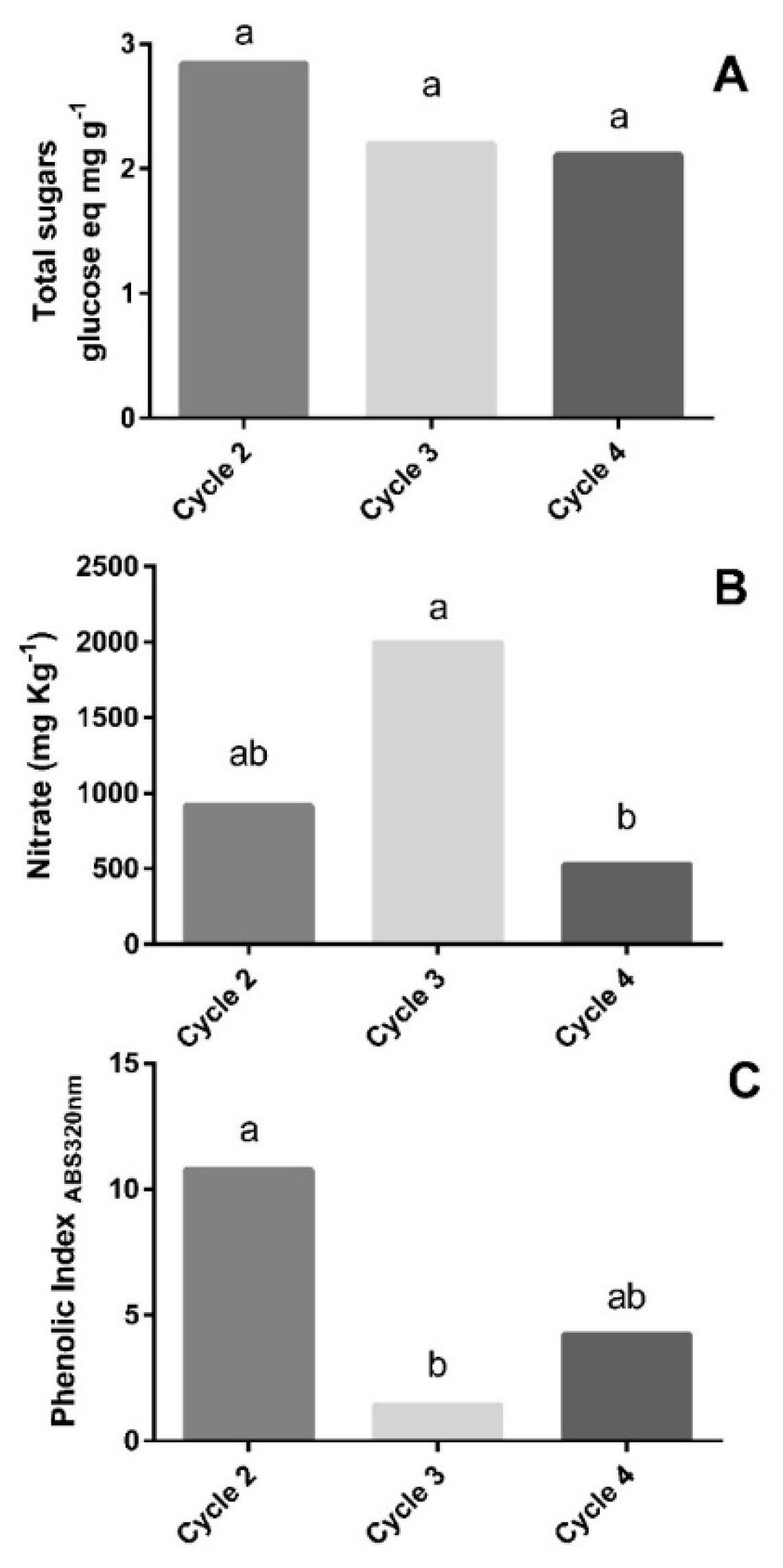
3.4. Quality and Physiological Evaluation of Lettuce under Different Growing Conditions
4. Discussion
4.1. Different Growing Conditions Affected Leaf Functionality
4.2. Effect of Different Growing Conditions on Biomass Accumulation and Energy Consumption
4.3. Effect of Different Growing Conditions on Lettuce Quality and Physiological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Bian, Z.; Jiang, N.; Grundy, S.; Lu, C. Uncovering LED light effects on plant growth: New angles and perspectives-LED light for improving plant growth, nutrition and energy-use efficiency. Acta Hortic. 2017, 1227, 491–498. [Google Scholar] [CrossRef]
- Han, T.; Vaganov, V.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Peng, L.; Zhang, C.; Yakovlev, A.N.; Zhong, Y.; et al. Improving “color rendering” of LED lighting for the growth of lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Q.C.; Song, W.P.; Wang, L.C.; Guo, W.Z.; Xue, X.Z. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Goins, G.D.; Ruffe, L.M.; Cranston, N.A.; Yorio, N.C.; Wheeler, R.M.; Sager, J.C. Salad crop production under different wavelengths of red light-emitting diodes (LEDs). In Proceedings of the 31st International Conference on Environmental Systems, Orlando, FA, USA, 9–12 July 2001; pp. 1–9. [Google Scholar]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Kim, H.H.; Wheeler, R.; Sager, J.; Norikane, J. Photosynthesis of lettuce exposed to different short-term light qualities. Environ. Control Biol. 2005, 43, 113–119. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2009, 907, 179–184. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Novičkovas, A.; Duchovskis, P. Supplementary red-LED lighting affects phytochemicals and nitrate of baby leaf lettuce. J. Food Agric. Environ. 2011, 9, 271–274. [Google Scholar]
- Samuolienė, G.; Urbonavičiūtė, A.; Duchovskis, P.; Bliznikas, Z.; Vitta, P.; Žukauskas, A. Decrease in nitrate concentration in leafy vegetables under a solid-state illuminator. HortScience 2009, 44, 1857–1860. [Google Scholar] [CrossRef]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Yeh, N.; Chung, J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kanematsu, Y.; Yoshikawa, N.; Okubo, T.; Takagaki, M. Environmental and resource use analysis of plant factories with energy technology options: A case study in Japan. J Clean. Prod. 2018, 186, 703–717. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. EPJ Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Avercheva, O.V.; Berkovich, Y.A.; Konovalova, I.O.; Radchenko, S.G.; Lapach, S.N.; Bassarskaya, E.M.; Kochetovaa, G.V.; Zhigalova, T.V.; Yakovlevad, O.T.; Tarakanov, I.G. Optimizing LED lighting for space plant growth unit: Joint effects of photon flux density, red to white ratios and intermittent light pulses. Life Sci. Space Res. 2016, 11, 29–42. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Ke, D.; Saltveit, M.E. Wound-induced ethylene production, phenolic metabolism and susceptibility to russet spotting in iceberg lettuce. Physiol. Plant. 1989, 76, 412–418. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Bulgari, R.; Cola, G.; Ferrante, A.; Franzoni, G.; Mariani, L.; Martinetti, L. Micrometeorological environment in traditional and photovoltaic greenhouses and effects on growth and quality of tomato (Solanum lycopersicum L.). Ital. J. Agrometeorol. 2015, 20, 27–38. [Google Scholar]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Qin, L.; Guo, S.; Ai, W.; Tang, Y. Selection of candidate salad vegetables for controlled ecological life support system. Adv. Space Res. 2008, 41, 768–772. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Gianino, D. An Adaptive Control Approach for Light-emitting Diode Lights Can Reduce the Energy Costs of Supplemental Lighting in Greenhouses. HortScience 2017, 52, 72–77. [Google Scholar] [CrossRef]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of temporally shifted irradiation of blue and red LED light on cos lettuce growth and morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant. 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A.; Cristino, M. Monitoring of nitrites and nitrates levels in leafy vegetables (spinach and lettuce): A contribution to risk assessment. J. Sci. Food Agric. 2014, 94, 773–778. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Samuoliené, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartaccia, F.M.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortic. Sci. 2010, 45, 1809–1814. [Google Scholar] [CrossRef]





| Cycle | Length (days) | Relative Humidity (RH %) | Light Period Temperature (°C) | Dark Period Temperature (°C) | Photosynthetic Photon Flux Density (PPFD) (µmol m−2 s−1) | Hours of Light | R:G + Y:B (%) * | Light Spectrum (nm) | Energy Consumption (Wh) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 50 ± 2 | 22 ± 1 | 19 ± 1 | 194.54 | 6 am–10 pm | 69.9:17.0:13.1 | 460; 470; 590; 630; 670 | 128 ± 1 |
| 2 | 31 | 50 ± 2 | 22 ± 1 | 19 ± 1 | 151.41 | 6 am–8 pm | 73.0:20.1:6.9 | 460; 470; 590; 630; 670; White 4000k | 120 ± 1 |
| 3 | 27 | 50 ± 2 | 20 ± 1 | 17 ± 1 | 165.89 | 6 am–8 pm | 77.1:17.9:5 | 460; 470; 590; 630; 670; White 4000k | 116 ± 1 |
| 4 | 28 | 50 ± 2 | 20 ± 1 | 17 ± 1 | 165.89/63.2 ** | 6 am–8 pm | 77.1:17.9:5/79.1:16.6:4.3 ** | 460; 470; 590; 630; 670; White 4000k | 116/70 ± 1 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loconsole, D.; Cocetta, G.; Santoro, P.; Ferrante, A. Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System. Sustainability 2019, 11, 841. https://doi.org/10.3390/su11030841
Loconsole D, Cocetta G, Santoro P, Ferrante A. Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System. Sustainability. 2019; 11(3):841. https://doi.org/10.3390/su11030841
Chicago/Turabian StyleLoconsole, Danilo, Giacomo Cocetta, Piero Santoro, and Antonio Ferrante. 2019. "Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System" Sustainability 11, no. 3: 841. https://doi.org/10.3390/su11030841
APA StyleLoconsole, D., Cocetta, G., Santoro, P., & Ferrante, A. (2019). Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System. Sustainability, 11(3), 841. https://doi.org/10.3390/su11030841







