Fragmented Riverine Habitats in Taiwan Have Spatio-Temporal Consequences, Re-Distributing Caprimulgus affinis into Urban Areas Leading to a Human–Wildlife Conflict
Abstract
:1. Introduction
2. Materials and Methods
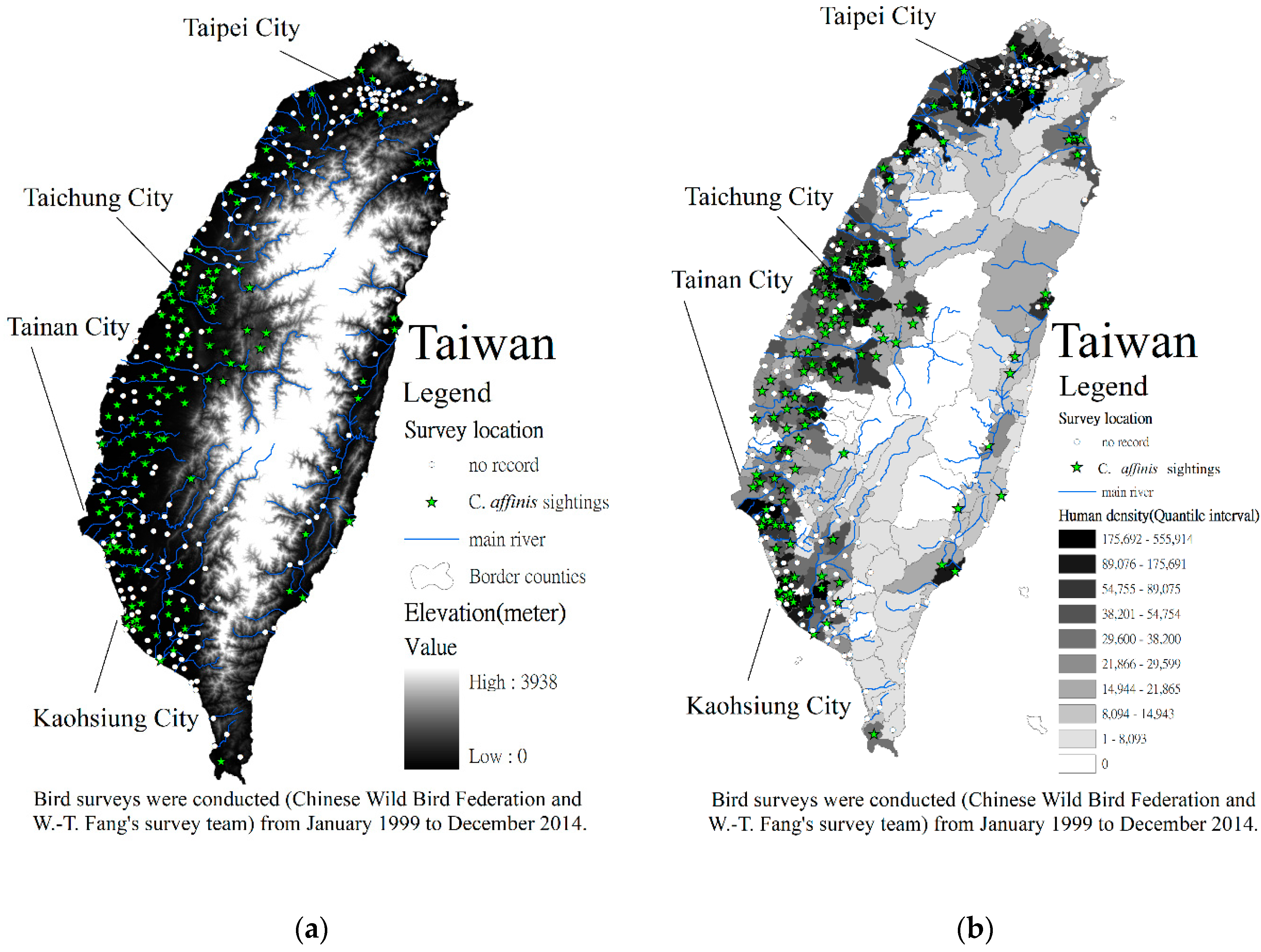
2.1. Study Area
2.2. Methods
3. Results and Discussion
3.1. Driving Forces of Diversity Detected Using Logistic Regression
3.2. Estimation of C. affinis Space Distribution Using Indicator Kriging Method
3.3. Estimation of C. affinis Space Distribution Using Regression Kriging Method
3.4. Time–Space Distribution of C. affinis Sightings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Names of All Rivers Included in This Survey. | ||||
| No. | River Names | Locations | Length of Riparian Section Monitored | Points of the Compass |
| 1 | Dongshan | Yilan County | 24 km (15 mi) | Northeast, NE |
| 2 | Lanyang | Yilan County | 73 km (45 mi) | NE |
| 3 | Yilan | Yilan County | 25 km (16 mi) | NE |
| 4 | Qingshui | Yilan County | 25 km (16 mi) | NE |
| 5 | Luodong | Yilan County | 21 km (13 mi) | NE |
| 6 | Shuang | New Taipei City | 27 km (17 mi) | North, N |
| 7 | Tamsui | New Taipei City, Taoyuan City, Taipei City, Hsinchu County | 159 km (99 mi) | N |
| 8 | Keelung | New Taipei City, Taipei City, Keelung City | 89.4 km (55.6 mi) | N |
| 9 | Xindian | New Taipei City, Taipei City | 82 km (51 mi) | N |
| 10 | Jingmei | New Taipei City, Taipei City | 28.1 km (17.5 mi) | N |
| 11 | Beishi | New Taipei City, Yilan County | 50 km (31 mi) | N |
| 12 | Nanshi | New Taipei City | 45 km (28 mi) | N |
| 13 | Dahan | New Taipei City, Taoyuan City, Hsinchu County | 135 km (84 mi) | N |
| 14 | Sanxia | New Taipei City | 22.6 km (14.0 mi) | N |
| 15 | Nankan | Taoyuan City | 31 km (19 mi) | Northwest, NW |
| 16 | Fengshan | Hsinchu County | 45 km (28 mi) | NW |
| 17 | Touqian | Hsinchu County | 63 km (39 mi) | NW |
| 18 | Houlong | Miaoli County | 58 km (36 mi) | West |
| 19 | Da’an | Miaoli County, Taichung City | 96 km (60 mi) | W |
| 20 | Dajia | Taichung City | 124 km (77 mi) | W |
| 21 | Dadu | Taichung City, Changhua County, Nantou County | 116 km (72 mi) | W |
| 22 | Maoluo | Taichung City, Changhua County, Nantou County | 47 km (29 mi) | W |
| 23 | Nankang | Taichung City | 34 km (21 mi) | W |
| 24 | Zhuoshui | Nantou County, Changhua County, Yunlin County | 186 km (116 mi) | W |
| 25 | Qingshui | Taichung City, Chiayi County, Tainan City | 51 km (32 mi) | W |
| 26 | Shuili | Taichung City | 19 km (12 mi) | W |
| 27 | Wanta | Taichung City | 37 km (23 mi) | W |
| 28 | Kashe | Nantou County | 47 km (29 mi) | W |
| 29 | Beigang | Yunlin County, Chiayi County | 82 km (51 mi) | W |
| 30 | Tahukou | Yunlin County | 33 km (21 mi) | W |
| 31 | Sandie | Chiayi County | 35 km (22 mi) | W |
| 32 | Puzi | Chiayi County | 75 km (47 mi) | W |
| 33 | Bazhang | Chiayi County, Tainan City | 81 km (50 mi) | W |
| 34 | Touqian | Tainan City | 28 km (17 mi) | Southwest, SW |
| 35 | Jishui | Tainan City | 65 km (40 mi) | SW |
| 36 | Guichong | Tainan City | 35 km (22 mi) | SW |
| 37 | Baishui | Tainan City | 20 km (12 mi) | SW |
| 38 | Tsengwen | Chiayi County, Tainan City | 130 km (81 mi) | SW |
| 39 | Houku | Nantou County, Tainan City | 51.4 km (31.9 mi) | SW |
| 40 | Yanshui | Tainan City | 41 km (25 mi) | SW |
| 41 | Erren | Tainan City, Kaohsiung City | 65 km (40 mi) | SW |
| 42 | Agongdian | Kaohsiung City | 38 km (24 mi) | SW |
| 43 | Fengshan | Kaohsiung City | 20 km (12 mi) | SW |
| 44 | Dianbao | Kaohsiung City | 32 km (20 mi) | SW |
| 45 | Gaoping | Kaohsiung City, Pingtung County | 171 km (106 mi) | SW |
| 46 | Qishan | Kaohsiung City, Pingtung County | 117 km (73 mi) | SW |
| 47 | Ailiao | Kaohsiung City, Pingtung County | 69 km (43 mi) | SW |
| 48 | Laonong | Kaohsiung City | 136 km (85 mi) | SW |
| 49 | Zhuokou | Kaohsiung City | 50 km (31 mi) | SW |
| 50 | Donggang | Pingtung County | 47 km (29 mi) | South, S |
| 51 | Linbian | Pingtung County | 42 km (26 mi) | S |
| 52 | Beinan | Taitung County | 84 km (52 mi) | East, E |
| 53 | Xiuguluan | Hualien County | 81 km (50 mi) | E |
| 54 | Fuyuan | Hualien County | 28 km (17 mi) | E |
| 55 | Fengping | Hualien County | 37 km (23 mi) | E |
| 56 | Lekuleku | Hualien County | 54 km (34 mi) | E |
| 57 | Hualian | Hualien County | 57 km (35 mi) | E |
| 58 | Mukua | Hualien County | 42 km (26 mi) | E |
| 59 | Shoufeng | Hualien County | 37 km (23 mi) | E |
| 60 | Wanli | Hualien County | 53 km (33 mi) | E |
References
- Swinhoe, R. The ornithology of Formosa, or Taiwan. Ibis 1863, 5, 250–311. [Google Scholar] [CrossRef]
- Zwart, M.C.; Baker, A.; McGowan, P.J.; Whittingham, M.J. The use of automated bioacoustic recorders to replace human wildlife surveys: An example using nightjars. PLoS ONE 2014, 9, e102770. [Google Scholar] [CrossRef]
- Camacho, C.; Palacios, S.; Sáez, P.; Sánchez, S.; Potti, J. Human-induced changes in landscape configuration influence individual movement routines: Lessons from a versatile, highly mobile species. PLoS ONE 2014, 9, e104974. [Google Scholar] [CrossRef]
- Ganpule, P. Roosting behaviour of Franklin’s Nightjar Caprimulgus affinis. Indian BIRDS 2010, 6, 92–94. [Google Scholar]
- MacKinnon, J.; Phillipps, K. A Field Guide to the Birds of China; Oxford University Press: Oxford, UK, 2000; p. 127. [Google Scholar]
- Myers, S. Birds of Borneo, 2nd ed.; Bloomsbury: London, UK, 2012; p. 168. [Google Scholar]
- Myers, S. Wildlife of Southeast Asia; Princeton University Press: Princeton, NJ, USA, 2016; p. 78. [Google Scholar]
- Sharps, K.; Henderson, I.; Conway, G.; Armour-Chelu, N.; Dolman, P.M. Home-range size and habitat use of European Nightjars Caprimulgus europaeus nesting in a complex plantation-forest landscape. Ibis 2015, 157, 260–272. [Google Scholar] [CrossRef]
- Lowe, A.; Rogers, A.; Durrant, K. Effect of human disturbance on long-term habitat use and breeding success of the European Nightjar, Caprimulgus europaeus. Avian Conserv. Ecol. 2014, 9. [Google Scholar] [CrossRef]
- Brigham, R.M.; Debus, S.J.; Geiser, F. Cavity selection for roosting, and roosting ecology of forest-dwelling Australian Owlet-nightjars (Aegotheles cristatus). Aust. J. Ecol. 1998, 23, 424–429. [Google Scholar] [CrossRef]
- Vilella, F.J.; Zwank, P.J. Geographic Distribution and Abundance of the Puerto Rican Nightjar (Distribución Geográfica y Abundancia del Guabairo Pequeño de Puerto Rico (Caprimulgus noctitherus). J. Field Ornithol. 1993, 223–238. [Google Scholar]
- Langston, R.; Liley, D.; Murison, G.; Woodfield, E.; Clarke, R. What effects do walkers and dogs have on the distribution and productivity of breeding European Nightjar Caprimulgus europaeus? Ibis 2007, 149, 27–36. [Google Scholar] [CrossRef]
- Delannoy, C.A. First nesting records of the Puerto Rican Nightjar and Antillean Nighthawk in a montane forest of western Puerto Rico. J. Field Ornithol. 2005, 76, 271–273. [Google Scholar] [CrossRef]
- Manegold, A. First evidence for a nightjar (Caprimulgidae Aves) in the early Pliocene of Langebaanweg, South Africa. Palaeobiodivers. Palaeoenviron. 2010, 90, 163–168. [Google Scholar] [CrossRef]
- Brigham, R.M.; Körtner, G.; Maddocks, T.A.; Geiser, F. Seasonal use of torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Physiol. Biochem. Zool. 2000, 73, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Doucette, L.I.; Brigham, R.M.; Pavey, C.R.; Geiser, F. Roost type influences torpor use by Australian owlet-nightjars. Naturwissenschaften 2011, 98, 845. [Google Scholar] [CrossRef]
- Harvey, W.G. Caprimulgus affinis as an urban species in Indonesia. Bull. Br. Ornithol. Club 1976, 96, 122–123. [Google Scholar]
- Hayes, W.K.; Bracey, E.D.; Price, M.R.; Robinette, V.; Gren, E.; Stahala, C. Population Status of Chuck-will’s-widow (Caprimulgus carolinensis) in the Bahamas. Wilson J. Ornithol. 2010, 122, 381–384. [Google Scholar] [CrossRef]
- Ashdown, R.A.; McKechnie, A.E. Environmental correlates of Freckled Nightjar (Caprimulgus tristigma) activity in a seasonal, subtropical habitat. J. Ornithol. 2008, 149, 615–619. [Google Scholar] [CrossRef]
- Clay, R.P.; López-Lanús, B.; Tobias, J.A.; Lowen, J.C.; Mazar Barnett, J. The display of the White-winged Nightjar. J. Field Ornithol. 2000, 71, 619–626. [Google Scholar] [CrossRef]
- Pratt, T.K. Evidence for a previously unrecognized species of Owlet-nightjar. Auk 2000, 117, 1–11. [Google Scholar] [CrossRef]
- Jiguet, F.; Williamson, T. Estimating local population size of the European Nightjar Caprimulgus europaeus using territory capture–recapture models. Bird Study 2010, 57, 509–514. [Google Scholar] [CrossRef]
- Perrins, C.; Crick, H. Influence of lunar cycle on laying dates of European Nightjars (Caprimulgus europaeus). Auk 1996, 113, 705–708. [Google Scholar] [CrossRef]
- Wichmann, G. Habitat use of nightjar (Caprimulgus europaeus) in an Austrian pine forest. J. Ornithol. 2004, 145, 69–73. [Google Scholar] [CrossRef]
- Fang, W.-T.; Cheng, B.-Y.; Shih, S.-S.; Chou, J.-Y.; Otte, M.L. Modeling driving forces of avian diversity in a spatial configuration surrounded by farm ponds. Paddy Water Environ. 2016, 14, 185–197. [Google Scholar] [CrossRef]
- Jonsson, M.; Strasevicius, D.; Malmqvist, B. Influences of river regulation and environmental variables on upland bird assemblage in northern Sweden. Ecol. Res. 2012, 27, 945–954. [Google Scholar] [CrossRef]
- Chiu, M.-C.; Kuo, M.-H.; Sun, Y.-H.; Hong, S.-Y.; Kuo, H.-C. Effects of flooding on avian top-predators and their invertebrate prey in a monsoonal Taiwan stream. Freshw. Biol. 2008, 53, 1335–1344. [Google Scholar] [CrossRef]
- Royan, A.; Hannah, D.M.; Reynolds, S.M.; Noble, D.G.; Sadler, J.P. Avian community responses to variability in river hydrology. PLoS ONE 2013, 8, e83221. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.B. Nonparametric tests against trend. Econom. J. Econom. Soc. 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Deutsch, C.V.; Journel, A.G. GSLIB: Geostatistical Software Library and User’s Guide. Hauptbd, 2nd ed.; Oxford University Press: New York, NY, USA, 1992; 340p. [Google Scholar]
- Hengl, T.; Heuvelink, G.B.; Rossiter, D.G. About regression-kriging: From equations to case studies. Comput. Geosci. 2007, 33, 1301–1315. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Cheng, B.-Y.; Chu, H.-J.; Chang, T.-K.; Yu, H.-L. Assessing how heavy metal pollution and human activity are related by using logistic regression and kriging methods. Geoderma 2011, 163, 275–282. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Cheng, B.-Y.; Shyu, G.-S.; Chang, T.-K. Combining a finite mixture distribution model with indicator kriging to delineate and map the spatial patterns of soil heavy metal pollution in Chunghua County, Central Taiwan. Environ. Pollut. 2010, 158, 235–244. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Chang, C.-R.; Chu, H.-J.; Cheng, B.-Y. Identifying the spatial mixture distribution of bird diversity across urban and suburban areas in the metropolis: A case study in Taipei Basin of Taiwan. Landsc. Urb. Plan. 2011, 102, 156–163. [Google Scholar] [CrossRef]
- Hirsch, R.M.; Slack, J.R.; Smith, R.A. Techniques of trend analysis for monthly water quality data. Water Resour. Res. 1982, 18, 107–121. [Google Scholar] [CrossRef]
- Cheng, B.-Y.; Liu, T.-C.; Shyu, G.-S.; Chang, T.-K.; Fang, W.-T. Analysis of trends in water quality: Constructed wetlands in metropolitan Taipei. Water Sci. Technol. 2011, 64, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Taipei Times. Chen Chu Calls for Increased Campus Security. Available online: http://www.taipeitimes.com/News/taiwan/archives/2010/06/01/2003474390 (accessed on 1 June 2010).
- Brazil, M. Birds of East Asia: China, Taiwan, Korea, Japan, and Russia; Princeton University Press: Princeton, NJ, USA, 2009; p. 270. [Google Scholar]
- Maa, T.-C.; Kuo, J.-S. Catalogue and bibliography of ticks and mites on vertebrates in Taiwan. Quart. J. Taiwan Mus. 1966, 19, 373–413. [Google Scholar]
- Hsu, F.-H.; Yao, C.-T.; Lin, S.R.-S.; Yang, C.-C.; Lai, S.-J. Avian species composition and distribution along elevation gradient in the Southern Taiwan. Endem. Species Res. 2004, 6, 41–66. [Google Scholar]
- Chen, C.-H.; Den, W. The value of green belts in urban sprawl: A case study of Taichung city, Taiwan. Int. J. GEOMATE 2017, 12, 147–152. [Google Scholar] [CrossRef]
- Jathar, G.; Anpat, S.; Bhangare, B.; Patil, D.J. Unusual congregation of Savaana nightjar, Caprimulgus affinis in a wheat field. J. Bombay Nat. Hist. Soc. 2014, 111, 138. [Google Scholar] [CrossRef]
- Chavan, S.P.; Jondhale, S.; Walke, D.; Jadhav, P. Habitat for camouflage is priority in preference besides harsh physical conditions in three species of Nightjar (Aves: Caprimulgiformes). Int. J. Fauna Biolog. Stud. 2017, 4, 5–10. [Google Scholar]





| Intercept | Standard Error | Wald b | Degrees of Freedom | Sig. c | Exponentiation of the Intercept (B) Coefficient | |
|---|---|---|---|---|---|---|
| Urban population | 0.003 | 0.001 | 5.46 | 1 | 0.019 * | 1.003 |
| Coastal proximity (the distance to the nearest ocean coast) | 0.069 | 0.014 | 22.35 | 1 | 0.000 *** | 1.071 |
| City elevation | −0.005 | 0.002 | 8.08 | 1 | 0.004 ** | 0.995 |
| Distance to a main river location a | - | - | - | - | - | - |
| Distance to an upstream river location | 0.000 | 0.000 | 12.95 | 1 | 0.000 *** | 1.000 |
| Constant | −2.677 | 0.506 | 27.93 | 1 | 0.000 | 0.069 |
| Model Type | C0 | C0 + C | Range | R2 | C/C0 + C | RSS | |
|---|---|---|---|---|---|---|---|
| First situational category | Exponential | 0.0063 | 0.2046 | 10,800 | 0.907 | 0.969 | 4.7E–4 |
| Second situational category | Exponential | 0.0116 | 0.0233 | 94,500 | 0.759 | 0.502 | 4.4E–4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.-Y.; Shyu, G.-S.; Wu, S.-C.; Lin, H.-H.; Hsu, C.-H.; LePage, B.A.; Fang, W.-T. Fragmented Riverine Habitats in Taiwan Have Spatio-Temporal Consequences, Re-Distributing Caprimulgus affinis into Urban Areas Leading to a Human–Wildlife Conflict. Sustainability 2019, 11, 1778. https://doi.org/10.3390/su11061778
Cheng B-Y, Shyu G-S, Wu S-C, Lin H-H, Hsu C-H, LePage BA, Fang W-T. Fragmented Riverine Habitats in Taiwan Have Spatio-Temporal Consequences, Re-Distributing Caprimulgus affinis into Urban Areas Leading to a Human–Wildlife Conflict. Sustainability. 2019; 11(6):1778. https://doi.org/10.3390/su11061778
Chicago/Turabian StyleCheng, Bai-You, Guey-Shin Shyu, Shi-Ching Wu, Hsiao-Hsien Lin, Chia-Hsuan Hsu, Ben A. LePage, and Wei-Ta Fang. 2019. "Fragmented Riverine Habitats in Taiwan Have Spatio-Temporal Consequences, Re-Distributing Caprimulgus affinis into Urban Areas Leading to a Human–Wildlife Conflict" Sustainability 11, no. 6: 1778. https://doi.org/10.3390/su11061778
APA StyleCheng, B.-Y., Shyu, G.-S., Wu, S.-C., Lin, H.-H., Hsu, C.-H., LePage, B. A., & Fang, W.-T. (2019). Fragmented Riverine Habitats in Taiwan Have Spatio-Temporal Consequences, Re-Distributing Caprimulgus affinis into Urban Areas Leading to a Human–Wildlife Conflict. Sustainability, 11(6), 1778. https://doi.org/10.3390/su11061778









