Grazing Exclusion, a Choice between Biomass Growth and Species Diversity Maintenance in Beijing—Tianjin Sand Source Control Project
Abstract
:1. Introduction
2. Materials and Methods
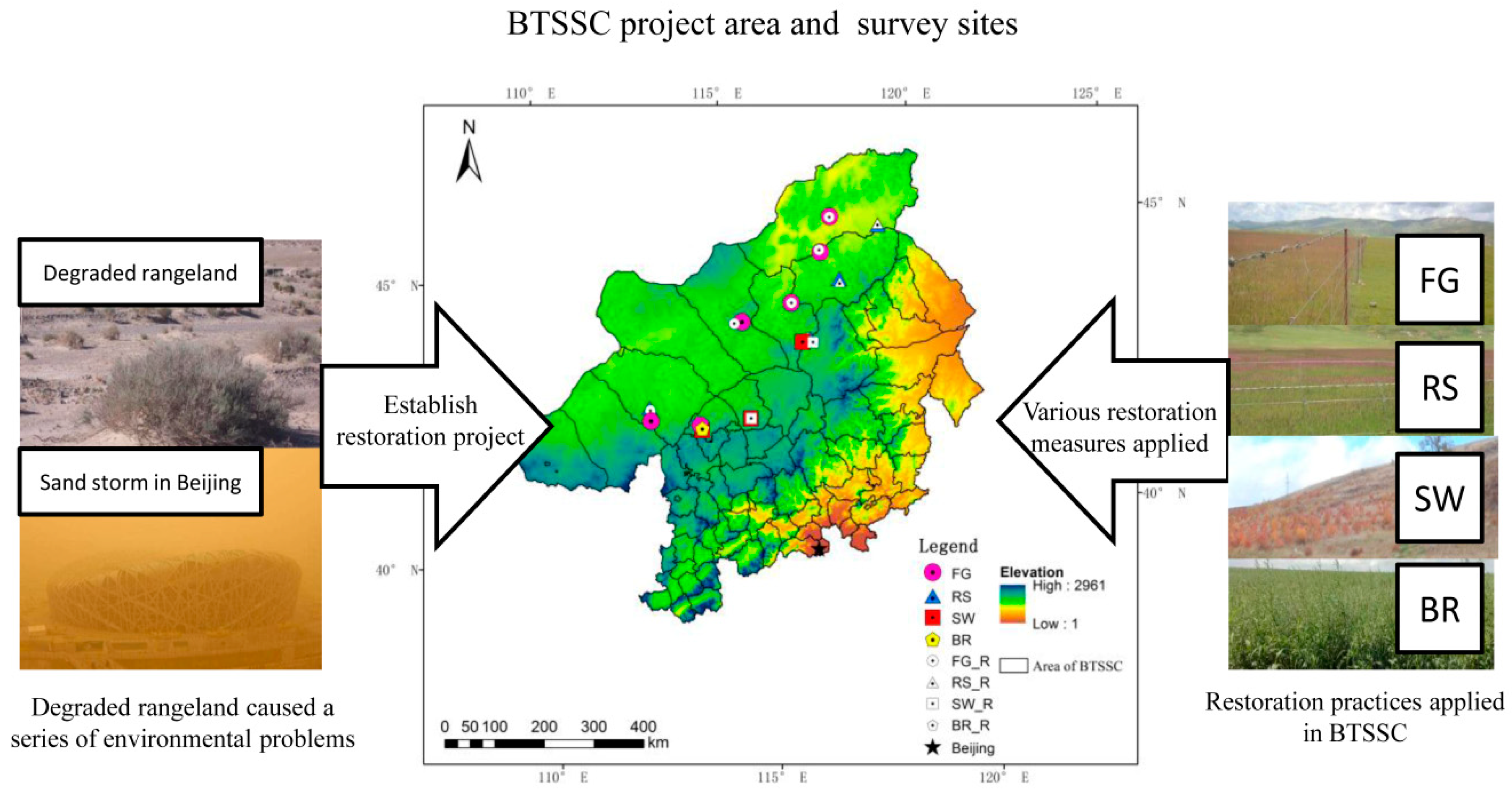
2.1. Study Area Description
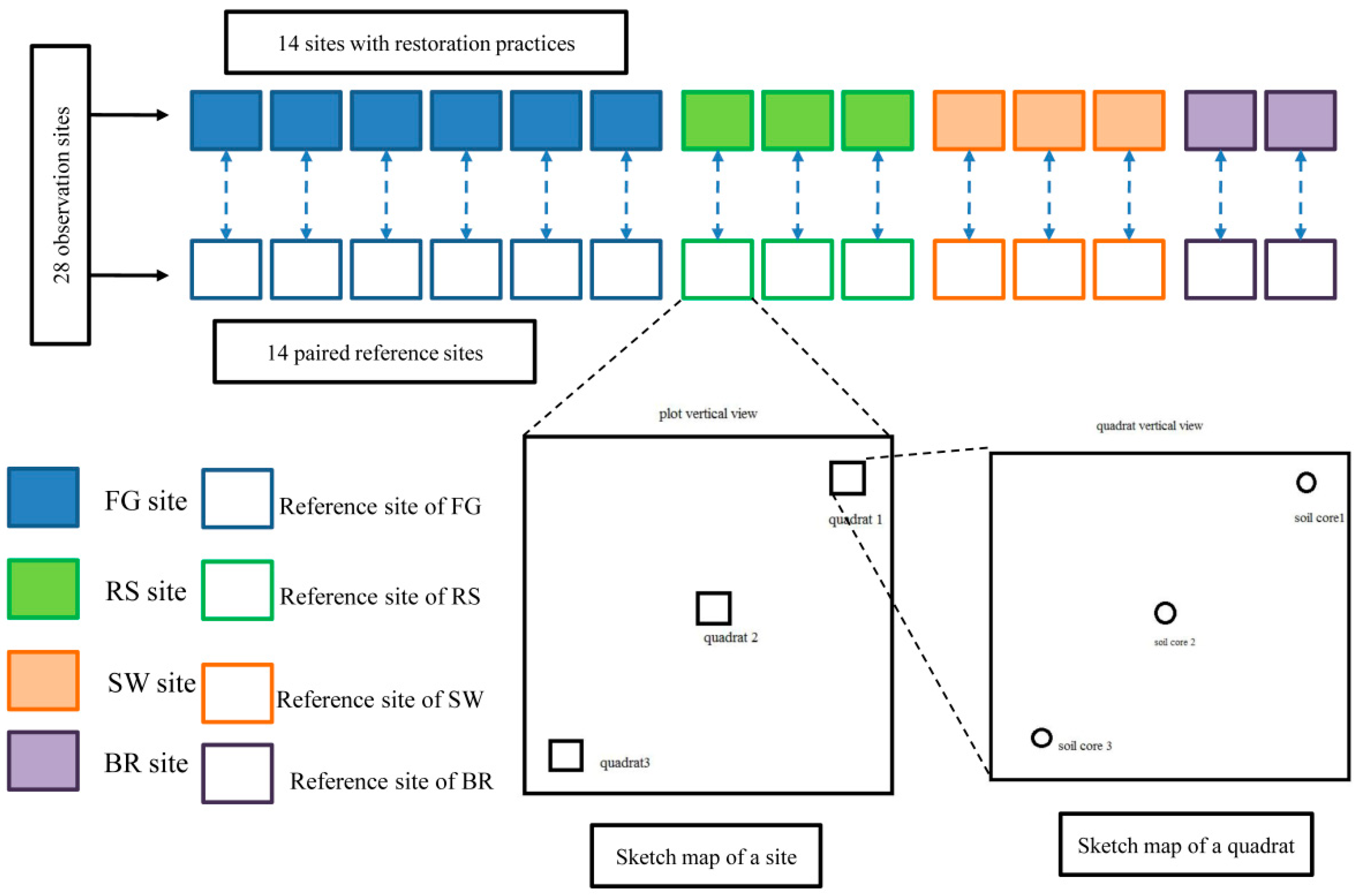
2.2. Plot Settings
2.3. Measures Applied in Restoration Practices
2.4. Community Features and Vegetation Analyses
2.5. Measurement of Above- and Below-Ground Biomass
2.6. Calculation of Species Diversity Indices
2.7. Data Analysis
3. Results
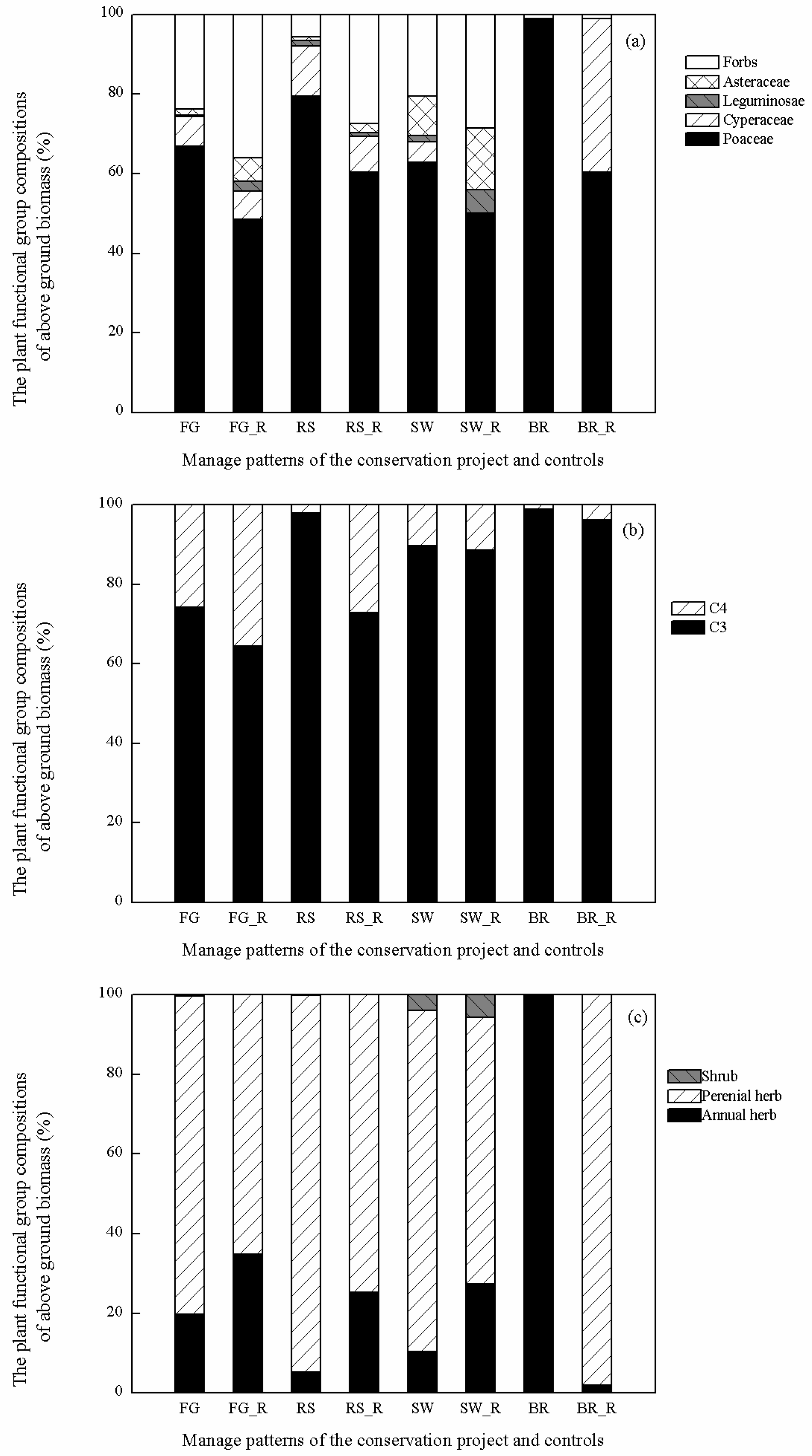
3.1. Influence of Restoration Practices on Community Structural Characteristics and Species Composition
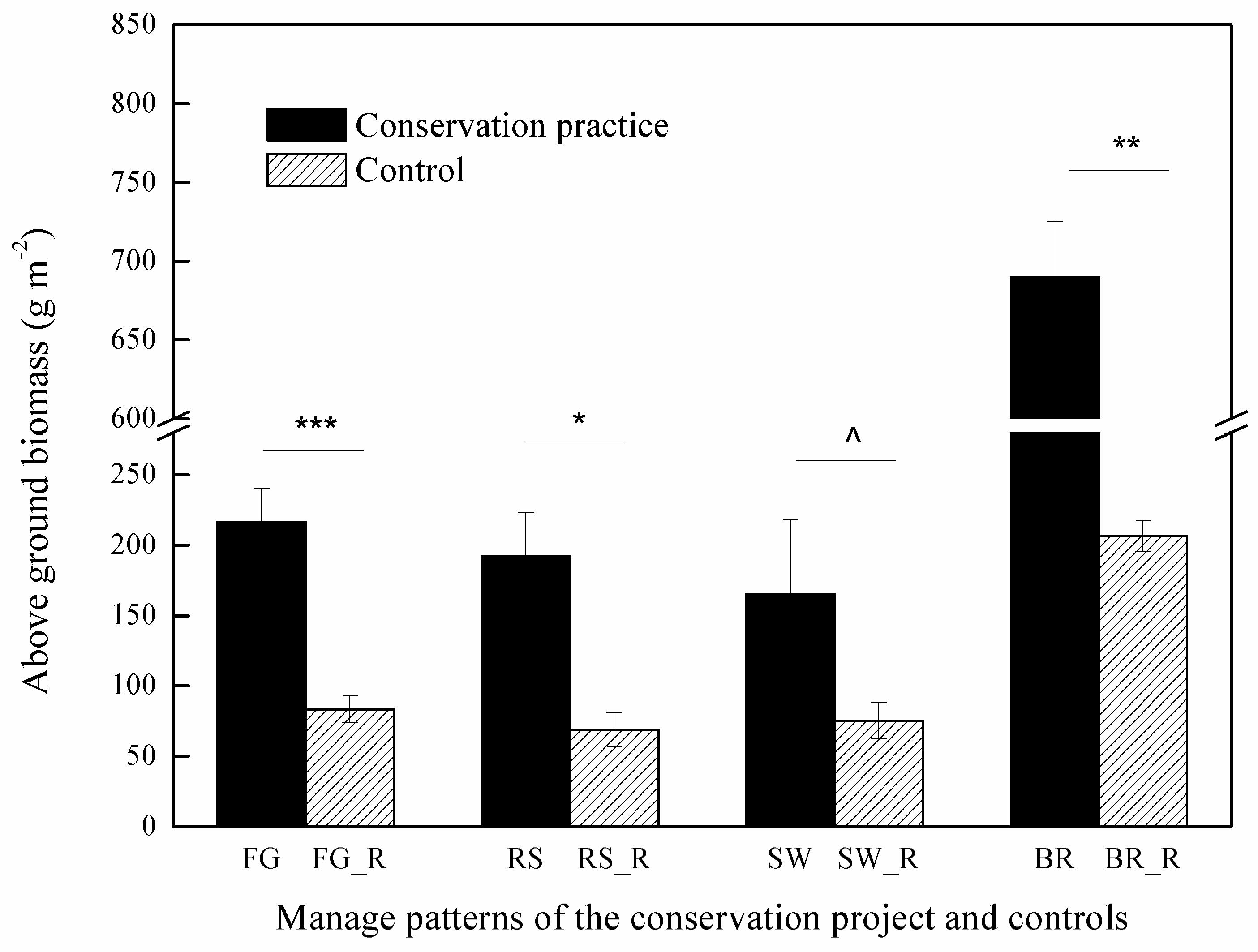
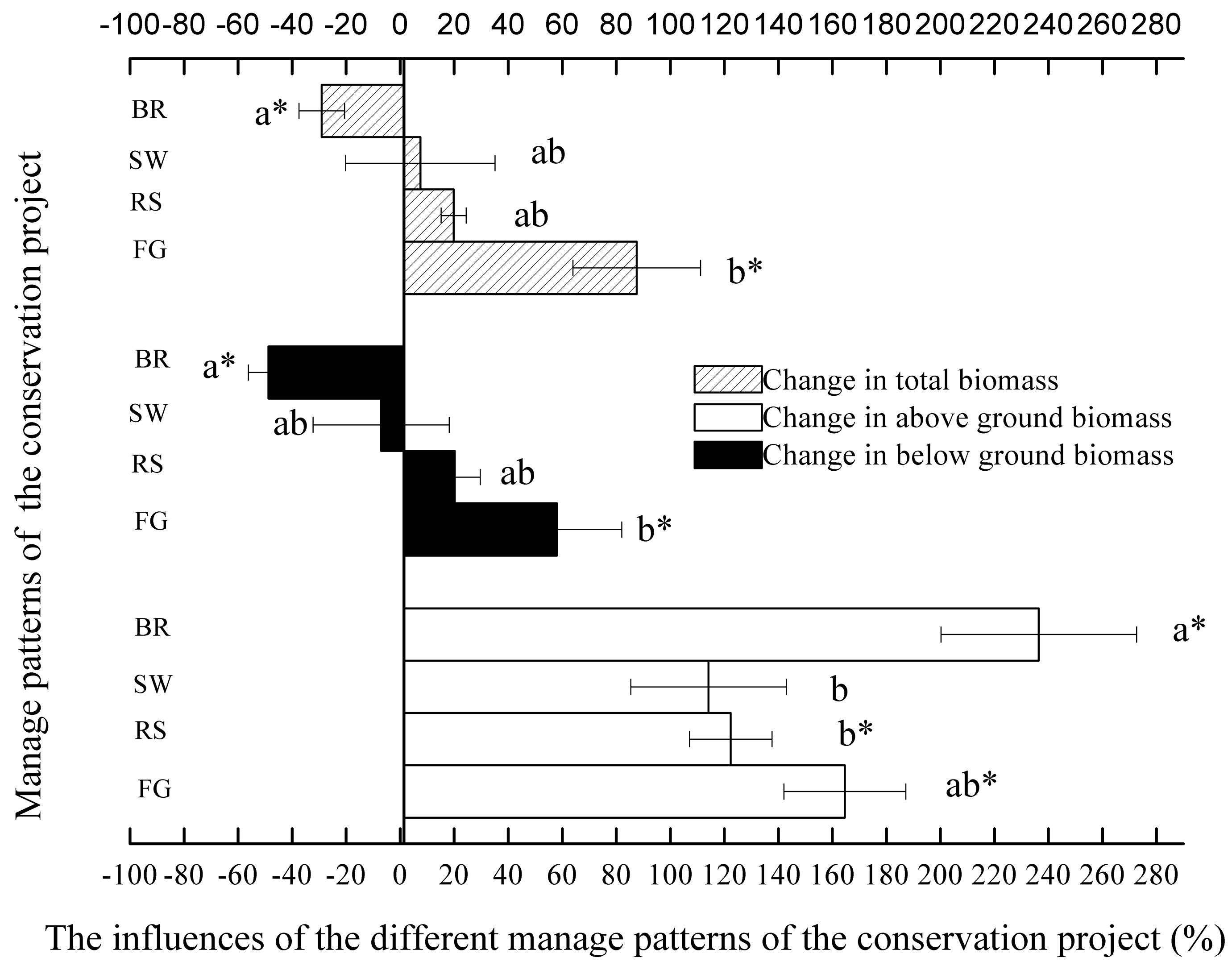
3.2. Influence of Restoration Practices on Above-Ground Biomass
3.3. Influence of Restoration Practices on Below-Ground Biomass
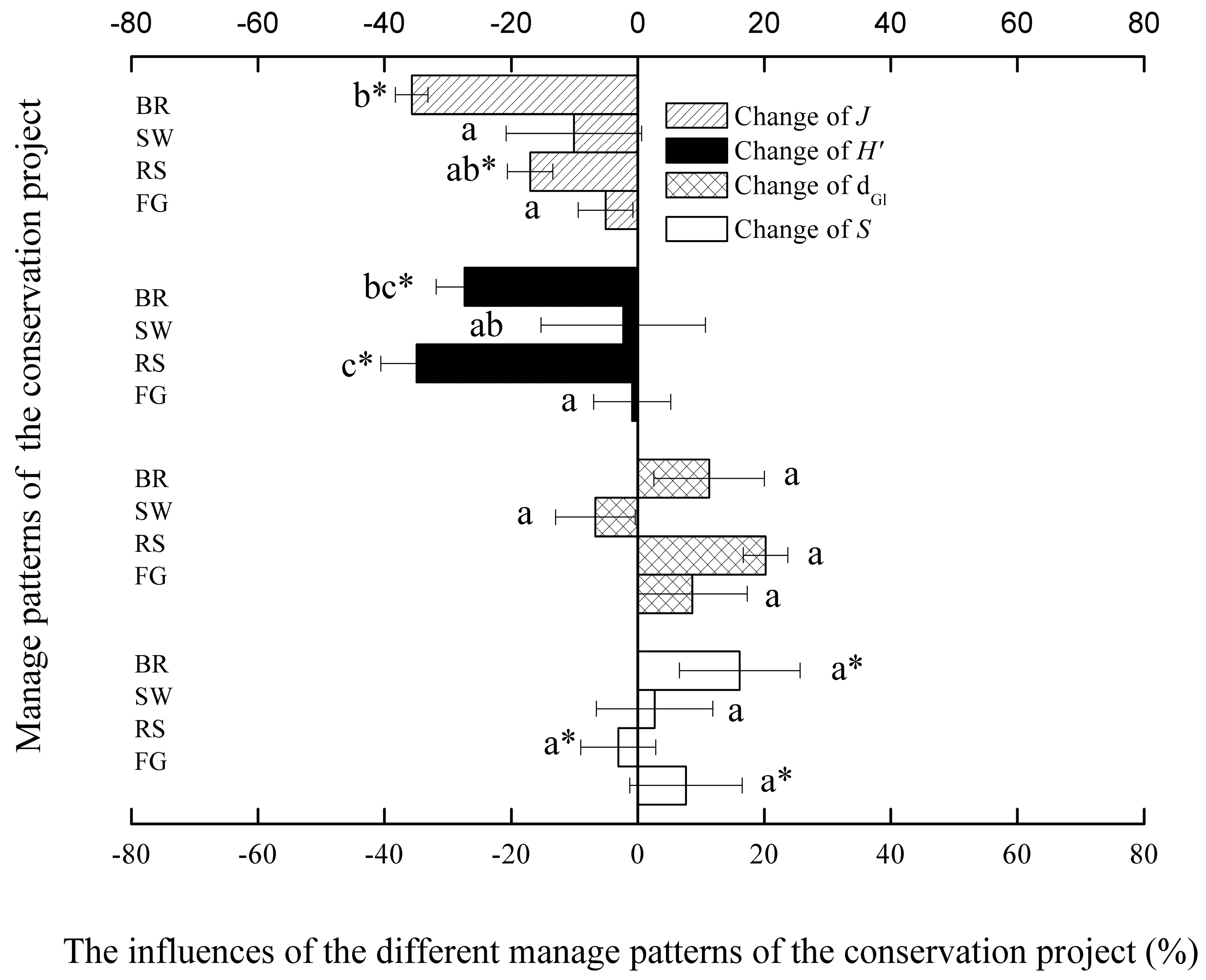
3.4. Influence of Restoration Practices on Species Diversity
3.5. Comparison of Different Effects of the Restoration Practices
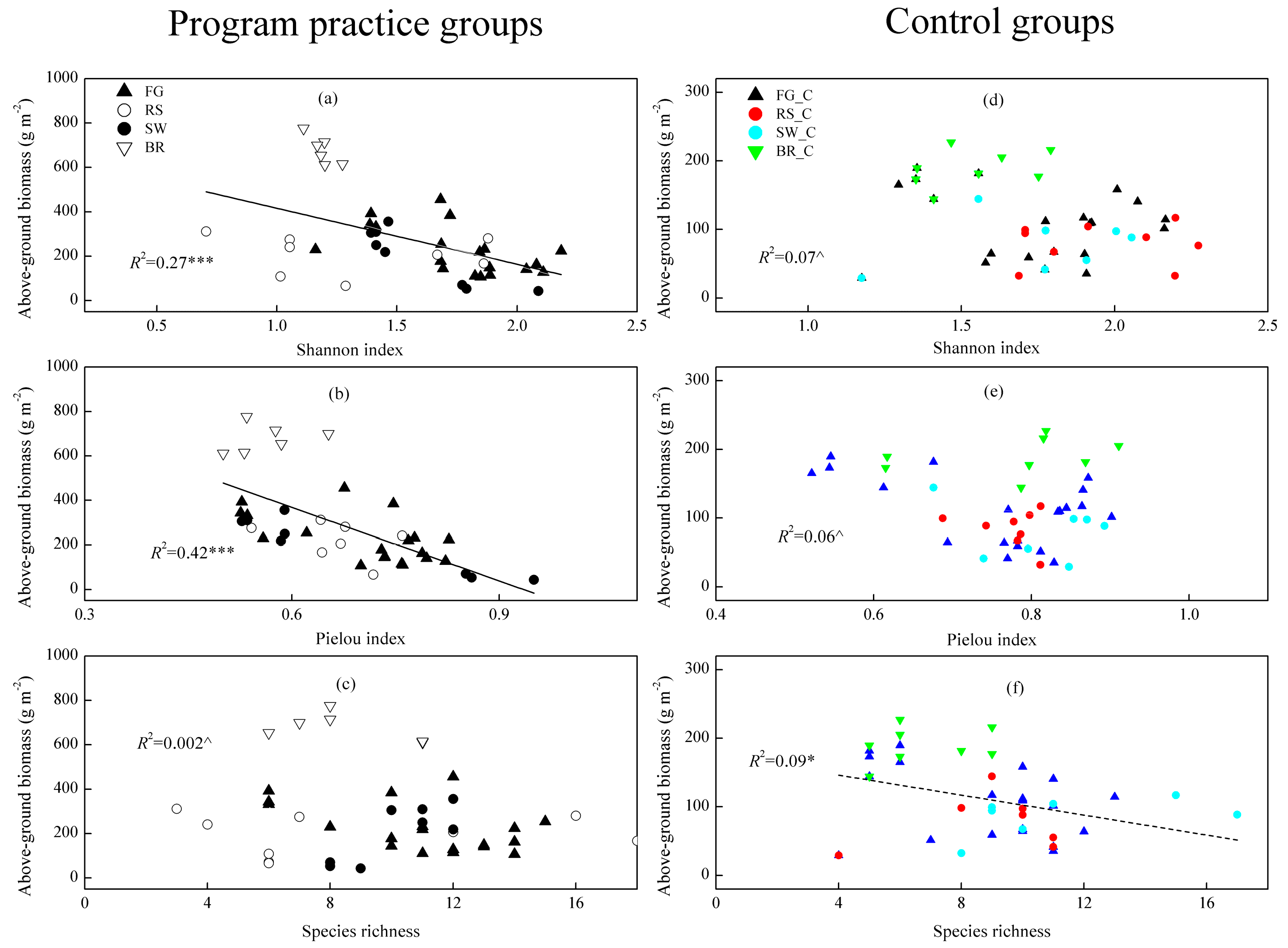
3.6. Relationship between Grassland Biomass and Diversity Inside and Outside the Implementation Areas of the Restoration Practices
4. Discussion
4.1. Influence of Restoration Practices on Plant Community Characteristics and Species Composition
4.2. Different Influence of the Typical Restoration Practices on Productivity
4.3. Influence of Implementing Restoration Practices on Plant Species Diversity
4.4. Implementation of Restoration Practices Alters the Relationship between Above-Ground Biomass and Species Diversity in Grasslands
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Practices | |
| FG | fenced and grazing exclusion |
| RS | seed replenishment |
| SW | integrated control of small watersheds |
| BR | basic ranch construction |
| Reference ecosystems | |
| FG_R | reference ecosystems of fenced and grazing exclusion |
| RS_R | reference ecosystems of seed replenishment |
| SW_R | reference ecosystems of integrated control of small watersheds |
| BR_R | reference ecosystems of basic ranch construction |
| Species diversity indices | |
| S | species richness |
| dGl | Gleason index |
| H’ | Shannon–Wiener index |
| J | Pielou evenness index |
| IV | species importance value |
| Others | |
| BTSSC | Beijing–Tianjin Sand Source Control |
Appendix A
References
- Wu, Z.; Wu, J.; He, B.; Liu, J.; Wang, Q.; Zhang, H.; Liu, Y. Drought offset ecological restoration program-induced increase in vegetation activity in the Beijing-Tianjin Sand Source Region, China. Environ. Sci. Technol. 2014, 48, 12108–12117. [Google Scholar] [CrossRef] [PubMed]
- Török, P.; Ambarlı, D.; Kamp, J.; Wesche, K.; Dengler, J. Step (pe) up! Raising the profile of the Palaearctic natural grasslands. Biodivers. Conserv. 2016, 25, 2187–2195. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, W.; Fan, J. Soil organic carbon dynamics in Xilingol grassland of northern China induced by the Beijing-Tianjin Sand Source Control Program. Front. Earth Sci. 2017, 11, 407–415. [Google Scholar] [CrossRef]
- Szewrański, S.; Świąder, M.; Kazak, J.K.; Katarzyna, T.D.; & Joost, V.H. Socio-environmental vulnerability mapping for environmental and flood resilience assessment: The case of ageing and poverty in the city of Wrocław, poland. Integr. Environ. Assess. Manag. 2018, 14, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.Y.; Wang, Q.; Zhen, H. National Remote Sensing Survey and Assessment of Ecological and Environmental Change (2000–2010); Science press: Beijing, China, 2014. [Google Scholar]
- Bryan, B.A.; Gao, L.; Ye, Y.; Sun, X.; Connor, J.D.; Crossman, N.D.; Stafford-Smith, M.; Wu, J.; He, C.; Yu, D.; et al. China’s response to a national land-system sustainability emergency. Nature 2018, 559, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Mausbach, M.J.; Dedrick, A.R. The length we go. J. Soil Water Conserv. 2004, 59, 96. [Google Scholar]
- Rodgers, R.D.; Hoffman, R.W. Prairie grouse population response to Conservation Reserve Program grasslands: An overview. In Proceedings of the Conservation Reserve Program National Conference, Planting for the Future, Fort Collins, CO, USA, 6–9 June 2004; pp. 120–128. [Google Scholar]
- Schroeder, M.A.; Vander Haegen, W.M. Response of greater sage-grouse to the Conservation Reserve Program in Washington State. Stud. Avian Biol. 2011, 38, 517–529. [Google Scholar]
- Brinson, M.M.; Eckles, S.D. US Department of Agriculture conservation program and practice effects on wetland ecosystem services: A synthesis. Ecol. Appl. 2011, 21, 116–127. [Google Scholar] [CrossRef]
- Fennessy, S.; Craft, C. Agricultural conservation practices increase wetland ecosystem services in the Glaciated Interior Plains. Ecol. Appl. 2011, 21, 46–64. [Google Scholar] [CrossRef]
- Zheng, H.; Robinson, B.E.; Liang, Y.-C.; Polasky, S.; Ma, D.-C.; Wang, F.-C.; Ruckelshaus, M.; Ouyang, Z.-Y.; Daily, G.C. Benefits, costs, and livelihood implications of a regional payment for ecosystem service program. Proc. Natl. Acad. Sci. USA 2013, 110, 16681–16686. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fan, J.; Hu, Z.; Shao, Q.; Harris, W. Comparison of evapotranspiration components and water-use efficiency among different land use patterns of temperate steppe in the Northern China pastoral-farming ecotone. Int. J. Biometeorol. 2016, 60, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, J.; Hu, Z. Comparison of Carbon-Use Efficiency Among Different Land-Use Patterns of the Temperate Steppe in the Northern China Pastoral Farming Ecotone. Sustainability 2018, 10, 487. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Jin, G.H. Responces of species diversity and aboveground biomass to nitrogen addition in fenced and grazed grassland on the Loess Plateau. Acta Prataculturae Sin. 2017, 26, 54–64. [Google Scholar]
- Liu, J.; Li, S.; Ouyang, Z.; Tam, C.; Chen, X. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. USA 2008, 105, 9477–9482. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, J.; Hu, Z.; Shao, Q.; Zhang, L.; Yu, H. Influence of land use patterns on evapotranspiration and its components in a temperate grassland ecosystem. Adv. Meteorol. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Z.; Li, S.; Guo, Q.; Liu, Z.; Zhang, L. Comparison of surface energy budgets and feedbacks to microclimate among different land use types in an agro-pastoral ecotone of northern China. Sci. Total Environ. 2017, 599–600, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Wu, S.H.; Zhao, D.S. Temporal-spatial Changes in Inner Mongolian Grassland Degradation during Past Three Decades. Agric. Sci. Technol. 2013, 4, 676–683. [Google Scholar]
- Pan, X.; Xu, L.; Yang, Z.; Yu, B. Payments for Ecosystem Services in China: Policy, practice, and progress. Ecosyst. Serv. 2017, 21, 109–119. [Google Scholar] [CrossRef]
- Shi, S.; Zou, X.Y.; Zhang, C.L. Investigation of vegetation restoration in the area of Beijing and Tianjin sand storm source control project. Sci. Soil Water Conserv. 2009, 7, 86–92. [Google Scholar]
- Li, Y.Z.; Fan, J.W.; Zhang, L.X. The impact of different land use and management on community composition, species diversity and productivity in a typical temperate grassland. Acta Prataculturae Sin. 2013, 22, 1–10. [Google Scholar]
- Deng, X.; Zhao, C.; Yan, H. Systematic Modeling of Impacts of Land Use and Land Cover Changes on Regional Climate: A Review. Adv. Meteorol. 2013, 2013, 1375–1383. [Google Scholar] [CrossRef]
- Li, X. A review of the international researches on land use/land cover change. Acta Geogr. Sin. 1996, 6, 553–558. [Google Scholar]
- Mu, S.; Zhou, S.; Chen, Y.; Li, J.; Ju, W.; Odeh, I. Assessing the impact of restoration-induced land conversion and management alternatives on net primary productivity in Inner Mongolian grassland, China. Glob. Planet. Chang. 2013, 108, 29–41. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Lin, G.; Zhang, W.; Miao, H.; Wei, L.; Huang, J.; Han, X. Energy balance and partition in Inner Mongolia steppe ecosystems with different land use types. Agric. For. Meteorol. 2009, 149, 1800–1809. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Cao, W.; Zhong, H.; Harris, W.; Gong, G.; Zhang, Y. Changes in multiple ecosystem services between 2000 and 2013 and their driving factors in the Grazing Withdrawal Program, China. Ecol. Eng. 2018, 116, 67–79. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Zhou, D.; Zhang, H. Ecological Protection and Restoration Program Reduced Grazing Pressure in the Three-River Headwaters Region, China. Rangel. Ecol. Manag. 2017, 70, 540–548. [Google Scholar] [CrossRef]
- Börner, J.; Baylis, K.; Corbera, E.; Ezzine-de-Blas, D.; Ferraro, P.J.; Honey-Rosés, J.; Lapeyre, R.; Persson, U.M.; Wunder, S. Emerging Evidence on the Effectiveness of Tropical Forest Conservation. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0159152 (accessed on 1 April 2019).
- Zeng, Q.; An, S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Li, Y.H. Influence of different stocking rates on plant diversity of Artemisia frigida community in Inner Mongolia steppe. Acta Bot. Sin. 2001, 43, 89–96. [Google Scholar]
- Wiesmair, M.; Otte, A.; Waldhardt, R. Relationships between plant diversity, vegetation cover, and site conditions: Implications for grassland conservation in the Greater Caucasus. Biodivers. Conserv. 2016, 26, 1–19. [Google Scholar] [CrossRef]
- Demalach, N.; Kadmon, R. Light competition explains diversity decline better than niche dimensionality. Funct. Ecol. 2017, 31, 1834–1838. [Google Scholar] [CrossRef]
- Mariotte, P.; Spotswood, E.N.; Farrer, E.C.; Suding, K.N. Positive litter feedbacks of an introduced species reduce native diversity and promote invasion in Californian grasslands. Appl. Veg. Sci. 2017, 20, 28–39. [Google Scholar] [CrossRef]
- Casado, M.A.; Castro, I.; Ramírez-Sanz, L.; Costa-Tenorio, M.; De Miguel, J.M.; Pineda, F.D. Herbaceous plant richness and vegetation cover in Mediterranean grasslands and shrublands. Plant. Ecol. 2004, 170, 83–91. [Google Scholar] [CrossRef]
- Pausas, J.G.; Ribeiro, E. Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 2017, 26, 889–897. [Google Scholar] [CrossRef]
- Valkó, O.; Kelemen, A.; Miglécz, T.; Török, P.; Deák, B.; Tóth, K.; Tóth, J.P.; Tóthmérész, B. Litter removal does not compensate detrimental fire effects on biodiversity in regularly burned semi-natural grasslands. Sci. Total Environ. 2018, 622–623, 783–789. [Google Scholar]
- Zhang, L.; Hu, Z.; Fan, J.; Zhou, D.; Tang, F. A meta-analysis of the canopy light extinction coefficient in terrestrial ecosystems. Front. Earth Sci. 2014, 8, 599–609. [Google Scholar] [CrossRef]
- Duffy, J.E.; Godwin, C.M.; Cardinale, B.J. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 2017, 549, 261. [Google Scholar] [CrossRef]
- Kemp, D.; King, W.M.; Gilmour, A.; Lodge, G.; Murphy, S.; Quigley, P.; Sanford, P.; Andrew, M. SGS Biodiversity Theme: Impact of plant biodiversity on the productivity and stability of grazing systems across southern Australia. Aust. J. Exp. Agric. 2003, 43, 961–975. [Google Scholar] [CrossRef]
- Humbert, J.Y.; Dwyer, J.M.; Andrey, A.; Arlettaz, R. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: A systematic review. Glob. Chang. Biol. 2016, 22, 110–120. [Google Scholar] [CrossRef]
- Kemp, D.R.; Guodong, H.; Xiangyang, H.; Michalk, D.L.; Fujiang, H.; Jianping, W.; Yingjun, Z. Innovative grassland management systems for environmental and livelihood benefits. Proc. Natl. Acad. Sci. USA 2013, 110, 8369–8374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demalach, N.; Zaady, E.; Kadmon, R. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 2017, 20, 60–69. [Google Scholar] [CrossRef] [PubMed]

| Land Manage Type | Sites/Plots | Cover (%) | Height (cm) | Species Composition (Constructive Species and Dominant Species) |
|---|---|---|---|---|
| FG | 6/18 | 73.1 ± 5.5 abc | 32.3 ± 3.2 b | Leymus chinensis *, Stipa grandis *, S. sareptana var. krylovii *, C. squarrosa, Kochia prostrata, Allium tenuissimum, Salsola collina |
| FG_R | 6/18 | 48.2 ± 2.3 d | 12.9 ± 1.2 c | S. sareptana var. krylovii *, Agropyron cristatum *, Artemisia frigida *, C. squarrosa *, L. chinensis, S. grandis, Salsola collina |
| RS | 3/9 | 83.3 ± 7.7 ab | 32.8 ± 3.9 b | L. chinensis *, Achnatherum sibiricum *, S. sareptana var. krylovii, A. cristatum, Carex sp. |
| RS_R | 3/9 | 58.9 ± 7.7 bcd | 11.6 ± 1.5 c | S. sareptana var. krylovii *, C. squarrosa *, Thymus mongolicus *, L. chinensis, Carex sp. |
| SW | 3/6 | 76.7 ± 7.5 abc | 22.1 ± 3.6 bc | L. chinensis *, Allium polyrhizum *, Neopallasia pectinata, Astragalus laxmannii, Potentilla bifurca, Salsola sp., Carex sp., Al. tenuissimum |
| SW_R | 3/6 | 51.7 ± 3.8 cd | 7.0 ± 1.6 c | S. sareptana var. krylovii *, P. bifurca *, N. pectinata, Astragalus scaberrimus, L. chinensis, Ar. frigida |
| BR | 2/6 | 98.6 ± 1.3 a | 82.5 ± 1.44 a | Avena sativa *, Setaria sp., Panicum miliaceum, P. bifurca * |
| BR_R | 2/6 | 75.0 ± 3.5 abc | 19.8 ± 0.63 bc | L. chinensis *, Carex sp., S. sareptana var. krylovii, A. cristatum |
| Land Use Type | FG | FG_R | RS | RS_R | SW | SW_R | BR | BR_R |
|---|---|---|---|---|---|---|---|---|
| Species richness S | 11.6 ± 0.4 * | 10.0 ± 0.5 | 8.6 ± 1.7 ** | 10.7 ± 1.2 | 10.0 ± 0.8 ^ | 9.0 ± 1. | 9.5 ± 0.8 * | 7.5 ± 0.8 |
| Gleason index dGl | 7.7 ± 0.7 ^ | 7.2 ± 0.6 | 11.4 ± 3.4 ^ | 13.1 ± 4.7 | 13.7 ± 2.4 ^ | 8.9 ± 1.5 | 6.8 ± 0.7 ^ | 6.2 ± 1.3 |
| Shannon-Wiener index H’ | 1.82 ± 0.06 ^ | 1.84 ± 0.06 | 1.28 ± 0.14 *** | 1.95 ± 0.08 | 1.66 ± 0.11 ^ | 1.78 ± 0.13 | 1.19± 0.03 * | 1.66 ± 0.07 |
| Pielou index J | 0.74 ± 0.02 ^ | 0.79 ± 0.02 | 0.64 ± 0.02bc ** | 0.78 ± 0.04 | 0.74 ± 0.07 ^ | 0.83 ± 0.02 | 0.53 ± 0.02 ** | 0.84 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fan, J.; Yu, H. Grazing Exclusion, a Choice between Biomass Growth and Species Diversity Maintenance in Beijing—Tianjin Sand Source Control Project. Sustainability 2019, 11, 1941. https://doi.org/10.3390/su11071941
Li Y, Fan J, Yu H. Grazing Exclusion, a Choice between Biomass Growth and Species Diversity Maintenance in Beijing—Tianjin Sand Source Control Project. Sustainability. 2019; 11(7):1941. https://doi.org/10.3390/su11071941
Chicago/Turabian StyleLi, Yuzhe, Jiangwen Fan, and Hailing Yu. 2019. "Grazing Exclusion, a Choice between Biomass Growth and Species Diversity Maintenance in Beijing—Tianjin Sand Source Control Project" Sustainability 11, no. 7: 1941. https://doi.org/10.3390/su11071941





