Effects of Physico-Chemical Parameters on Actinomycetes Communities during Composting of Agricultural Waste
Abstract
:1. Introduction
2. Material and Methods
2.1. Composting and Sampling
2.2. Physico-Chemical Parameters Analyses
2.3. DNA Extraction and PCR-DGGE
2.4. qPCR Analysis
2.5. Data Analysis
3. Results and Discussion
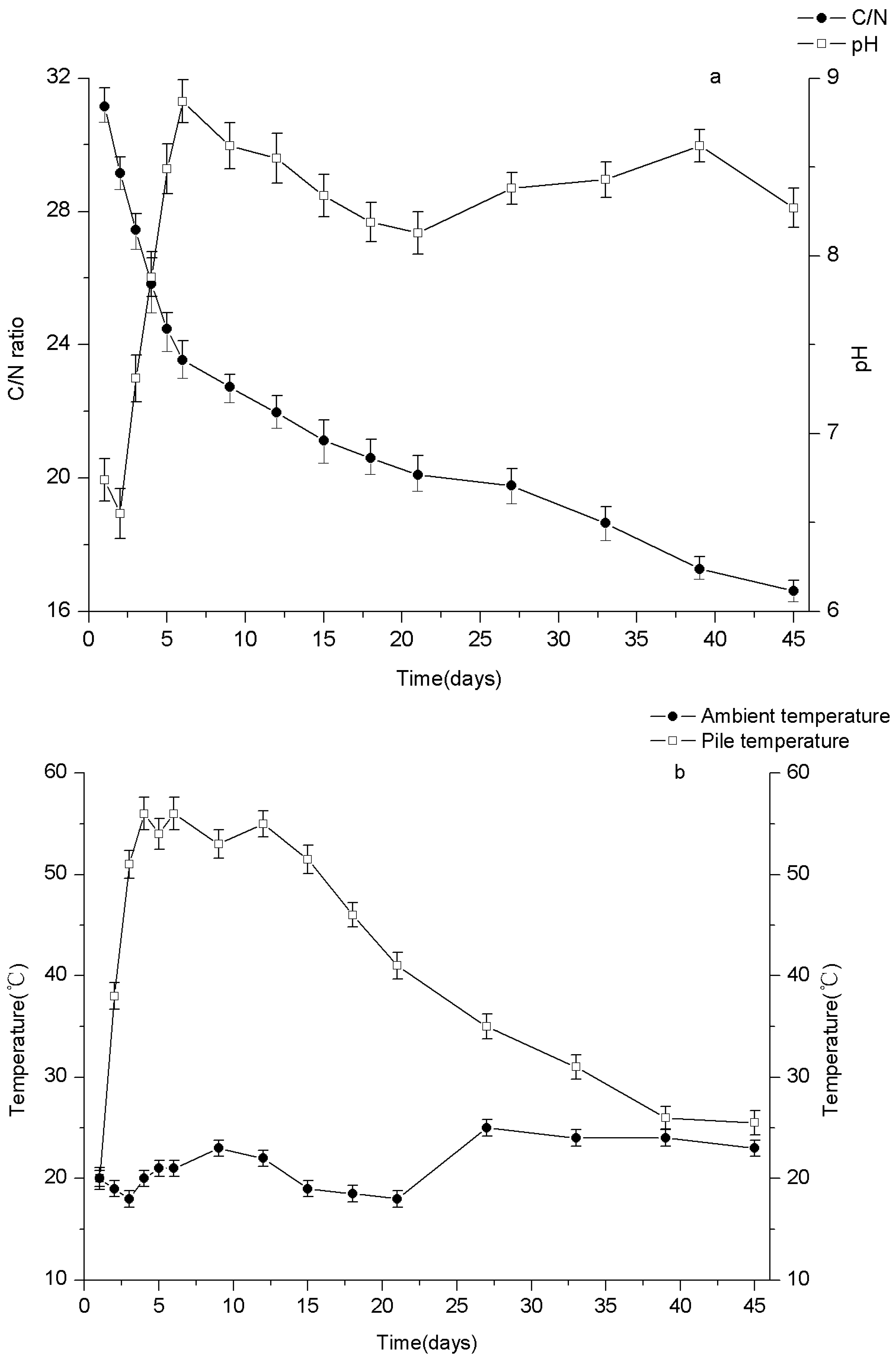
3.1. The Changes of Physico-Chemical Parameters
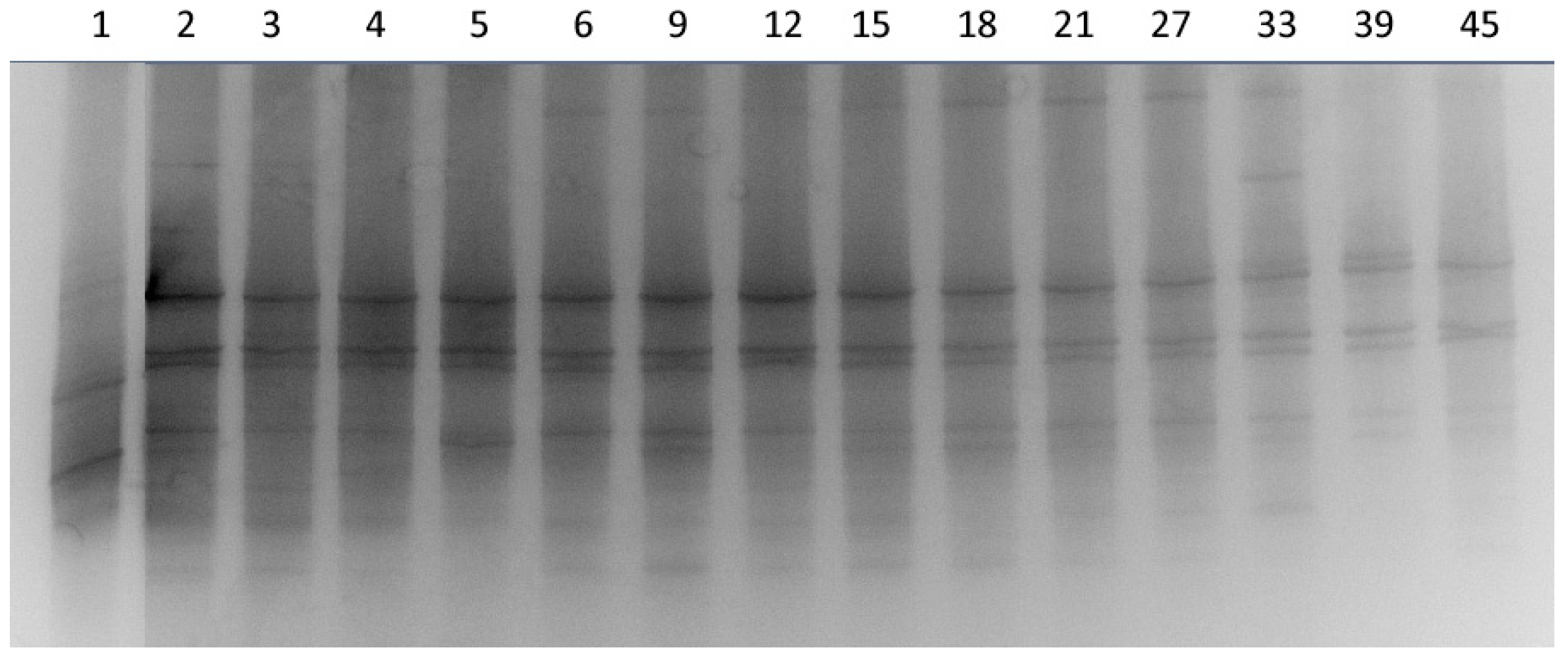
3.2. DGGE Analysis and Quantitative Analysis
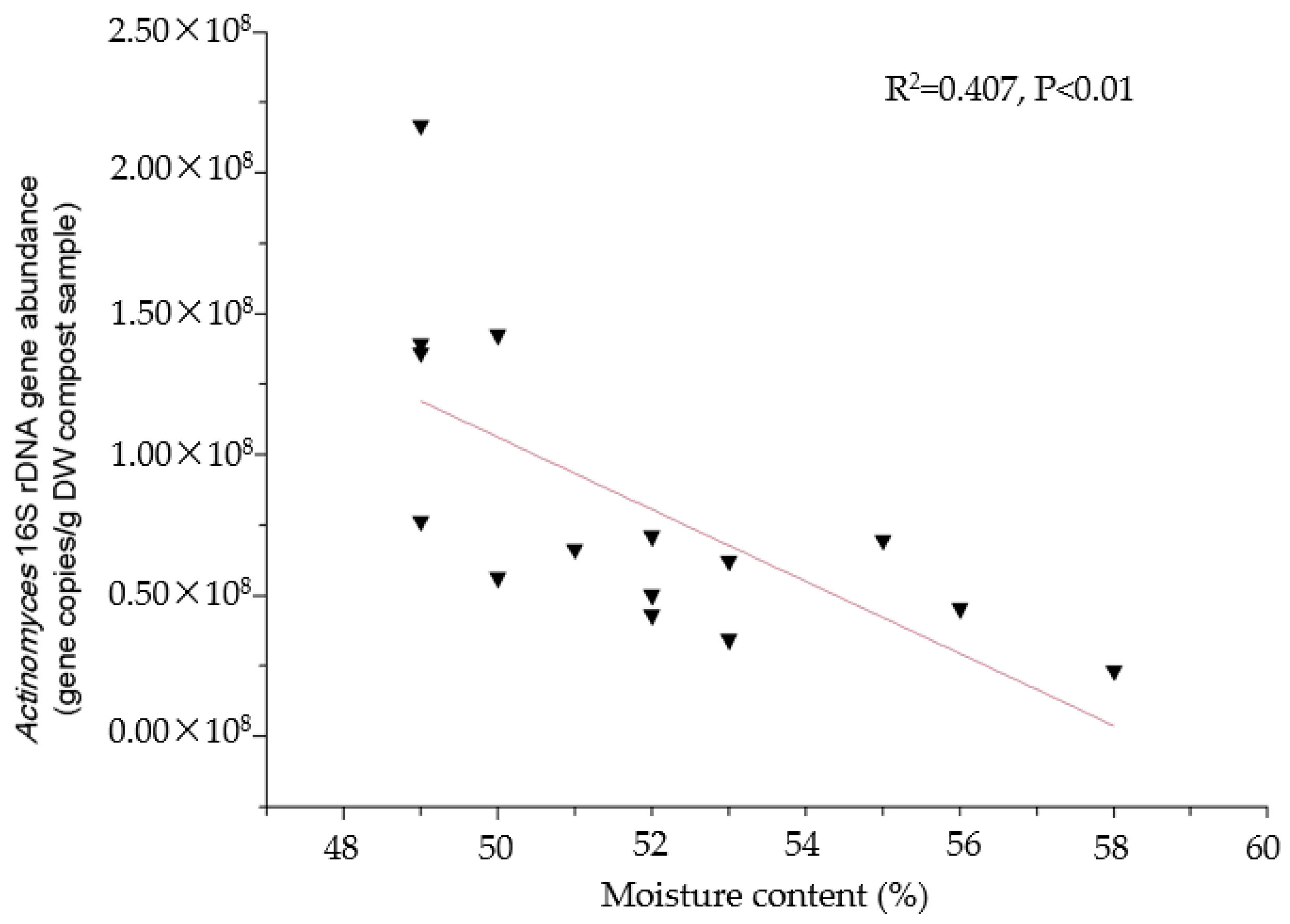
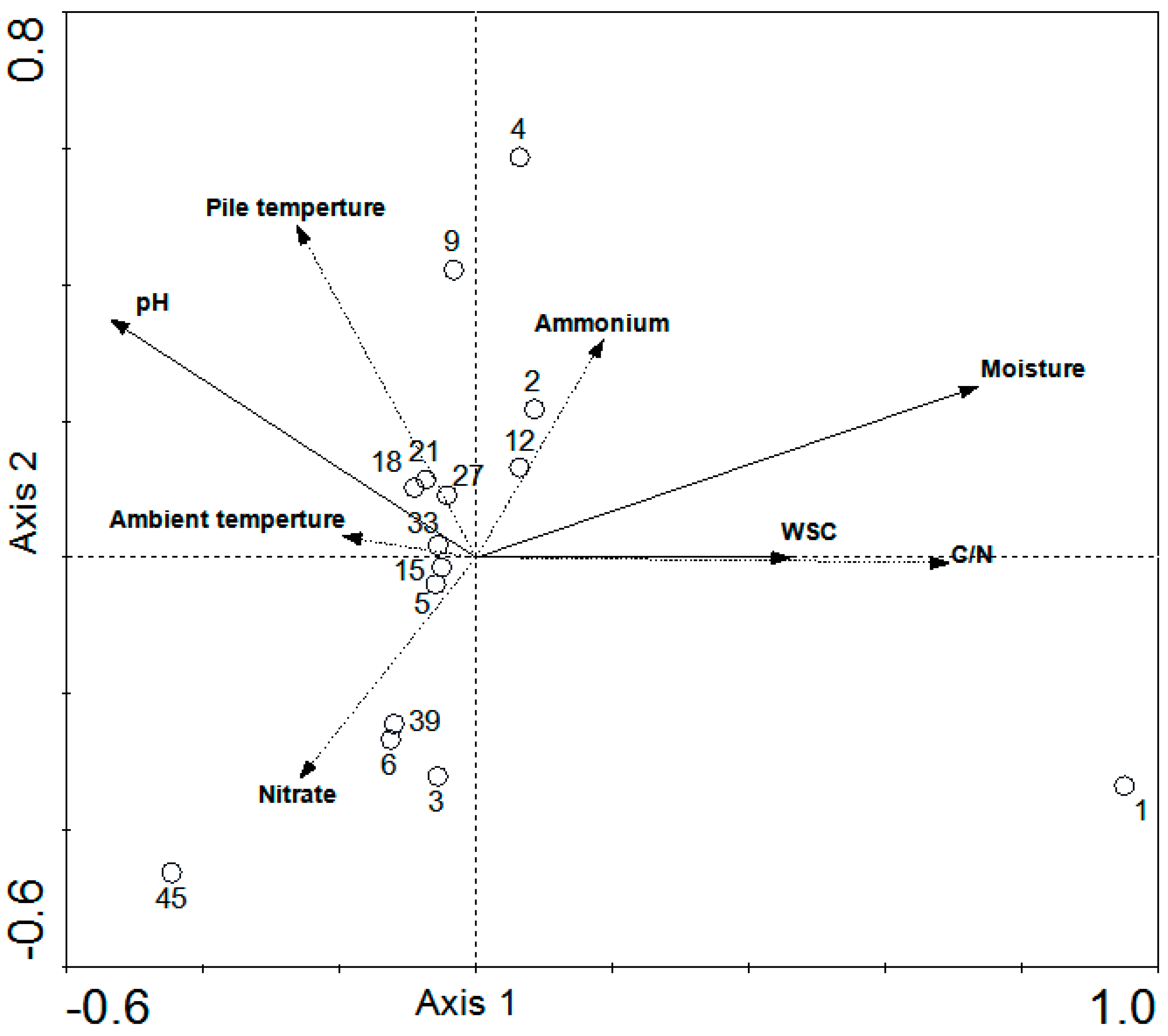
3.3. Redundancy Analyses and Regression Analysis
3.4. Forward Selections and Variation Partitioning
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, Y.; Zeng, G.M.; Yang, Z.H.; Ma, Y.H.; Huang, C.; Xu, Z.Y.; Huang, J.; Fan, C.Z. Changes in the actinomycetal communities during continuous thermophilic composting as revealed by denaturing gradient gel electrophoresis and quantitative PCR. Bioresour. Technol. 2011, 102, 1383–1388. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Li, H.; Yu, Y.; Huang, H. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Li, Y.; Wu, Y.; Zhu, F.; Zeng, G.; Zhang, J.; Li, H. Application of Fenton pretreatment on the degradation of rice straw by mixed culture of, Phanerochaete chrysosporium, and, Aspergillus niger. Ind. Crops Prod. 2018, 112, 290–295. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Li, Y.; Zeng, G.; Zhang, J.; Huang, A.; Zhang, J.; Ma, S.; Tan, X.; Xu, W.; et al. Study of the rice straw biodegradation in mixed culture of Trichoderma viride and Aspergillus niger by GC-MS and FTIR. Environ. Sci. Pollut. Res. Int. 2015, 22, 9807–9815. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2010, 53, 349–410. [Google Scholar]
- Godden, B.; Ball, A.S.; Helvenstein, P.; Mccarthy, A.J.; Penninckx, M.J. Towards elucidation of the lignin degradation pathway in actinomycetes. Microbiology 1992, 138, 2441–2448. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, W.; Li, Y.; Zhang, J.; Zeng, G.; Huang, A.; Huang, J. Nitrite reductase genes as functional markers to investigate diversity of denitrifying bacteria during agricultural waste composting. Appl. Microbiol. Biotechnol. 2014, 98, 4233–4243. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Das, K.; McClendon, R. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Paetsch, L.; Mueller, C.W.; Rumpel, C.; Houot, S.; Kögel-Knabner, I. Urban waste composts enhance OC and N stocks after long-term amendment but do not alter organic matter composition. Agric. Ecosyst. Environ. 2016, 223, 211–222. [Google Scholar] [CrossRef]
- Sundberg, C.; Yu, D.; Franke-Whittle, I.; Kauppi, S.; Smars, S.; Insam, H.; Romantschuk, M.; Jonsson, H. Effects of pH and microbial composition on odour in food waste composting. Waste Manag. 2013, 33, 204–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioeng. 2007, 104, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Shahi, A.; Ozbayram, E.G.; Ince, B.; Ince, O. Use of PCR-DGGE based molecular methods to assessment of microbial diversity during anaerobic treatment of antibiotic combinations. Bioresour. Technol. 2015, 192, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Camara, B.; De los Rios, A.; Urizal, M.; de Buergo, M.A.; Varas, M.J.; Fort, R.; Ascaso, C. Characterizing the microbial colonization of a dolostone quarry: Implications for stone biodeterioration and response to biocide treatments. Microb. Ecol. 2011, 62, 299–313. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Wu, Y.; Chen, Y.; Zeng, G.; Zhang, J.; Li, H. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour. Technol. 2017, 243, 347–355. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Huang, D.; Liu, L.; Shu, X.; Yin, H.; Li, X. A novel PCR-DGGE-based method for identifying plankton 16S rDNA for the diagnosis of drowning. Forensic Sci. Int. 2008, 176, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Matussek, A.; Stark, L.; Dienus, O.; Aronsson, J.; Mernelius, S.; Lofgren, S.; Lindgren, P.E. Analyzing multiclonality of Staphylococcus aureus in clinical diagnostics using spa-based denaturing gradient gel electrophoresis. J. Clin. Microbiol. 2011, 49, 3647–3648. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Chauhan, A.; Jain, R.K. Integrative approaches for assessing the ecological sustainability of in situ bioremediation. FEMS Microbiol. Rev. 2009, 33, 324–375. [Google Scholar] [CrossRef]
- Ruan, A.D.; Liu, C.X. Analysis of effect of nicotine on microbial community structure in sediment usinpog PCR-DGGE fingerprinting. Water Sci. Eng. 2015, 8, 309–314. [Google Scholar] [CrossRef]
- Tatsadjieu, N.L.; Maïworé, J.; Hadjia, M.B.; Loiseau, G.; Montet, D.; Mbofung, C.M.F. Study of the microbial diversity of Oreochromis niloticus of three lakes of Cameroon by PCR-DGGE: Application to the determination of the geographical origin. Food Control 2010, 21, 673–678. [Google Scholar] [CrossRef]
- Corbisier, P.; Pinheiro, L.; Mazoua, S.; Kortekaas, A.M.; Chung, P.Y.; Gerganova, T.; Roebben, G.; Emons, H.; Emslie, K. DNA copy number concentration measured by digital and droplet digital quantitative PCR using certified reference materials. Anal. Bioanal. Chem. 2015, 407, 1831–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ma, S.; Li, Y.; Yan, M.; Zeng, G.; Zhang, J.; Zhang, J.; Tan, X. Microbiological study on bioremediation of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) contaminated soil by agricultural waste composting. Appl. Microbiol. Biotechnol. 2016, 100, 9709–9718. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, P.; Santoro, A. Fuzzy clusterwise linear regression analysis with symmetrical fuzzy output variable. Comput. Stat. Date Anal. 2006, 51, 287–313. [Google Scholar] [CrossRef]
- Zeng, G.; Yu, M.; Chen, Y.; Huang, D.; Zhang, J.; Huang, H.; Jiang, R.; Yu, Z. Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour. Technol. 2010, 101, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, G.; Zhang, J.; Chen, Y.; Yu, M.; Lu, L.; Li, H.; Zhu, Y.; Yuan, Y.; Huang, A. Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl. Microbiol. Biotechnol. 2015, 99, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Xiao, Y.; Zeng, G.M.; Xu, Z.Y.; Liu, Y. Comparison of methods for total community DNA extraction and purification from compost. Appl. Microbiol. Biotechnol. 2007, 74, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of fungi, bacteria and actinomycetes on leaves decomposing in a stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef]
- Roesti, D.; Gaur, R.; Johri, B.; Imfeld, G.; Sharma, S.; Kawaljeet, K.; Aragno, M. Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol. Biochem. 2006, 38, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Gilbride, K.A.; Frigon, D.; Cesnik, A.; Gawat, J.; Fulthorpe, R.R. Effect of chemical and physical parameters on a pulp mill biotreatment bacterial community. Water Res. 2006, 40, 775–787. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Novo, A.; André, S.; Viana, P.; Nunes, O.C.; Manaia, C.M. Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res. 2013, 47, 1875–1887. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Tian, Y.; Lin, G.; Zheng, T. Using population dynamics analysis by DGGE to design the bacterial consortium isolated from mangrove sediments for biodegradation of PAHs. Int. Biodeterior. Biodegrad. 2011, 65, 269–275. [Google Scholar] [CrossRef]
- Ovreås, L.; Forney, L.; Daae, F.L.; Torsvik, V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Straathof, A.L.; Chincarini, R.; Comans, R.N.J.; Hoffland, E. Dynamics of soil dissolved organic carbon pools reveal both hydrophobic and hydrophilic compounds sustain microbial respiration. Soil Biol. Biochem. 2014, 79, 109–116. [Google Scholar] [CrossRef]
- Maeda, K.; Morioka, R.; Osada, T. Effect of covering composting piles with mature compost on ammonia emission and microbial community structure of composting process. J. Environ. Qual. 2009, 38, 598–606. [Google Scholar] [CrossRef]
- Wei, L.; Shutao, W.; Jin, Z.; Tong, X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zeng, G.; Yang, Z.; Shi, W.; Huang, C.; Fan, C.; Xu, Z. Continuous thermophilic composting (CTC) for rapid biodegradation and maturation of organic municipal solid waste. Bioresour. Technol. 2009, 100, 4807–4813. [Google Scholar] [CrossRef]


| Materials | Moisture Content % | Organic Materials % | C% | N% | C/N |
|---|---|---|---|---|---|
| Rice straw | 0.1173 | 0.7380 | 0.4280 | 0.0088 | 48.7600 |
| Bran | 0.1406 | 0.8174 | 0.4741 | 0.0412 | 11.5000 |
| Vegetables | 0.7906 | 0.1676 | 0.0972 | 0.0050 | 19.5500 |
| soil | 0.2613 | 0.0950 | 0.0551 | 0.0024 | 22.6700 |
| Primer | Primer Sequence |
|---|---|
| F243 | 5′-GGA TGA GCC CGC GGC CTA-3′ |
| R1378 | 5′-CGG TGT GTA CAA GGC CCG GGA ACG-3′ |
| F984GC | 5′-GC-AAC GCG AAG AAC CTT AC-3′ |
| Axis | Eigen Value | Species-Environment Correlation | Cumulative % Variation of Species | Cumulative % Variation of Species-Environment | Sum of All Canonical Eigen Values |
|---|---|---|---|---|---|
| Axis 1 | 0.664 | 0.986 | 66.4 | 76.7 | 0.866 |
| Axis 2 | 0.078 | 0.88 | 74.2 | 85.7 | |
| Axis 3 | 0.072 | 0.912 | 81.4 | 94 | |
| Axis 4 | 0.028 | 0.883 | 84.2 | 97.3 |
| Parameters Included in the Model | Eigen Value | % Variation Explains Solely | F Value | p Value |
|---|---|---|---|---|
| moisture | 0.196 | 19.6 | 7.79 | 0.002 |
| pH | 0.053 | 5.3 | 2.71 | 0.03 |
| WSC | 0.028 | 2.8 | 6.58 | 0.002 |
| All the above together | 0.454 | 45.4 | 3.047 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Y.; Chen, Y.; Wu, Y.; Zhang, C.; Peng, Z.; Liu, Y.; Wang, S.; Xu, R.; Zeng, Z. Effects of Physico-Chemical Parameters on Actinomycetes Communities during Composting of Agricultural Waste. Sustainability 2019, 11, 2229. https://doi.org/10.3390/su11082229
Li Y, Chen Y, Chen Y, Wu Y, Zhang C, Peng Z, Liu Y, Wang S, Xu R, Zeng Z. Effects of Physico-Chemical Parameters on Actinomycetes Communities during Composting of Agricultural Waste. Sustainability. 2019; 11(8):2229. https://doi.org/10.3390/su11082229
Chicago/Turabian StyleLi, Yuanping, Yanrong Chen, Yaoning Chen, Yanxin Wu, Chun Zhang, Zhen Peng, Yihuan Liu, Sha Wang, Ran Xu, and Ziping Zeng. 2019. "Effects of Physico-Chemical Parameters on Actinomycetes Communities during Composting of Agricultural Waste" Sustainability 11, no. 8: 2229. https://doi.org/10.3390/su11082229





