From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes
Abstract
:1. Introduction
2. CNT Synthesis Methods
3. Bio-Feedstocks for CNT Synthesis
3.1. Liquid Precursors
3.1.1. Camphor Oil and Camphor
3.1.2. Castor Oil
3.1.3. Eucalyptus Oil
3.1.4. Palm Oil
3.1.5. Sesame Oil
3.1.6. Sunflower Oil
3.1.7. Turpentine Oil
3.2. Solid Precursors
4. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Brady, G.J.; Way, A.J.; Safron, N.S.; Evensen, H.T.; Gopalan, P.; Arnold, M.S. Quasi-ballistic carbon nanotube array transistors with current density exceeding Si and GaAs. Sci. Adv. 2016, 2, e1601240. [Google Scholar] [CrossRef] [Green Version]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical Properties and Applications of Carbon Nanotube Structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Miao, M. Electrical conductivity of pure carbon nanotube yarns. Carbon 2011, 49, 3755–3761. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Lian, F.; Llinas, J.P.; Li, Z.; Estrada, D.; Pop, E. Thermal conductivity of chirality-sorted carbon nanotube networks. Appl. Phys. Lett. 2016, 108, 103101. [Google Scholar] [CrossRef]
- Che, J.; Çagin, T.; Goddard, W.A. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Verdejo, R.; Khayet, M.; de Zarate, J.M.O.; Essalhi, M.; Lopez-Manchado, M.A. Thermal conductivity of carbon nanotubes and graphene in epoxy nanofluids and nanocomposites. Nanoscale Res. Lett. 2011, 6, 610. [Google Scholar] [CrossRef] [Green Version]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 6479. [Google Scholar] [CrossRef] [PubMed]
- Sammalkorpi, M.; Krasheninnikov, A.; Kuronen, A.; Nordlund, K.; Kaski, K. Mechanical properties of carbon nanotubes with vacancies and related defects. Phys. Rev. B 2004, 70, 245416. [Google Scholar] [CrossRef] [Green Version]
- Kulik, A.J.; Kis, A.; Lukic, B.; Lee, K.; Forró, L. Mechanical Properties of Carbon Nanotubes. In Fundamentals of Friction and Wear; Gnecco, E., Meyer, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 583–600. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozena, A.H.; Kim, M.; Powell, L.R.; Wang, Y. Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects. Nat. Rev. Chem. 2019, 3, 375–392. [Google Scholar] [CrossRef]
- He, X.; Htoon, H.; Doorn, S.K.; Pernice, W.H.P.; Pyatkov, F.; Krupke, R.; Jeantet, A.; Chassagneux, Y.; Voisin, C. Carbon nanotubes as emerging quantum-light sources. Nat. Mater. 2018, 17, 663–670. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Advancement in Photothermal Effect of Carbon Nanotubes by Grafting of Poly(amidoamine) and Deposition of CdS Nanocrystallites. Ind. Eng. Chem. Res. 2018, 57, 7826–7833. [Google Scholar] [CrossRef]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef]
- Lin, M.F.; Shung, K.W.K. Plasmons and optical properties of carbon nanotubes. Phys. Rev. B 1994, 50, 17744–17747. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Gaviria Rojas, W.A.; Hersam, M.C. Chirality-Enriched Carbon Nanotubes for Next-Generation Computing. Adv. Mater. 2020, n/a, 1905654. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-M.; Zhang, Z.; Wang, S. Carbon nanotube electronics: Recent advances. Mater. Today 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Avouris, P.; Appenzeller, J.; Martel, R.; Wind, S.J. Carbon nanotube electronics. Proc. IEEE 2003, 91, 1772–1784. [Google Scholar] [CrossRef]
- Cwirzen, A.; Habermehl-Cwirzen, K.; Penttala, V. Surface decoration of carbon nanotubes and mechanical properties of cement/carbon nanotube composites. Adv. Cem. Res. 2008, 20, 65–73. [Google Scholar] [CrossRef]
- Chen, S.J.; Collins, F.G.; Macleod, A.J.N.; Pan, Z.; Duan, W.H.; Wang, C.M. Carbon nanotube–cement composites: A retrospect. IES J. Part A Civ. Struct. Eng. 2011, 4, 254–265. [Google Scholar] [CrossRef]
- Yu, X.; Kwon, E. A carbon nanotube/cement composite with piezoresistive properties. Smart Mater. Struct. 2009, 18, 055010. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Saliev, T. The advances in biomedical applications of carbon nanotubes. C—J. Carbon Res. 2019, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The advances of carbon nanotubes in cancer diagnostics and therapeutics. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Janas, D. Towards monochiral carbon nanotubes: A review of progress in the sorting of single-walled carbon nanotubes. Mater. Chem. Front. 2018, 2, 36–63. [Google Scholar] [CrossRef]
- Tersoff, J. Contact resistance of carbon nanotubes. Appl. Phys. Lett. 1999, 74, 2122–2124. [Google Scholar] [CrossRef]
- Walker, J.S.; Fagan, J.A.; Biacchi, A.J.; Kuehl, V.A.; Searles, T.A.; Hight Walker, A.R.; Rice, W.D. Global Alignment of Solution-Based Single-Wall Carbon Nanotube Films via Machine-Vision Controlled Filtration. Nano Lett. 2019, 19, 7256–7264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, N.; Nakamura, M.; Ghosh, S.; Kim, D.; Chen, H.; Katagiri, A.; Yomogida, Y.; Gao, W.; Yanagi, K.; Kono, J. Groove-Assisted Global Spontaneous Alignment of Carbon Nanotubes in Vacuum Filtration. Nano Lett. 2020, 20, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, P.J.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 2018, 13, 634–641. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef]
- Baccile, N. Nanomaterials from Renewable Resources. In Nanomaterials: A Danger or a Promise? A Chemical and Biological Perspective; Brayner, R., Fiévet, F., Coradin, T., Eds.; Springer: London, UK, 2013; pp. 335–356. [Google Scholar] [CrossRef]
- Vivekanandhan, S.; Schreiber, M.; Muthuramkumar, S.; Misra, M.; Mohanty, A.K. Carbon nanotubes from renewable feedstocks: A move toward sustainable nanofabrication. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef] [Green Version]
- John, G.; Vemula, P.K. Design and development of soft nanomaterials from biobased amphiphiles. Soft Matter 2006, 2, 909–914. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Boneberg, B.S.; Machado, G.D.; Santos, D.F.; Gomes, F.; Faria, D.J.; Gomes, L.A.; Santos, F.A. Biorefinery of lignocellulosic biopolymers. Rev. Eletrônica Científica Da UERGS 2016, 2, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ando, Y. Camphor–a botanical precursor producing garden of carbon nanotubes. Diam. Relat. Mater. 2003, 12, 998–1002. [Google Scholar] [CrossRef]
- Porro, S.; Musso, S.; Giorcelli, M.; Tagliaferro, A.; Dalal, S.H.; Teo, K.B.K.; Jefferson, D.A.; Milne, W.I. Study of CNTs and nanographite grown by thermal CVD using different precursors. J. Non-Cryst. Solids 2006, 352, 1310–1313. [Google Scholar] [CrossRef]
- Raziah, A.Z.; Junizah, A.R.; Saifuddin, N. Synthesis of carbon nanotubes using natural carbon precursor: Castor oil. AIP Conf. Proc. 2012, 1482, 564–567. [Google Scholar] [CrossRef]
- Holmgren, J.; Gosling, C.; Couch, K.; Kalnes, T.; Marker, T.; McCall, M.; Marinangeli, R. Refining biofeedstock innovations. Pet. Technol. Q. 2007, 12, 119. [Google Scholar]
- Awasthi, A.; Bhaskar, T. Chapter 11—Combustion of Lignocellulosic Biomass. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels (Second Edition); Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 267–284. [Google Scholar] [CrossRef]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Chapter Five—Conversion of Lignocellulosic Biomass Into Platform Chemicals for Biobased Polyurethane Application. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 161–213. [Google Scholar]
- Abdullah, N.; Sulaiman, F. The oil palm wastes in Malaysia. Biomass Now-Sustain. Growth Use 2013, 1, 75–93. [Google Scholar]
- Dungani, R.; Aditiawati, P.; Aprilia, S.; Yuniarti, K.; Karliati, T.; Suwandhi, I.; Sumardi, I. Biomaterial from Oil Palm Waste: Properties, Characterization and Applications. Palm Oil 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Shi, Z.; Lian, Y.; Liao, F.H.; Zhou, X.; Gu, Z.; Zhang, Y.; Iijima, S.; Li, H.; Yue, K.T.; Zhang, S.-L. Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 2000, 61, 1031–1036. [Google Scholar] [CrossRef]
- Huang, L.; Wu, B.; Chen, J.; Xue, Y.; Liu, Y.; Kajiura, H.; Li, Y. Synthesis of single-walled carbon nanotubes by an arc-discharge method using selenium as a promoter. Carbon 2011, 49, 4792–4800. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Y.; Yang, Z.; Wei, L.; Wang, Y.; Zhang, Y. Arc synthesis of double-walled carbon nanotubes in low pressure air and their superior field emission properties. Carbon 2013, 58, 92–98. [Google Scholar] [CrossRef]
- Das, R.; Shahnavaz, Z.; Ali, M.E.; Islam, M.M.; Abd Hamid, S.B. Can We Optimize Arc Discharge and Laser Ablation for Well-Controlled Carbon Nanotube Synthesis? Nanoscale Res. Lett. 2016, 11, 510. [Google Scholar] [CrossRef] [Green Version]
- Arepalli, S. Laser Ablation Process for Single-Walled Carbon Nanotube Production. J. Nanosci. Nanotechnol. 2004, 4, 317–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Iijima, S. Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl. Phys. Lett. 1998, 73, 3827–3829. [Google Scholar] [CrossRef]
- Scott, C.D.; Arepalli, S.; Nikolaev, P.; Smalley, R.E. Growth mechanisms for single-wall carbon nanotubes in a laser-ablation process. Appl. Phys. A 2001, 72, 573–580. [Google Scholar] [CrossRef]
- Yudasaka, M.; Komatsu, T.; Ichihashi, T.; Iijima, S. Single-wall carbon nanotube formation by laser ablation using double-targets of carbon and metal. Chem. Phys. Lett. 1997, 278, 102–106. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Ji, D.; Liu, Y.; Li, L.; Yuan, D.; Zhang, Z.; Ren, J.; Lefler, M.; Wang, B.; et al. One-pot synthesis of nanostructured carbon materials from carbon dioxide via electrolysis in molten carbonate salts. Carbon 2016, 106, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Li, F.-F.; Lau, J.; González-Urbina, L.; Licht, S. One-Pot Synthesis of Carbon Nanofibers from CO2. Nano Lett. 2015, 15, 6142–6148. [Google Scholar] [CrossRef]
- Novoselova, I.A.; Oliinyk, N.F.; Volkov, S.V.; Konchits, A.A.; Yanchuk, I.B.; Yefanov, V.S.; Kolesnik, S.P.; Karpets, M.V. Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2231–2237. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Ge, J.; Zhu, J.; Han, Z.; Jiao, S. Electrochemical deposition of carbon nanotubes from CO2 in CaCl2–NaCl-based melts. J. Mater. Chem. A 2017, 5, 6219–6225. [Google Scholar] [CrossRef]
- Johnson, M.; Ren, J.; Lefler, M.; Licht, G.; Vicini, J.; Liu, X.; Licht, S. Carbon nanotube wools made directly from CO2 by molten electrolysis: Value driven pathways to carbon dioxide greenhouse gas mitigation. Mater. Today Energy 2017, 5, 230–236. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H. Synthesis of Carbon Nanotube Through Sonochemical Process Under Ambient Conditions. J. Nanosci. Nanotechnol. 2009, 9, 5940–5945. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Libera, J.A.; Yoshimura, M. Hydrothermal synthesis of multiwall carbon nanotubes. J. Mater. Res. 2011, 15, 2591–2594. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, X.; Fan, K.; Peng, T.; Wei, B. Hydrothermal synthesis of single-walled carbon nanotube–TiO2 hybrid and its photocatalytic activity. Appl. Surf. Sci. 2013, 270, 238–244. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, K.; Verma, V.; Bhatti, H.S. Low-temperature hydrothermal synthesis and functionalization of multiwalled carbon nanotubes. Indian J. Phys. 2016, 90, 139–148. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öncel, Ç.; Yürüm, Y. Carbon Nanotube Synthesis via the Catalytic CVD Method: A Review on the Effect of Reaction Parameters. Fuller. Nanotub. Carbon Nanostruct. 2006, 14, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, S.B.; Andrews, R.; Qian, D.; Rao, A.M.; Mao, Z.; Dickey, E.C.; Derbyshire, F. Model of carbon nanotube growth through chemical vapor deposition. Chem. Phys. Lett. 1999, 315, 25–30. [Google Scholar] [CrossRef]
- Lim, Y.D.; Avramchuck, A.V.; Grapov, D.; Tan, C.W.; Tay, B.K.; Aditya, S.; Labunov, V. Enhanced Carbon Nanotubes Growth Using Nickel/Ferrocene-Hybridized Catalyst. ACS Omega 2017, 2, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, E.C.; Daramola, M.O.; Iyuke, S.E. Production of carbon nanotube yarns via floating catalyst chemical vapor deposition: Effect of synthesis temperature on electrical conductivity. Results Phys. 2019, 15, 102705. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Preparation of carbon nanotube by rotary CVD on Ni nano-particle precipitated cBN using nickelocene as a precursor. Mater. Lett. 2011, 65, 367–370. [Google Scholar] [CrossRef]
- Weissker, U.; Hampel, S.; Leonhardt, A.; Büchner, B. Carbon nanotubes filled with ferromagnetic materials. Materials 2010, 3, 4387–4427. [Google Scholar] [CrossRef] [Green Version]
- Janas, D.; Koziol, K.K. Carbon nanotube fibers and films: Synthesis, applications and perspectives of the direct-spinning method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Zakarya, S.A.; Sarijo, S.H.; Zainal, Z. Parameter optimisation of carbon nanotubes synthesis via hexane decomposition over minerals generated from Anadara granosa shells as the catalyst support. J. Nanomater. 2012, 2012, 525616. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.; Hussein, M.Z.; Yusof, N.A.; Abu Bakar, F. Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials 2019, 9, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, Y.; Hu, L.; Qian, W.-Z.; Luo, G.-H.; Wei, F. Synthesis of thin-walled carbon nanotubes from methane by changing the Ni/Mo ratio in a Ni/Mo/MgO catalyst. New Carbon Mater. 2008, 23, 319–325. [Google Scholar] [CrossRef]
- Ramírez Rodríguez, F.; López, B.L.; Giraldo, L.F. Single Wall Carbon Nanotubes Synthesis through Methane Chemical Vapor Deposition over MCM-41–Co Catalysts: Variables Optimization. C—J. Carbon Res. 2018, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube growth inhibition in floating catalyst based chemical vapor deposition and its application in flexible circuit fabrication. Carbon 2017, 116, 40–49. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Yahyazadeh, A.; Khoshandam, B. Carbon nanotube synthesis via the catalytic chemical vapor deposition of methane in the presence of iron, molybdenum, and iron–molybdenum alloy thin layer catalysts. Results Phys. 2017, 7, 3826–3837. [Google Scholar] [CrossRef]
- Maruyama, T.; Kondo, H.; Ghosh, R.; Kozawa, A.; Naritsuka, S.; Iizumi, Y.; Okazaki, T.; Iijima, S. Single-walled carbon nanotube synthesis using Pt catalysts under low ethanol pressure via cold-wall chemical vapor deposition in high vacuum. Carbon 2016, 96, 6–13. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhang, L.-H.; Zhong, X.-H.; Windle, A.H. Synthesis of high purity single-walled carbon nanotubes from ethanol by catalytic gas flow CVD reactions. Nanotechnology 2007, 18, 225604. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Huczko, A.; Byszewski, P.; Domańska, M.; Rümmeli, M.H.; Gemming, T.; Lange, H. Systematic Studies on Carbon Nanotubes Synthesis from Aliphatic Alcohols by the CVD Floating Catalyst Method. Fuller. Nanotub. Carbon Nanostruct. 2009, 17, 298–307. [Google Scholar] [CrossRef]
- Ordoñez-Casanova, E.G.; Román-Aguirre, M.; Aguilar-Elguezabal, A.; Espinosa-Magaña, F. Synthesis of carbon nanotubes of few walls using aliphatic alcohols as a carbon source. Materials 2013, 6, 2534–2542. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.A.; Marquez, A.; Pérez, A.; Duarte-Moller, A. Simple method to synthesize functionalized carbon nanotubes employing cobalt nitrate and acetone by using spray pyrolysis deposition technique. Adv. Mater. Sci. Eng. 2012, 2012, 258673. [Google Scholar] [CrossRef] [Green Version]
- Melezhik, A.V.; Smykov, M.A.; Filatova, E.Y.; Shuklinov, A.V.; Stolyarov, R.A.; Larionova, I.S.; Tkachov, A.G. Synthesis of carbon nanotubes from acetone. Theor. Found. Chem. Eng. 2013, 47, 435–443. [Google Scholar] [CrossRef]
- Luo, T.; Liu, J.; Chen, L.; Zeng, S.; Qian, Y. Synthesis of helically coiled carbon nanotubes by reducing ethyl ether with metallic zinc. Carbon 2005, 43, 755–759. [Google Scholar] [CrossRef]
- Hou, B.; Xiang, R.; Inoue, T.; Einarsson, E.; Chiashi, S.; Shiomi, J.; Miyoshi, A.; Maruyama, S. Decomposition of ethanol and dimethyl ether during chemical vapor deposition synthesis of single-walled carbon nanotubes. Jpn. J. Appl. Phys. 2011, 50, 065101. [Google Scholar]
- Tang, C.; Bando, Y.; Golberg, D.; Xu, F. Structure and nitrogen incorporation of carbon nanotubes synthesized by catalytic pyrolysis of dimethylformamide. Carbon 2004, 42, 2625–2633. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Mian, S.A.; Ali, G. An overview of the recent progress in the synthesis and applications of carbon nanotubes. C—J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Carole, E.B.; Cedric, B. Carbon Nanotube Synthesis: A Review. Int. J. Chem. React. Eng. 2005, 3. [Google Scholar] [CrossRef]
- Zhang, B.; Piao, G.; Zhang, J.; Bu, C.; Xie, H.; Wu, B.; Kobayashi, N. Synthesis of carbon nanotubes from conventional biomass-based gasification gas. Fuel Process. Technol. 2018, 180, 105–113. [Google Scholar] [CrossRef]
- Januszewicz, K.; Klugmann-Radziemska, E. Synthesis of reduced graphene oxide nanosheets using nanofibers from methane and biogas thermal decomposition with various catalysts. Chem. Pap. 2018, 72, 1991–1999. [Google Scholar] [CrossRef] [Green Version]
- TermehYousefi, A.; Bagheri, S.; Shinji, K.; Rouhi, J.; Rusop Mahmood, M.; Ikeda, S. Fast synthesis of multilayer carbon nanotubes from camphor oil as an energy storage material. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Salifairus, M.; Rusop, M. Synthesis of carbon nanotubes by chemical vapour deposition of camphor oil over ferrocene and aluminum isopropoxide catalyst. Adv. Mater. Res. 2013, 667, 213–217. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Mohammad, M.; Zobir, S.A.M.; Asli, N.A.; Bakar, S.A.; Abdullah, S.; Yahya, S.Y.S.; Mahmood, M.R. Synthesis and nucleation-growth mechanism of almost catalyst-free carbon nanotubes grown from Fe-filled sphere-like graphene-shell surface. J. Nanostruct. Chem. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ando, Y. Single-wall and multi-wall carbon nanotubes from camphor—A botanical hydrocarbon. Diam. Relat. Mater. 2003, 12, 1845–1850. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Krishna, K.M.; Sharon, M. Fullerenes from camphor: A natural source. Phys. Rev. Lett. 1994, 72, 3182–3185. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. A simple method of producing aligned carbon nanotubes from an unconventional precursor—Camphor. Chem. Phys. Lett. 2003, 374, 521–526. [Google Scholar] [CrossRef]
- Kumar, M.; Kakamu, K.; Okazaki, T.; Ando, Y. Field emission from camphor—Pyrolyzed carbon nanotubes. Chem. Phys. Lett. 2004, 385, 161–165. [Google Scholar] [CrossRef]
- Ghosh, K.; Kumar, M.; Maruyama, T.; Ando, Y. Micro-structural, electron-spectroscopic and field-emission studies of carbon nitride nanotubes grown from cage-like and linear carbon sources. Carbon 2009, 47, 1565–1575. [Google Scholar] [CrossRef]
- Boncel, S.; Pattinson, S.W.; Geiser, V.; Shaffer, M.S.; Koziol, K.K. En route to controlled catalytic CVD synthesis of densely packed and vertically aligned nitrogen-doped carbon nanotube arrays. Beilstein J. Nanotechnol. 2014, 5, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ando, Y. Controlling the diameter distribution of carbon nanotubes grown from camphor on a zeolite support. Carbon 2005, 43, 533–540. [Google Scholar] [CrossRef]
- Musso, S.; Fanchini, G.; Tagliaferro, A. Growth of vertically aligned carbon nanotubes by CVD by evaporation of carbon precursors. Diam. Relat. Mater. 2005, 14, 784–789. [Google Scholar] [CrossRef]
- Musso, S.; Porro, S.; Giorcelli, M.; Chiodoni, A.; Ricciardi, C.; Tagliaferro, A. Macroscopic growth of carbon nanotube mats and their mechanical properties. Carbon 2007, 45, 1133–1136. [Google Scholar] [CrossRef]
- Pavese, M.; Musso, S.; Bianco, S.; Giorcelli, M.; Pugno, N. An analysis of carbon nanotube structure wettability before and after oxidation treatment. J. Phys. Condens. Matter 2008, 20, 474206. [Google Scholar] [CrossRef] [Green Version]
- Somani, S.P.; Somani, P.R.; Tanemura, M.; Lau, S.P.; Umeno, M. Carbon nanofibers and multiwalled carbon nanotubes from camphor and their field electron emission. Curr. Appl. Phys. 2009, 9, 144–150. [Google Scholar] [CrossRef]
- Awasthi, K.; Kumar, R.; Raghubanshi, H.; Awasthi, S.; Pandey, R.; Singh, D.; Yadav, T.; Srivastava, O. Synthesis of nano-carbon (nanotubes, nanofibres, graphene) materials. Bull. Mater. Sci. 2011, 34, 607. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Afre, R.A.; Soga, T.; Jimbo, T. A simple method of producing single-walled carbon nanotubes from a natural precursor: Eucalyptus oil. Mater. Lett. 2007, 61, 3768–3770. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol Res. 2015, 48, 7. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, A.; Shinohara, H. Control of diameter distribution of single-walled carbon nanotubes using the zeolite-CCVD method at atmospheric pressure. Carbon 2005, 43, 431–436. [Google Scholar] [CrossRef]
- Zhao, W.; Basnet, B.; Kim, I.J. Carbon nanotube formation using zeolite template and applications. J. Adv. Ceram. 2012, 1, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Soga, T.; Tanemura, M.; Zamri, M.; Jimbo, T.; Katoh, R.; Sumiyama, K. Vertically aligned carbon nanotubes from natural precursors by spray pyrolysis method and their field electron emission properties. Appl. Phys. A 2009, 94, 51–56. [Google Scholar] [CrossRef]
- Henson, I.E. 1—A Brief History of the Oil Palm. In Palm Oil; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 1–29. [Google Scholar] [CrossRef]
- Suriani, A.B.; Azira, A.A.; Nik, S.F.; Md Nor, R.; Rusop, M. Synthesis of vertically aligned carbon nanotubes using natural palm oil as carbon precursor. Mater. Lett. 2009, 63, 2704–2706. [Google Scholar] [CrossRef]
- Maryam, M.; Suriani, A.; Shamsudin, M.; Rusop Mahmood, M. Synthesis of Carbon Nanotubes from Palm Oil Precursor by Aerosol-Assisted Catalytic CVD method. Appl. Mech. Mater. 2012, 229–231, 247–251. [Google Scholar] [CrossRef]
- Suriani, A.B.; Asli, N.A.; Salina, M.; Mamat, M.H.; Aziz, A.A.; Falina, A.N.; Maryam, M.; Shamsudin, M.S.; Md Nor, R.; Abdullah, S.; et al. Effect of Iron and Cobalt Catalysts on The Growth of Carbon Nanotubes from Palm Oil Precursor. IOP Conf. Ser. Mater. Sci. Eng. 2013, 46, 012014. [Google Scholar] [CrossRef] [Green Version]
- Kudin, T.I.T.; Zainal, N.F.A.; Ali, A.M.M.; Abdullah, S.; Rusop, M.; Sulaiman, M.A.; Yahya, M.Z.A. Electrochemical performance of anode material from palm oils derived carbon nanotubes for lithium ion batteries. Mater. Res. Innov. 2009, 13, 269–271. [Google Scholar] [CrossRef]
- Robaiah, M.; Rusop, M.; Abdullah, S.; Khusaimi, Z.; Azhan, H.; Fadzlinatul, M.Y.; Salifairus, M.J.; Asli, N.A. Synthesis of carbon nanotubes from palm oil on stacking and non-stacking substrate by thermal-CVD method. AIP Conf. Proc. 2018, 1963, 020027. [Google Scholar] [CrossRef]
- Ram, R.; Catlin, D.; Romero, J.; Cowley, C. Sesame: New approaches for crop improvement. In Advances New Crop; Timber Press: Portland, OR, USA, 1990; pp. 225–228. [Google Scholar]
- Kumar, R.; Singh, R.K.; Kumar, P.; Dubey, P.K.; Tiwari, R.S.; Srivastava, O.N. Clean and Efficient Synthesis of Graphene Nanosheets and Rectangular Aligned-Carbon Nanotubes Bundles Using Green Botanical Hydrocarbon Precursor: Sesame Oil. Sci. Adv. Mater. 2014, 6, 76–83. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Tiwari, R.S. Growth analysis and high-yield synthesis of aligned-stacked branched nitrogen-doped carbon nanotubes using sesame oil as a natural botanical hydrocarbon precursor. Mater. Des. 2016, 94, 166–175. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, R.M.; Awasthi, K.; Tiwari, R.S.; Srivastava, O.N. Effect of nitrogen variation on the synthesis of vertically aligned bamboo-shaped C–N nanotubes using sunflower oil. Int. J. Nanosci. 2011, 10, 809–813. [Google Scholar] [CrossRef]
- Afre, R.A.; Soga, T.; Jimbo, T.; Kumar, M.; Ando, Y.; Sharon, M. Growth of vertically aligned carbon nanotubes on silicon and quartz substrate by spray pyrolysis of a natural precursor: Turpentine oil. Chem. Phys. Lett. 2005, 414, 6–10. [Google Scholar] [CrossRef]
- Afre, R.A.; Soga, T.; Jimbo, T.; Kumar, M.; Ando, Y.; Sharon, M.; Somani, P.R.; Umeno, M. Carbon nanotubes by spray pyrolysis of turpentine oil at different temperatures and their studies. Microporous Mesoporous Mater. 2006, 96, 184–190. [Google Scholar] [CrossRef]
- Ghosh, P.; Soga, T.; Afre, R.A.; Jimbo, T. Simplified synthesis of single-walled carbon nanotubes from a botanical hydrocarbon: Turpentine oil. J. Alloy. Compd. 2008, 462, 289–293. [Google Scholar] [CrossRef]
- Ghosh, P.; Soga, T.; Ghosh, K.; Afre, R.A.; Jimbo, T.; Ando, Y. Vertically aligned N-doped carbon nanotubes by spray pyrolysis of turpentine oil and pyridine derivative with dissolved ferrocene. J. Non-Cryst. Solids 2008, 354, 4101–4106. [Google Scholar] [CrossRef]
- Awasthi, K.; Kumar, R.; Tiwari, R.S.; Srivastava, O.N. Large scale synthesis of bundles of aligned carbon nanotubes using a natural precursor: Turpentine oil. J. Exp. Nanosci. 2010, 5, 498–508. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, E.; Mao, B.; Su, Z.; Chen, L.; Xu, L. Obtaining carbon nanotubes from grass. Nanotechnology 2005, 16, 1192–1195. [Google Scholar] [CrossRef]
- Cho, W.S.; Hamada, E.; Kondo, Y.; Takayanagi, K. Synthesis of carbon nanotubes from bulk polymer. Appl. Phys. Lett. 1996, 69, 278–279. [Google Scholar] [CrossRef]
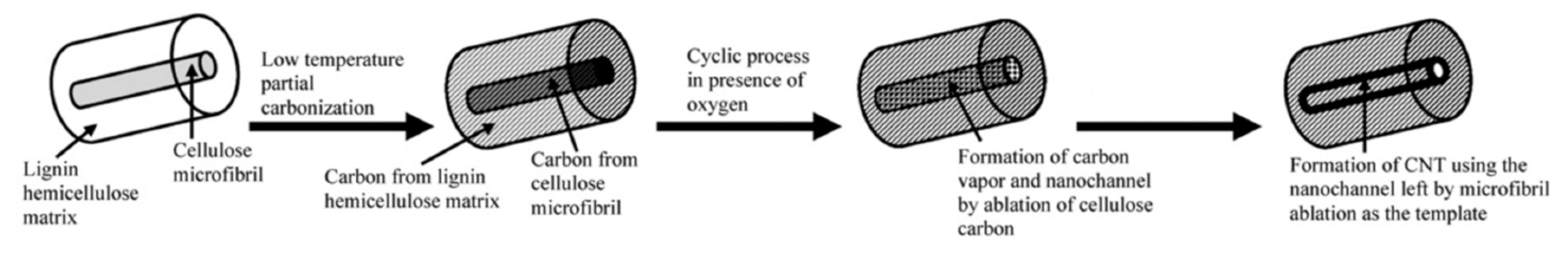
- Goodell, B.; Xie, X.; Qian, Y.; Daniel, G.; Peterson, M.; Jellison, J. Carbon Nanotubes Produced from Natural Cellulosic Materials. J. Nanosci. Nanotechnol. 2008, 8, 2472–2474. [Google Scholar] [CrossRef]
- Xie, X.; Goodell, B.; Daniel, G.; Qian, Y.; Jellison, J.; Peterson, M. Carbonization of wood and nanostructures formed from the cell wall. Int. Biodeterior. Biodegrad. 2009, 63, 933–935. [Google Scholar] [CrossRef]
- Xie, X.; Goodell, B.; Qian, Y.; Daniel, G.; Zhang, D.; Nagle, D.C.; Peterson, M.L.; Jellison, J. A method for producing carbon nanotubes directly from plant materials. For. Prod. J. 2009, 59, 26. [Google Scholar]
- Ma, Y.; Lu, N.; Lu, Y.; Guan, J.-N.; Qu, J.; Liu, H.-Y.; Cong, Q.; Yuan, X. Comparative Study of Carbon Materials Synthesized “Greenly” for 2-CP Removal. Sci. Rep. 2016, 6, 29167. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Cong, Q.; Luo, C.; Yuan, X. Adsorption and photocatalytic degradation of bisphenol A by low-cost carbon nanotubes synthesized using fallen leaves of poplar. RSC Adv. 2013, 3, 961–965. [Google Scholar] [CrossRef]
- Rümmeli, M.H.; Bachmatiuk, A.; Börrnert, F.; Schäffel, F.; Ibrahim, I.; Cendrowski, K.; Simha-Martynkova, G.; Plachá, D.; Borowiak-Palen, E.; Cuniberti, G.; et al. Synthesis of carbon nanotubes with and without catalyst particles. Nanoscale Res. Lett. 2011, 6, 303. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Luo, C.; Cong, Q.; Yuan, X. Carbon nanotubes and Cu–Zn nanoparticles synthesis using hyperaccumulator plants. Environ. Chem. Lett. 2012, 10, 153–158. [Google Scholar] [CrossRef]
- Liu, H.; Ren, M.; Qu, J.; Feng, Y.; Song, X.; Zhang, Q.; Cong, Q.; Yuan, X. A cost-effective method for recycling carbon and metals in plants: Synthesizing nanomaterials. Environ. Sci. Nano 2017, 4, 461–469. [Google Scholar] [CrossRef]




| Precursor | Conditions T, cat. | Product | Diameter [nm] | Quality ID/IG | Ref. |
|---|---|---|---|---|---|
| Camphor oil | 750–850 °C, ferrocene | MWCNTs | 30–75 | 0.89 | [105] |
| 700–900 °C, ferrocene and aluminum isopropoxide | MWCNTs and SWCNTs | N/A | ~0.90 | [106] | |
| 875 °C, ferrocene 1 | MWCNTs | N/A | 0.791–0.998 | [107] | |
| Camphor | 875 °C, ferrocene | MWCNTs and SWCNTs | 25–50 (MWCNTs), ~1.2 (SWCNTs) | High purity | [47] |
| 800–1050 °C, ferrocene | MWCNTs and SWCNTs | 10–50 (MWCNTs), ~1.2 (SWCNTs) | 0.50 | [108] | |
| 900 °C, ferrocene | MWCNTs | 20–40 | N/A | [110] | |
| 850 °C, ferrocene | MWCNTs | N/A | N/A | [111] | |
| 800 °C, ferrocene 2 | N-MWCNTs and MWCNTs | 10–50 | 0.66–0.98 | [112] | |
| 500–1000 °C, Fe-Co on Y-type zeolites | MWCNTs and SWCNTs | 5–10 (MWCNTs), 0.8–1.2 (SWCNTs) | High purity (SWCNTs) | [114] | |
| 650–1100 °C, ferrocene | MWCNTs | ~7 | N/A | [115] | |
| 850 °C, ferrocene | MWCNTs | 10–100 | N/A | [116] | |
| 850 °C, ferrocene | MWCNTs | 30–80 | N/A | [117] | |
| 700–800 °C, Co and Fe-Co films | MWCNTs and CNF | 35–150 | ~0.80–1.00 | [118] | |
| 850 °C, ferrocene | MWCNTs | ~80 | N/A | [48] | |
| Castor oil | 850 °C, ferrocene 3 | MWCNTs and N-MWCNTs | 20–80 | N/A | [119] |
| 300–400 °C, ferrocene 4 | MWCNTs | 7–50 | N/A | [49] | |
| Eucalyptus oil | 850 °C, Fe-Co on Y-type zeolites | SWCNTs | 0.71–1.71 | High purity | [120] |
| 700 °C, ferrocene | MWCNTs | 15–25 | ~1.0 | [124] | |
| Palm oil | 750 °C, ferrocene | MWCNTs and SWCNTs | 0.6–1.2 (SWCNTs) | 0.52 | [126] |
| 700 °C, ferrocene | MWCNTs | ~30 | 0.73 | [127] | |
| 750 °C, Fe and Co films | MWCNTs | ~30 (Fe cat.), ~90 (Co cat.) | 0.65–0.78 | [128] | |
| 700 °C, Ni and Co | MWCNTs | ~20 | N/A | [129] | |
| 750–950 °C, ferrocene | MWCNTs | 20–70 | 0.89–0.95 | [130] | |
| Sesame oil | 800 °C, ferrocene | MWCNTs | N/A | N/A | [132] |
| 900 °C, ferrocene 5 | N-MWCNTs | 30–60 | 1.02–1.26 | [133] | |
| Sunflower oil | 800–850 °C, ferrocene 3 | N-MWCNTs | 20–40 | ~1.00 | [134] |
| Turpentine oil | 700 °C, ferrocene | MWCNTs | 50–100 | ~0.50–1.00 | [135] |
| 500–900 °C, Fe-Co | MWCNTs | N/A | ~0.30–1.10 | [136] | |
| 800–850 °C, Fe-Co | MWCNTs and SWCNTs | 0.8–1.5 (SWCNTs) | High purity (SWCNTs) | [137] | |
| 700 °C, ferrocene 6 | N-MWCNTs | 7–30 | ~1.10 | [138] | |
| 700–900 °C, ferrocene | MWCNTs | 15–45 | 0.245 | [139] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janas, D. From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes. Sustainability 2020, 12, 4115. https://doi.org/10.3390/su12104115
Janas D. From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes. Sustainability. 2020; 12(10):4115. https://doi.org/10.3390/su12104115
Chicago/Turabian StyleJanas, Dawid. 2020. "From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes" Sustainability 12, no. 10: 4115. https://doi.org/10.3390/su12104115
APA StyleJanas, D. (2020). From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes. Sustainability, 12(10), 4115. https://doi.org/10.3390/su12104115