Comparison of Soil Properties and Organic Components in Infusions According to Different Aerial Appearances of Tea Plantations in Central Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Analysis of the Soil and Tea Leaves
2.3. Soil Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Soil pH, ECw and SOC
3.2. Available N and Available P
3.3. Exchangeable Cations
3.4. Total Concentrations of Soil Cu and Zn
3.5. WAS
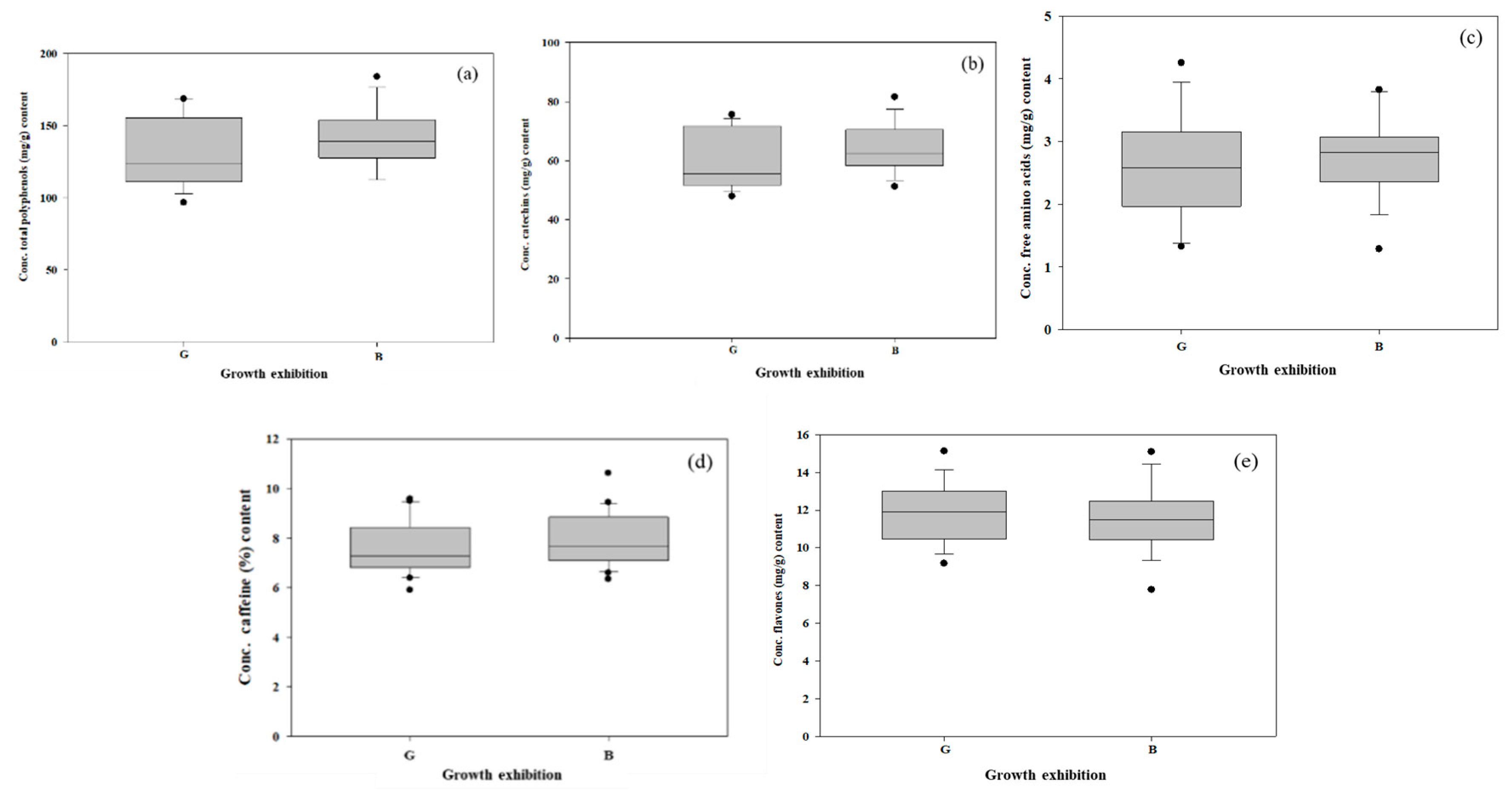
3.6. Organic Compounds in Tea Infusions
4. Discussion
4.1. Critical Soil Properties in Tea Cultivation
4.2. Total Score of SQ
4.3. Relationship between Soil and Infusion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| G | Good growth exhibition |
| B | Bad growth exhibition |
| SOC | Soil organic carbon |
| SOM | Soil organic matter |
| ECw | Electrical conductivity (Wsoil/Vwater = 1/5) |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| Ca | Calcium |
| Mg | Magnesium |
| Cu | Copper |
| Zn | Zinc |
| FAAs | Free amino acids |
| WAS | Soil wet aggregate stability |
| WHC | Water-holding capacity |
| MDS | Total minimum data set |
| SQ | Soil quality |
| SSFs | Standardized scoring functions |
| L | Lower threshold |
| B1 | Baseline |
| U | Upper threshold |
| B2 | Lower baseline |
| B3 | Upper baseline |
| Optimal | Optimum value |
| TARI Taiwan | Taiwan Agricultural Research Institute of Taiwan |
| EPA Taiwan | Environmental Protection Administration of Taiwan |
References
- Hajiboland, R. Environmental and nutritional requirements for tea cultivation. Folia Hortic. 2017, 29, 199–220. [Google Scholar] [CrossRef]
- Chen, Z.S.; Hseu, Z.Y.; Tsai, C.C. The Soils of Taiwan; Springer: Dordrecht, The Netherlands, 2015; p. 127. [Google Scholar]
- Willson, K.C.; Clifford, M.N. Tea: Cultivation to Consumption; Chapman and Hall: London, UK, 1992; pp. 87–321. [Google Scholar]
- Dang, M.V. Soil–plant nutrient balance of tea crops in the northern mountainous region, Vietnam. Agric. Ecosyst. Environ. 2005, 105, 413–418. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L.; Sandamali, J. Assessment of potential land suitability for tea (Camellia sinensis (L.) O. Kuntze) in Sri Lanka using a GIS-based multi-criteria approach. Agriculture 2019, 9, 148. [Google Scholar] [CrossRef]
- Han, W.Y. The major nutrient limiting factors in tea soils and development of series tea speciality fertilizers. J. Tea Sci. 2002, 22, 70–74. [Google Scholar]
- Yan, P.; Shen, C.; Fan, L.C.; Li, X.; Zhang, L.P.; Zhang, L.; Han, W.Y. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, M.K.; Zhuang, S.Y.; Chiang, P.N. Chemical and physical properties of rhizosphere and bulk soils of three tea plants cultivated in Ultisols. Geoderma 2006, 136, 378–387. [Google Scholar] [CrossRef]
- Chintala, R.; McDonald, L.M.; Bryan, W.B. Effect of soil water and nutrients on productivity of Kentucky bluegrass system in acidic soils. J. Plant Nutr. 2012, 35, 288–303. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.K.; Wang, N.; Li, X.H. Soil acidification of alfisols as influenced by tea cultivation in eastern china. Pedosphere 2010, 20, 799–806. [Google Scholar] [CrossRef]
- Konishi, S. Stimulatory effects of aluminium on tea plant growth. In Proceedings of the Transactions 14th International Congress of Soil Science, Kyoto, Japan, 12–18 August 1990. [Google Scholar]
- Oates, K.M.; Kamprath, E.J. Soil Acidity and Liming: I. Effect of the Extracting Solution Cation and pH on the Removal of Aluminum from Acid Soils 1. Soil Sci. Soc. Am. J. 1983, 47, 686–689. [Google Scholar] [CrossRef]
- Wang, R.H.; Zhu, X.F.; Qian, W.; Tang, H.Y.; Jiang, J.; Yu, Y.C.; Xu, R.K. Effect of tea polyphenols on copper adsorption and manganese release in two variable-charge soils. J. Geochem. Explor. 2018, 190, 374–380. [Google Scholar] [CrossRef]
- Behera, S.K.; Shukla, A.K. Spatial distribution of surface soil acidity, electrical conductivity, soil organic carbon content and exchangeable potassium, calcium and magnesium in some cropped acid soils of India. Land Degrad. Dev. 2015, 26, 71–79. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, H.X.; Yang, C.L.; Wang, Y.D.; Xue, H.; Niu, Y.F. Rates of soil acidification in tea plantations and possible causes. Agric. Ecosyst. Environ. 2016, 233, 60–66. [Google Scholar] [CrossRef]
- Hayatsu, M.; Kosuge, N. Effects of difference in fertilization treatments on nitrification activity in tea soils. Soil Sci. Plant Nutr. 1993, 39, 373–378. [Google Scholar] [CrossRef]
- Van Dang, M. Effects of Tea Cultivation on Soil Quality in the Northern Mountainous Zone, Vietnam. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2002. [Google Scholar]
- Cañasveras, J.C.; Barron, V.; Del Campillo, M.C.; Torrent, J.; Gomez, J.A. Estimation of aggregate stability indices in Mediterranean soils by diffuse reflectance spectroscopy. Geoderma 2010, 158, 78–84. [Google Scholar] [CrossRef]
- Mudau, F.N.; Soundy, P.; Du Toit, E.S. Nitrogen, phosphorus, and potassium nutrition increases growth and total polyphenol concentrations of bush tea in a shaded nursery environment. HortTechnology 2007, 17, 107–110. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, X.; Wang, X.; Yu, Y. Studies on Phosphorus Nutrition in Red Soil of Tea Field. In Tea Science Research Proceedings; Tea Research Institute of Chinese Academy of Agricultural Science, Shanghai Scientific and Technological Publisher: Shanghai, China, 1992. [Google Scholar]
- Zhang, Y.L.; Luo, S.H.; Zeng, Y.H.; Peng, F.Y. Study on nutrient scale of sufficiency or deficiency in tea soils in hunan province and fertilizing recommendation. J. Tea Sci. 1997, 17, 161–170. [Google Scholar]
- Li, J.; Xia, J.G. Summary on nitrogen (N), phosphorus (P), potassium (K) and tea quality. Chin. Sci. Bull. 2005, 21, 62–65. [Google Scholar]
- Ruan, J.; Wu, X.; Härdter, R. Effects of potassium and magnesium nutrition on the quality components of different types of tea. J. Sci. Food Agric. 1999, 79, 47–52. [Google Scholar] [CrossRef]
- Venkatesan, S.; Ganapathy, M.N.K. Impact of nitrogen and potassium fertiliser application on quality of CTC teas. Food Chem. 2004, 84, 325–328. [Google Scholar] [CrossRef]
- Ruan, J. Quality-Related Constituents in Tea (Camellia sinensis (L.) O. Kuntze) as Affected by the form and Concentration of Nitrogen and the Supply of Chloride. Ph.D. Thesis, Christian-Albrechts Universität Kiel, Kiel, Germany, February 2005. [Google Scholar]
- Zhao, H.; Zhang, S.; Zhang, Z. Relationship between multi-element composition in tea leaves and in provenance soils for geographical traceability. Food Control 2017, 76, 82–87. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA and ASA Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Murer, E.J.; Baumgarten, A.; Eder, G.; Gerzabek, M.H.; Kandeler, E.; Rampazzo, N. An Improved Sieving Machine for Estimation of Soil Aggregate Stability (SAS). In Soil Structure/Soil Biota Interrelationships; Brussaardm, L., Kooistra, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 539–547. [Google Scholar]
- Ou, S.M.; Liu, S.L.; Tsai, Y.S.; Chen, K.R. Characteristics of Taiwan Tiehkuanyin tea identified by physicochemical and stepwise discriminant analyses. Taiwan Tea Res. Bull. 2004, 23, 133–144. [Google Scholar]
- Huang, B.; Shi, X.; Yu, D.; Öborn, I.; Blombäck, K.; Pagella, T.F.; Wang, H.; Sun, W.; Sinclair, F.L. Environmental assessment of small-scale vegetable farming systems in peri-urban areas of the Yangtze River Delta Region, China. Agric. Ecosyst. Environ. 2006, 112, 391–402. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Liu, P.P.; Shi, J.; Gao, Y.; Wang, Q.S.; Yin, J.F. Quality development and main chemical components of Tieguanyin oolong teas processed from different parts of fresh shoots. Food Chem. 2018, 249, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Parkin, T.B.; Eash, N.S. Defining soil quality for a sustainable environment. In Methods for Assessing Soil Quality; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; CAB International: Wallingford, UK, 1997; pp. 345–355. [Google Scholar]
- Karlen, D.L.; Andrews, S.S.; Doran, J.W. Soil quality: Current concepts and applications. Adv. Agron. 2001, 74, 1–40. [Google Scholar]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Gil-Vázquez, J.M.; Delgado-Iniesta, M.J.; Marín-Sanleandro, P.; Blanco-Bernardeau, A.; Ortiz-Silla, R. Establishing an index and identification of limiting parameters for characterizing soil quality in Mediterranean ecosystems. Catena 2015, 131, 35–45. [Google Scholar] [CrossRef]
- Karlen, D.L.; Stott, D.E. A framework for evaluating physical and chemical indicators of soil quality in Iowa. In Methods for Assessing Soil Quality; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; CAB International: Wallingford, UK, 1994; pp. 53–72. [Google Scholar]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, D.L.; Hartz, T.K.; Horwath, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On-farm assessment of soil quality in California’s central valley. Agron. J. 2002, 94, 12–23. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, M.Y.; Asio, V.B.; Chen, Z.S. Using a soil quality index to assess the effects of applying swine manure compost on soil quality under a crop rotation system in Taiwan. Soil Sci. 2006, 171, 210–222. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Gamero, C.A.; Rodrigues, J.G.L.; Mirás-Avalos, J.M. Determination of the quality index of a Paleudult under sunflower culture and different management systems. Soil Tillage Res. 2011, 112, 167–174. [Google Scholar] [CrossRef]
- Rahmanipour, F.; Marzaioli, R.; Bahrami, H.A.; Fereidouni, Z.; Bandarabadi, S.R. Assessment of soil quality indices in agricultural lands of Qazvin Province. Iran. Ecol. Indic. 2014, 40, 19–26. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Cohen, J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ. Psychol. Meas. 1973, 33, 613–619. [Google Scholar] [CrossRef]
- Hudson, W.D. Correct Formulation of the Kappa-Coefficient of Agreement. Photogramm. Eng. Remote Sens. 1987, 53, 421–422. [Google Scholar]
- Perroca, M.G.; Gaidzinski, R.R. Avaliando a confiabilidade interavaliadores de um instrumento para classificação de pacientes: Coeficiente Kappa. Rev. Esc. Enferm. USP 2003, 37, 72–80. [Google Scholar] [CrossRef][Green Version]
- Souza, F.B.D.; Souza, É.D.J.C.D.; Garcia, M.C.D.M.; Madeira, K. A fuzzy logic-based expert system for substrate selection for soil construction in land reclamation. REM 2018, 71, 553–559. [Google Scholar] [CrossRef]
- Lai, H.Y. Negative effects of chelants on soil qualities of five soil series. Int. J. Phytoremediat. 2015, 17, 228–234. [Google Scholar] [CrossRef]
- Periago, M.V.; Diniz, R.C.; Pinto, S.A.; Yakovleva, A.; Correa-Oliveira, R.; Diemert, D.J.; Bethony, J.M. The Right Tool for the Job: Detection of Soil-Transmitted Helminths in Areas Co-endemic for Other Helminths. PLoS Negl. Trop. Dis. 2015, 9, e0003967. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, C.; Li, X.; Zhang, T.; Wang, X. Soil quality evaluation for navel orange production systems in central subtropical China. Soil Tillage Res. 2016, 155, 225–232. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xi, M.; Zhao, Q.; Lu, Q.; Jia, J. Soil quality assessment of coastal wetlands in the Yellow River Delta of China based on the minimum data set. Ecol. Indic. 2016, 66, 458–466. [Google Scholar] [CrossRef]
- Lin, Y.; Deng, H.; Du, K.; Li, J.; Lin, H.; Chen, C.; Fisher, L.; Wu, C.; Hong, T.; Zhang, G. Soil quality assessment in different climate zones of China’s Wenchuan earthquake affected region. Soil Tillage Res. 2017, 165, 315–324. [Google Scholar] [CrossRef]
- Lin, L.L. Evaluation of different soil conservation practices on a tea plantation in Taiwan. In Soil Erosion; American Society of Agricultural and Biological Engineers: Honolulu, HI, USA, 2001; pp. 187–190. [Google Scholar]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Juhos, K.; Czigány, S.; Madarász, B.; Ladányi, M. Interpretation of soil quality indicators for land suitability assessment—A multivariate approach for Central European arable soils. Ecol. Indic. 2019, 99, 261–272. [Google Scholar] [CrossRef]
- Lal, R.; Stewart, B.A. Food Security and Soil Quality; CRC: Boca Raton, FL, USA, 2010; p. 418. [Google Scholar]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of soil quality index using three methods. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef]
- Lin, Z.H.; Qi, Y.P.; Chen, R.B.; Zhang, F.Z.; Chen, L.S. Effects of phosphorus supply on the quality of green tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Millard, P.; Grelet, G.A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef]
- Jayaganesh, S.; Venkatesan, S. Impact of magnesium sulphate on biochemical and quality constituents of black tea. Am. J. Food Technol. 2010, 5, 31–39. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric. 2012, 92, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Jones, D.L. Soil acidification used as a management strategy to reduce nitrate losses from agricultural land. Soil Biol. Biochem. 2005, 37, 867–875. [Google Scholar] [CrossRef]
- Yu, P.; Yeo, A.S.; Low, M.Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef]
- Sidari, M.; Panuccio, M.R.; Muscolo, A. Influence of acidity on growth and biochemistry of Pennisetum clandestinum. Biol. Plant. 2004, 48, 133–136. [Google Scholar] [CrossRef]
- Lucassen, E.C.H.E.T.; Bobbink, R.; Smolders, A.J.P.; Van der Ven, P.J.M.; Lamers, L.P.M.; Roelofs, J.G.M. Interactive effects of low pH and high ammonium levels responsible for the decline of Cirsium dissectum (L.) Hill. Plant Ecol. 2003, 165, 45–52. [Google Scholar] [CrossRef]
- Sharma, K.L.; Shankar, G.M.; Chandrika, D.S.; Grace, J.K.; Sharma, S.K.; Thakur, H.S.; Jain, M.P.; Sharma, R.A.; Chary, G.R.; Srinivas, K.; et al. Effects of Conjunctive Use of Organic and Inorganic Sources of Nutrients on Soil Quality Indicators and Soil Quality Index in Sole Maize, Maize plus Soybean, and Sole Soybean Cropping Systems in Hot Semi-arid Tropical Vertisol. Commun. Soil Sci. Plant Anal. 2014, 45, 2118–2140. [Google Scholar] [CrossRef]
- Plaster, E.J. Soil Science and Management, 6th ed.; Delmar, Cengage Learning: Clifton Park, NY, USA, 2014; pp. 246–279. [Google Scholar]



| Indicators | Expected Range | Unit | Type of Curve | Lower Threshold | Baseline | Upper Threshold | Lower Baseline | Upper Baseline | Optimum Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| SSF | Type | L | B1 | U | B2 | B3 | Optimal | |||
| Chemical properties | ||||||||||
| SSF Score | 0 | 0.4 | 0.6 | 0.8 | 1 | |||||
| Exchangeable K | 100–1000 | mg/kg | 5 | Optimum | 100 | - | 800 | 350 | 650 | 500 |
| Available N | 3–50 | mg/kg | 5 | Optimum | 3 | - | 50 | 12 | 38 | 25 |
| pH | 3–7.7 | 5 | Optimum | 3 | - | 7.7 | 4.2 | 6.5 | 5 | |
| ECw | 0–2 | dS/m | 5 | Optimum | 0 | - | 2 | 0.25 | 1.8 | 1 |
| Available P | 7.5–200 | mg/kg | 5 | Optimum | 10 | - | 300 | 60 | 200 | 100 |
| SSF Score | 1 | 0.5 | 0 | |||||||
| Cu | 0–20 | mg/kg | 9 | Less is better | 0 | 10 | 20 | - | - | - |
| Zn | 0–50 | mg/kg | 9 | Less is better | 0 | 25 | 50 | - | - | - |
| SSF Score | 0 | 0.5 | 1 | |||||||
| SOC | 0–5 | % | 3 | More is better | 0 | 0.87 | 1.74 | - | - | - |
| Exchangeable Ca | 0–300 | mg/kg | 3 | More is better | 0 | 300 | 800 | - | - | - |
| Exchangeable Mg | 0–200 | mg/kg | 3 | More is better | 0 | 75 | 200 | - | - | - |
| Physical properties | ||||||||||
| Wet aggregate stability | 15–70 | % | 3 | More is better | 15 | 30 | 70 | - | - | - |
| Soil Property | Mean | Minimum | Maximum | Pearson Correlation 1,2 | Sig | t-Test | Median | Std. Deviation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | B | G | B | G | B | G-B | G | B | G | B | G | B | ||
| Exchangeable K | 219.03 | 195.55 | 48.66 ± 0.00 | 75.90 ± 0.92 | 643.82 ± 9.24 | 658.48 ± 4.59 | 0.66 ** | 0.00 | 14.85 | 12.78 | 209.22 | 152.45 | 108.39 | 112.41 |
| Available N | 23.95 | 18.60 | 0.00 ± 0.00 | 0.00 ± 0.01 | 165.13 ± 0.00 | 364.66 ± 0.00 | 0.71 ** | 0.00 | 5.47 | 2.76 | 13.76 | 8.60 | 32.17 | 49.55 |
| pH | 3.84 | 4.08 | 3.29 ± 0.00 | 3.33 ± 0.01 | 4.77 ± 0.01 | 4.87 ± 0.08 | 0.55 ** | 0.00 | 76.14 | 73.56 | 3.87 | 4.13 | 0.37 | 0.41 |
| ECw | 0.32 | 0.22 | 0.05 ± 0.00 | 0.05 ± 0.00 | 1.01 ± 0.00 | 0.78 ± 0.01 | 0.73 ** | 0.00 | 10.52 | 8.97 | 0.22 | 0.15 | 0.22 | 0.18 |
| Available P | 169.4 | 180.49 | 2.53 ± 0.61 | 6.53 ± 0.00 | 640.81 ± 6.22 | 721.12 ± 3.11 | 0.61 ** | 0.00 | 7.78 | 9.01 | 126.27 | 143.46 | 159.94 | 147.16 |
| Cu | 15.94 | 16.18 | 4.55 ± 0.06 | 7.34 ± 0.12 | 35.68 ± 0.05 | 25.87 ± 0.13 | 0.34 * | 0.01 | 18.05 | 25.38 | 15.43 | 15.65 | 6.49 | 4.69 |
| Zn | 32.08 | 34.07 | 14.05 ± 0.17 | 22.12 ± 1.6 | 53.80 ± 2.71 | 63.64 ± 1.35 | 0.24 | 0.08 | 27.87 | 31.567 | 31.85 | 32.13 | 8.46 | 7.93 |
| Soil organic carbon | 1.23 | 1.16 | 0.33 ± 0.05 | 0.43 ± 0.02 | 4.88 ± 0.18 | 3.35 ± 0.13 | 0.57 ** | 0.00 | 12.69 | 18.711 | 1.09 | 1.15 | 0.71 | 0.46 |
| Exchangeable Ca | 173.58 | 219.8 | 17.24 ± 0.86 | 15.21 ± 1.04 | 816.92 ± 5.74 | 787.28 ± 16.04 | 0.37 ** | 0.01 | 7.53 | 8.15 | 131.58 | 148.78 | 169.39 | 198.25 |
| Exchangeable Mg | 118.94 | 122.06 | 34.78 ± 5.59 | 39.12 ± 2.60 | 412.05 ± 22.16 | 460.75 ± 6.49 | 0.68 ** | 0.00 | 12.4 | 11.83 | 99.87 | 97.13 | 70.49 | 75.79 |
| Wet aggregate stability | 45.76 | 45.87 | 29.64 ± 0.56 | 20.84 ± 6.67 | 65.69 ± 1.64 | 66.33 ± 5.61 | 0.55 ** | 0.00 | 37.95 | 36.14 | 44.93 | 45.70 | 8.8609 | 9.3253 |
| Description | Total Polyphenols (mg/g) | Catechins (mg/g) | Free amino Acids (mg/g) | Caffeine (%) | Flavones (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| G | B | G | B | G | B | G | B | G | B | |
| Mean | 131.65 | 140.48 | 59.08 | 63.67 | 2.45 | 2.71 | 7.60 | 7.94 | 11.53 | 11.40 |
| Minimum | 96.69 ± 23.44 | 112.48 ± 15.07 | 47.94 ± 9.70 | 49.18 ± 0.84 | 1.13 ± 0.07 | 1.12 ± 0.05 | 5.89 ± 0.24 | 6.34 ± 0.25 | 8.29 ± 0.13 | 7.78 ± 0.81 |
| Maximum | 168.52 ± 4.47 | 183.91 ± 20.09 | 75.59 ± 0.68 | 81.55 ± 0.97 | 4.25 ± 0.23 | 3.85 ± 0.04 | 9.57 ± 1.07 | 10.61 ± 0.15 | 15.12 ± 0.11 | 15.09 ± 0.11 |
| Pearson Correlation 1,2 | 0.57 ** | 0.68 ** | 0.54 * | 0.65 ** | 0.61 ** | |||||
| Sig. (2-tailed) | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | |||||
| Pair 1 G-B | −1.97 | −2.79 | −1.45 | −1.69 | 0.37 | |||||
| Sig. (2-tailed) | 0.06 | 0.01 | 0.16 | 0.11 | 0.71 | |||||
| t-test | 25.53 | 31.74 | 28.30 | 31.12 | 12.47 | 15.84 | 32.57 | 33.38 | 31.31 | 28.69 |
| Sig. (2-tailed) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Element | Fleiss’ Kappa 1 | SE kappa (Asymptotic Standard Error) | Z | p-Value | Interpretation | Weight |
|---|---|---|---|---|---|---|
| Exchangeable K | 0.62 | 0.19 | 3.33 | 0.00 | Substantial agreement | 4 |
| Available N | 0.08 | 0.17 | 0.45 | 0.66 | Slight agreement | 1 |
| pH | 0.64 | 0.21 | 3.01 | 0.00 | Substantial agreement | 4 |
| ECw | 0.45 | 0.21 | 2.13 | 0.03 | Moderate agreement | 3 |
| Available P | 0.32 | 0.21 | 1.52 | 0.13 | Fair agreement | 2 |
| Cu | 0.43 | 0.15 | 2.79 | 0.01 | Moderate agreement | 3 |
| Zn | 0.41 | 0.2 | 2.10 | 0.04 | Moderate agreement | 3 |
| Soil organic carbon | 0.48 | 0.09 | 5.06 | 0.00 | Moderate agreement | 3 |
| Exchangeable Ca | 0.33 | 0.2 | 1.69 | 0.09 | Fair agreement | 2 |
| Exchangeable Mg | 0.55 | 0.16 | 3.47 | 0.00 | Moderate agreement | 3 |
| Wet aggregate stability | 0.86 | 0.20 | 2.46 | 0.00 | Almost perfect agreement | 5 |
| Content | Mean | Pearson Correlation 1,2 | Sig. (2-tailed) | Content | Mean | Pearson Correlation 1,2 | Sig. (2-tailed) |
|---|---|---|---|---|---|---|---|
| Exchangeable K | 206.03 | Available P | 173.52 | ||||
| Free amino acids | 2.67 | −0.28 | 0.11 | Free amino acids | 2.67 | −0.36 * | 0.03 |
| Total polyphenols | 136.99 | −0.04 | 0.81 | Total polyphenols | 136.99 | −0.08 | 0.64 |
| Catechins | 62.04 | −0.13 | 0.45 | Catechins | 62.04 | −0.05 | 0.77 |
| Caffeine | 8 | −0.14 | 0.41 | Caffeine | 8 | −0.02 | 0.9 |
| Flavones | 11.73 | −0.29 | 0.09 | Flavones | 11.73 | −0.13 | 0.46 |
| Available N | 21.15 | Cu | 16.1 | ||||
| Free amino acids | 2.67 | −0.16 | 0.35 | Free amino acids | 2.67 | 0.42 * | 0.01 |
| Total polyphenols | 136.99 | −0.21 | 0.22 | Total polyphenols | 136.99 | −0.2 | 0.25 |
| Catechins | 62.04 | −0.2 | 0.25 | Catechins | 62.04 | −0.33 | 0.05 |
| Caffeine | 8 | −0.15 | 0.39 | Caffeine | 8 | −0.22 | 0.2 |
| Flavones | 11.73 | −0.29 | 0.08 | Flavones | 11.73 | −0.05 | 0.77 |
| pH | 3.96 | Zn | 32.95 | ||||
| Free amino acids | 2.67 | 0.37 * | 0.03 | Free amino acids | 2.67 | 0.2 | 0.25 |
| Total polyphenols | 136.99 | 0.16 | 0.37 | Total polyphenols | 136.99 | −0.26 | 0.12 |
| Catechins | 62.04 | 0.05 | 0.78 | Catechins | 62.04 | −0.17 | 0.31 |
| Caffeine | 8 | 0.15 | 0.38 | Caffeine | 8 | 0.14 | 0.43 |
| Flavones | 11.73 | 0.25 | 0.14 | Flavones | 11.73 | −0.38 * | 0.02 |
| ECw | 0.27 | Soil organic carbon | 1.19 | ||||
| Free amino acids | 2.67 | −0.53 ** | 0 | Free amino acids | 2.67 | −0.55 ** | 0 |
| Total polyphenols | 136.99 | −0.18 | 0.3 | Total polyphenols | 136.99 | −0.03 | 0.86 |
| Catechins | 62.04 | −0.1 | 0.55 | Catechins | 62.04 | −0.16 | 0.34 |
| Caffeine | 8 | −0.15 | 0.39 | Caffeine | 8 | −0.1 | 0.58 |
| Flavones | 11.73 | −0.36 * | 0.03 | Flavones | 11.73 | −0.12 | 0.47 |
| Exchangeable Ca | 195.88 | Wet aggregate stability | 45.72 | ||||
| Free amino acids | 2.67 | −0.10 | 0.58 | Free amino acids | 2.67 | −0.25 | 0.14 |
| Total polyphenols | 136.99 | 0.05 | 0.74 | Total polyphenols | 136.99 | −0.07 | 0.71 |
| Catechins | 62.04 | 0.02 | 0.93 | Catechins | 62.04 | −0.14 | 0.42 |
| Caffeine | 8 | 0.09 | 0.59 | Caffeine | 8 | −0.28 | 0.1 |
| Flavones | 11.73 | 0.02 | 0.92 | Flavones | 11.73 | −0.28 | 0.1 |
| Exchangeable Mg | 118.93 | ||||||
| Free amino acids | 2.67 | −0.39 * | 0.02 | ||||
| Total polyphenols | 136.99 | 0.01 | 0.98 | ||||
| Catechins | 62.04 | −0.07 | 0.7 | ||||
| Caffeine | 8 | 0.03 | 0.84 | ||||
| Flavones | 11.73 | −0.08 | 0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tongsiri, P.; Tseng, W.-Y.; Shen, Y.; Lai, H.-Y. Comparison of Soil Properties and Organic Components in Infusions According to Different Aerial Appearances of Tea Plantations in Central Taiwan. Sustainability 2020, 12, 4384. https://doi.org/10.3390/su12114384
Tongsiri P, Tseng W-Y, Shen Y, Lai H-Y. Comparison of Soil Properties and Organic Components in Infusions According to Different Aerial Appearances of Tea Plantations in Central Taiwan. Sustainability. 2020; 12(11):4384. https://doi.org/10.3390/su12114384
Chicago/Turabian StyleTongsiri, Prapasiri, Wen-Yu Tseng, Yuan Shen, and Hung-Yu Lai. 2020. "Comparison of Soil Properties and Organic Components in Infusions According to Different Aerial Appearances of Tea Plantations in Central Taiwan" Sustainability 12, no. 11: 4384. https://doi.org/10.3390/su12114384
APA StyleTongsiri, P., Tseng, W.-Y., Shen, Y., & Lai, H.-Y. (2020). Comparison of Soil Properties and Organic Components in Infusions According to Different Aerial Appearances of Tea Plantations in Central Taiwan. Sustainability, 12(11), 4384. https://doi.org/10.3390/su12114384







