Personal Growth and Psychobiological Stress Responsiveness to the Trier Social Stress Test in Students
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Mental Stress Test
2.4. Questionnaire
2.4.1. Personal Growth
2.4.2. Perceived Health
2.4.3. Positive and Negative Affect
2.4.4. Perceived Stress
2.5. Physiological Indicators
2.5.1. Salivary Free-MHPG
2.5.2. HF and HR
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
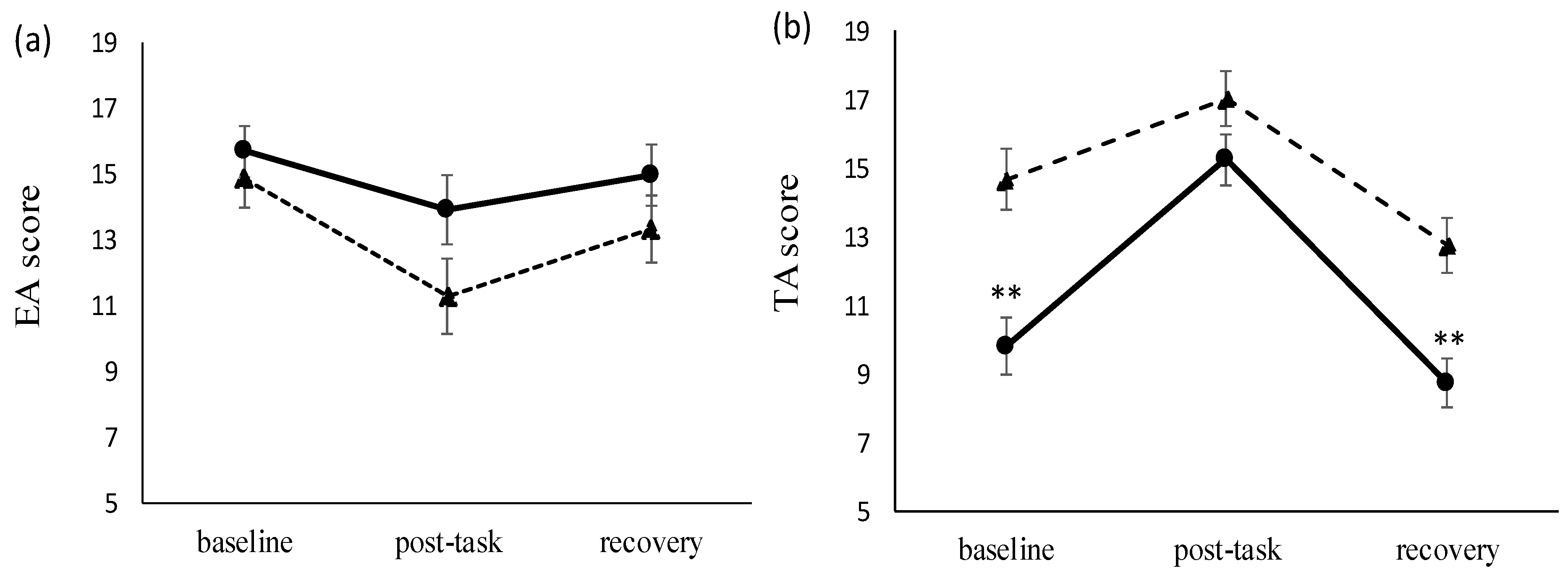
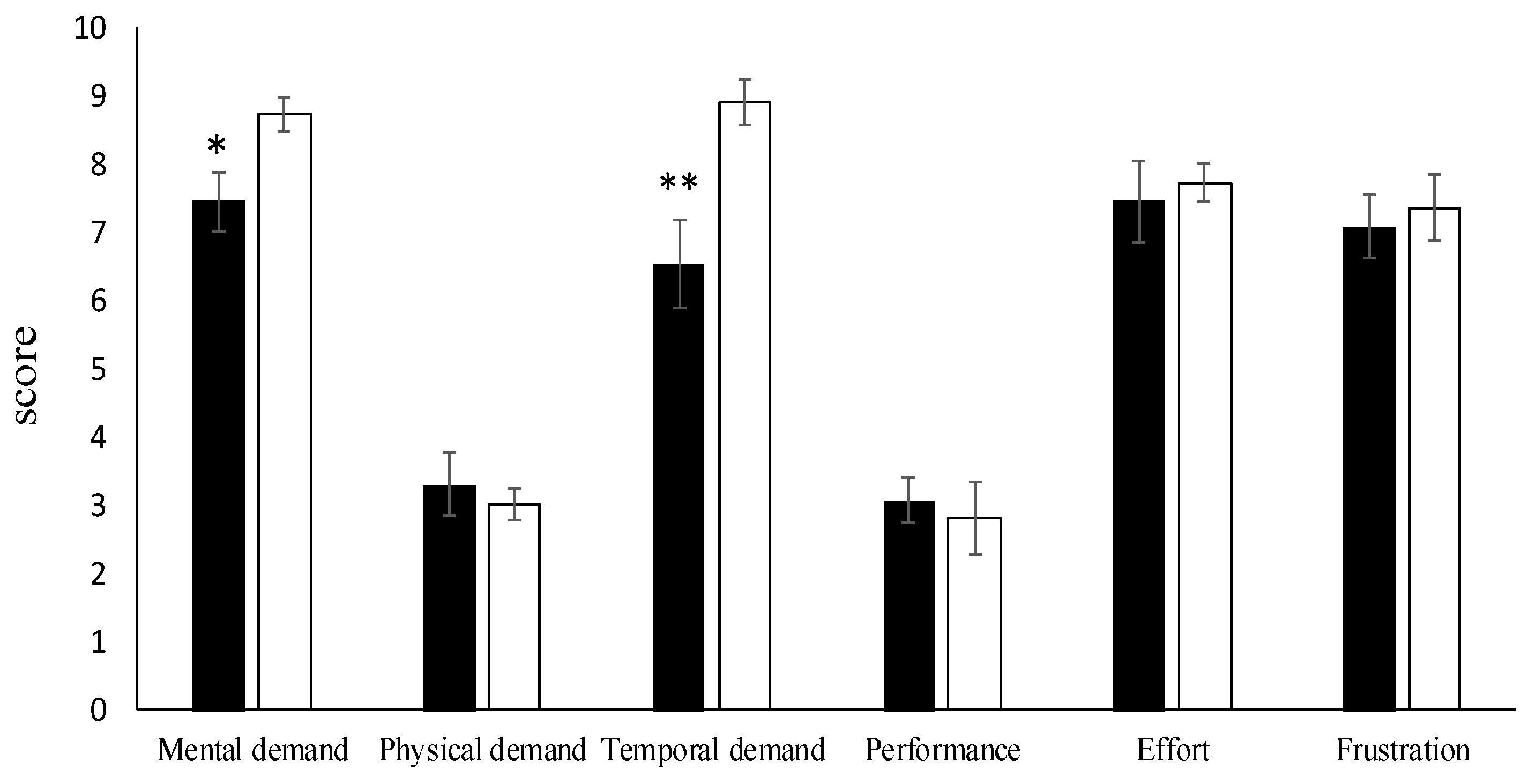
3.2. Perceived Stress
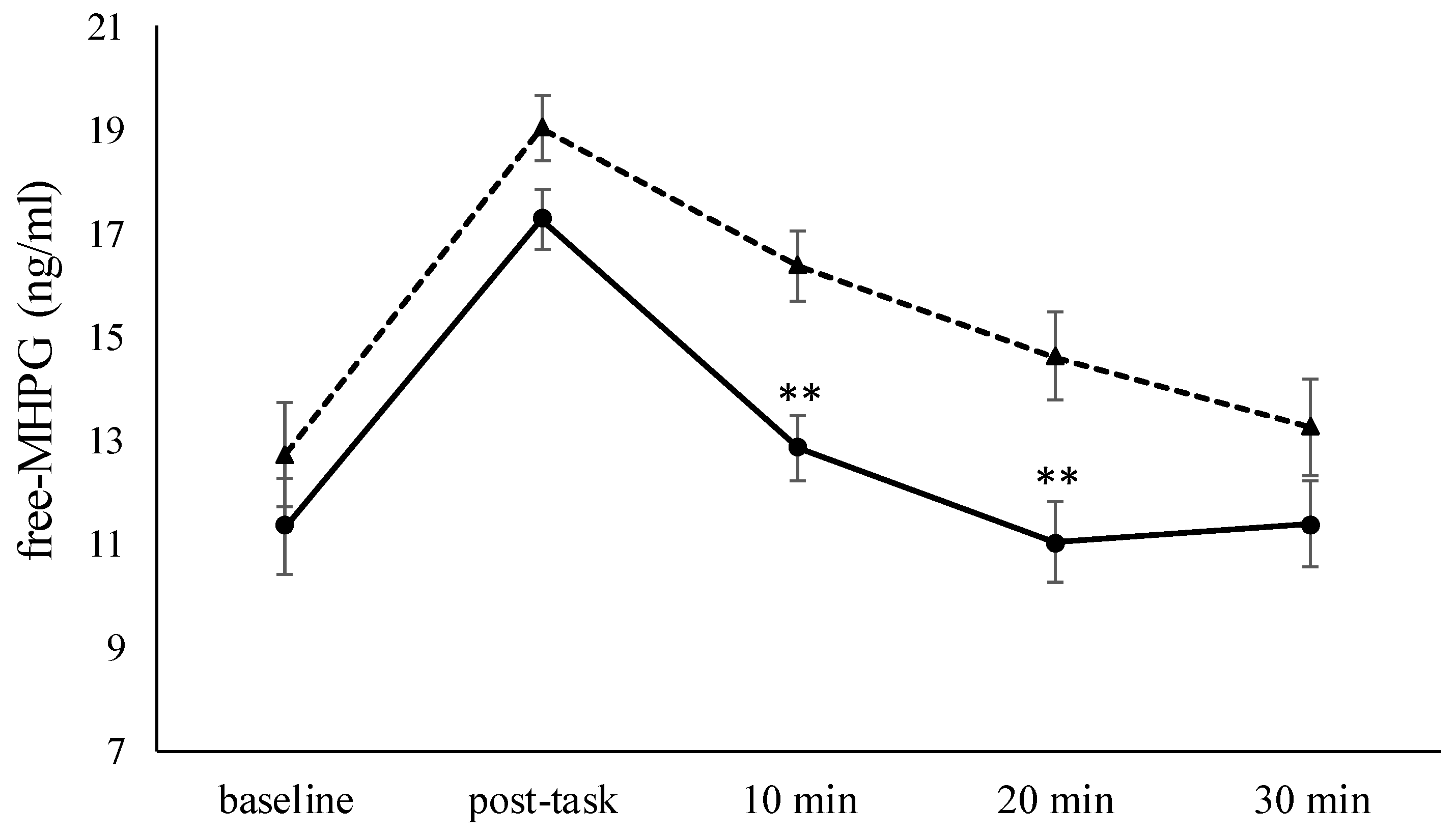
3.3. Salivary Free-MHPG
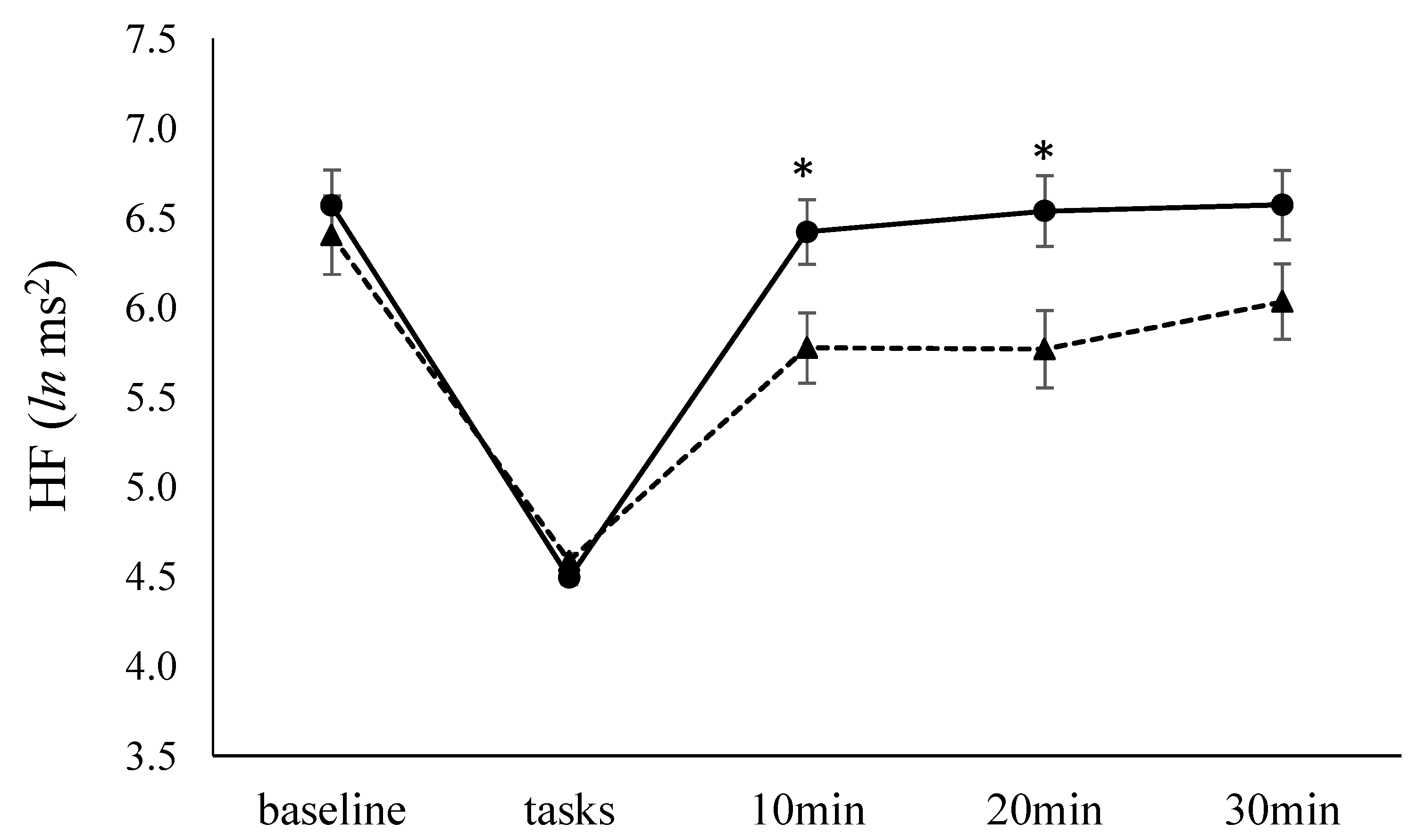
3.4. HF and HR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyerson, D.A.; Grant, K.E.; Carter, J.S.; Kilmer, R.P. Posttraumatic growth among children and adolescents: A systematic review. Clin. Psychol. Rev. 2011, 31, 949–964. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, C.P.P.; Damásio, B.F.; Tobo, P.R.; Kamei, H.H.; Koller, S.H. Systematic Review about Personal Growth Initiative. Ann. Psicol. 2016, 32, 770–782. [Google Scholar] [CrossRef]
- Brandel, M.; Vescovelli, F.; Ruini, C. Beyond Ryff’s scale: Comprehensive measures of eudaimonic well-being in clinical populations. A systematic review. Clin. Psychol. Psychother. 2017, 24, O1524–O1546. [Google Scholar] [CrossRef] [PubMed]
- Ryff, C.D.; Singer, B.H. Know thyself and become what you are: A eudaimonic approach to psychological well-being. J. Happiness Stud. 2008, 9, 13–39. [Google Scholar] [CrossRef]
- Ryan, R.M.; Huta, V.; Deci, E.L. Living well: A self-determination theory perspective on eudaimonia. J. Happiness Stud. 2008, 9, 139–170. [Google Scholar] [CrossRef]
- Waterman, A.S. Two Conceptions of Happiness: Contrasts of Personal Expressiveness (Eudaimonia) and Hedonic Enjoyment. J. Personal. Soc. Psychol. 1993, 64, 678–691. [Google Scholar] [CrossRef]
- Waterman, A.S. Reconsidering happiness: A eudaimonist’s perspective. J. Posit. Psychol. 2008, 3, 234–252. [Google Scholar] [CrossRef]
- Ryff, C.D.; Keyes, C.L.M. The Structure of Psychological Well-Being Revisited. J. Personal. Soc. Psychol. 1995, 69, 719–727. [Google Scholar] [CrossRef]
- Monteiro, S.; Torres, A.; Morgadinho, R.; Pereira, A. Psychosocial Outcomes in Young Adults with Cancer: Emotional Distress, Quality of Life and Personal Growth. Arch. Psychiatr. Nurs. 2013, 27, 299–305. [Google Scholar] [CrossRef]
- McCarthy, M. Psychological Sense of Community and Student Burnout. J. Coll. Stud. Dev. 1990, 31, 211–216. [Google Scholar]
- Seligman, M.E.; Csikszentmihalyi, M. Positive psychology. An introduction. Am. Psychol. 2000, 55, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.K.; Kubzansky, L.D. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol. Bull. 2012, 138, 655–691. [Google Scholar] [CrossRef] [PubMed]
- Kubzansky, L.D.; Huffman, J.C.; Boehm, J.K.; Hernandez, R.; Kim, E.S.; Koga, H.K.; Feig, E.H.; Lloyd-Jones, D.M.; Seligman, M.E.P.; Labarthe, D.R. Positive Psychological Well-Being and Cardiovascular Disease: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.Z.; Slavich, G.M.; Thaker, P.H.; Goodheart, M.J.; Bender, D.P.; Dahmoush, L.; Farley, D.M.; Markon, K.E.; Penedo, F.J.; Lubaroff, D.M.; et al. Eudaimonic well-being and tumor norepinephrine in patients with epithelial ovarian cancer. Cancer 2015, 121, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Aldridge-Gerry, A.; Spiegel, D. Posttraumatic growth and diurnal cortisol slope among women with metastatic breast cancer. Psychoneuroendocrinology 2014, 44, 83–87. [Google Scholar] [CrossRef]
- Ryff, C.D.; Singer, B.H.; Love, G.D. Positive health: Connecting well-being with biology. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1383–1394. [Google Scholar] [CrossRef]
- Fredrickson, B.L.; Grewen, K.M.; Coffey, K.A.; Algoe, S.B.; Firestine, A.M.; Arevalo, J.M.G.; Ma, J.; Cole, S.W. A functional genomic perspective on human well-being. Proc. Natl. Acad. Sci. USA 2013, 110, 13684–13689. [Google Scholar] [CrossRef]
- Fredrickson, B.L.; Grewen, K.M.; Algoe, S.B.; Firestine, A.M.; Arevalo, J.M.G.; Ma, J.; Cole, S.W. Psychological well-being and the human conserved transcriptional response to adversity. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Kitayama, S.; Akutsu, S.; Uchida, Y.; Cole, S.W. Work, meaning, and gene regulation: Findings from a Japanese information technology firm. Psychoneuroendocrinology 2016, 72, 175–181. [Google Scholar] [CrossRef]
- Boyle, C.C.; Cole, S.W.; Dutcher, J.M.; Eisenberger, N.I.; Bower, J.E. Changes in eudaimonic well-being and the conserved transcriptional response to adversity in younger breast cancer survivors. Psychoneuroendocrinology 2019, 103, 173–179. [Google Scholar] [CrossRef]
- Seeman, T.; Merkin, S.S.; Goldwater, D.; Cole, S.W. Intergenerational mentoring, eudaimonic well-being and gene regulation in older adults: A pilot study. Psychoneuroendocrinology 2020, 111, 104468. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.P.; Schwarz, E.; McKinley, P.S.; Weinstein, M.; Love, G.; Ryff, C.; Mroczek, D.; Choo, T.H.; Lee, S.; Seeman, T. Vagally-mediated heart rate variability and indices of well-being: Results of a nationally representative study. Health Psychol. 2017, 36, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tsenkova, V.K.; Love, G.D.; Singer, B.H.; Ryff, C.D. Socioeconomic status and psychological well-being predict cross-time change in glycosylated hemoglobin in older women without diabetes. Psychosom. Med. 2007, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Ryff, C.D.; Dienberg Love, G.; Urry, H.L.; Muller, D.; Rosenkranz, M.A.; Friedman, E.M.; Davidson, R.J.; Singer, B. Psychological well-being and ill-being: Do they have distinct or mirrored biological correlates? Psychother. Psychosom. 2006, 75, 85–95. [Google Scholar] [CrossRef]
- Friedman, E.M.; Hayney, M.; Love, G.D.; Singer, B.H.; Ryff, C.D. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007, 26, 305–313. [Google Scholar] [CrossRef]
- Bower, J.E.; Low, C.A.; Moskowitz, J.T.; Sepah, S.; Epel, E. Benefit Finding and Physical Health: Positive Psychological Changes and Enhanced Allostasis. Soc. Personal. Psychol. Compass 2008, 2, 223–244. [Google Scholar] [CrossRef]
- Bower, J.E.; Moskowitz, J.T.; Epel, E. Is Benefit Finding Good for Your Health? Curr. Dir. Psychol. Sci. 2009, 18, 337–341. [Google Scholar] [CrossRef]
- Epel, E.S.; McEwen, B.S.; Ickovics, J.R. Embodying psychological thriving: Physical thriving in response to stress. J. Soc. Issues 1998, 54, 301–322. [Google Scholar] [CrossRef]
- Di Fabio, A. Positive healthy organizations: Promoting well-being, meaningfulness, and sustainability in organizations. Front. Psychol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Di Fabio, A.; Tsuda, A. The psychology of Harmony and Harmonization: Advancing the perspectives for the psychology of sustainability and sustainable development. Sustainability 2018, 10, 4726. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The “Trier social stress test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ryff, C.D. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J. Personal. Soc. Psychol. 1989, 57, 1069–1081. [Google Scholar] [CrossRef]
- Nishida, Y. Diverse life-styles and psychological well-being in adult women. Jpn. Assoc. Educ. Psychol. 2000, 48, 433–443. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Daibo, I. Japanese Version of the General Health Questionnaire; Nihon Bunka Kagakusha: Tokyo, Japan, 1985. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Kawahito, J.; Otsuka, Y.; Kaida, K.; Nakata, A. Reliability and validity of the Japanese version of 20-item Positive and Negative Affect Schedule. Hiroshima Psychol. Res. 2011, 225–240. [Google Scholar] [CrossRef]
- Matthews, G.; Jones, D.M.; Chamberlain, A.G. Refining the measurement of mood: The UWIST Mood Adjective Checklist. Br. J. Psychol. 1990, 81, 17–42. [Google Scholar] [CrossRef]
- Okamura, H.; Tsuda, A.; Yajima, J. Stress state questionnaire. In Stress Scale Guidebook; Public Research Center, Ed.; Jitsumukyoiku-Shuppan: Tokyo, Japan, 2004; pp. 214–220. [Google Scholar]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. Adv. Psychol. 1988, 52, 139–183. [Google Scholar] [CrossRef]
- Yajima, J.; Tsuda, A.; Yamada, S.; Tanaka, M. Determination of saliva free-3-methoxy-4-hydroxyphenylglycol in normal volunteers using gas chromatography mass spectrometry. Biogr. Amine 2001, 16, 173–183. [Google Scholar]
- Allen, J.J.B.; Chambers, A.S.; Towers, D.N. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biol. Psychol. 2007, 74, 243–262. [Google Scholar] [CrossRef]
- Rosenthal, R.; Rosnow, R.L. Contrast Analysis: Focused Comparisons in the Analysis of Variance; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Okamura, H.; Tsuda, A.; Yajima, J.; Mark, H.; Horiuchi, S.; Toyoshima, N.; Matsuishi, T. Short sleeping time and psychobiological responses to acute stress. Int. J. Psychophysiol. 2010, 78, 209–214. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tsuda, A.; Okamura, H.; Yajima, J.; Steptoe, A. Differential Elicitation of the Salivary 3-Methoxy-4-Hydroxyphenylglycol (MHPG) Responses by Mental Stress Testing. Jpn. J. Behav. Med. 2010, 16, 31–38. [Google Scholar] [CrossRef]
- Nater, U.M.; La Marca, R.; Florin, L.; Moses, A.; Langhans, W.; Koller, M.M.; Ehlert, U. Stress-induced changes in human salivary alpha-amylase activity—Associations with adrenergic activity. Psychoneuroendocrinology 2006, 31, 49–58. [Google Scholar] [CrossRef] [PubMed]
- White, J.M. Effects of relaxing music on cardiac autonomic balance and anxiety after acute myocardial infarction. Am. J. Crit. Care 1999, 8, 220–230. [Google Scholar] [CrossRef]
- Schiweck, C.; Piette, D.; Berckmans, D.; Claes, S.; Vrieze, E. Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychol. Med. 2019, 49, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Brosschot, J.F. Prolonged stress-related cardiovascular activation: Is there any? Ann. Behav. Med. 2005, 30, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Chatkoff, D.K.; Maier, K.J.; Javaid, J.; Hammoud, M.K.; Munkrishna, P. Dispositional hostility and gender differentially relate to cognitive appraisal, engagement, and cardiovascular reactivity across cognitive and emotional laboratory tasks. Personal. Individ. Differ. 2009, 47, 122–126. [Google Scholar] [CrossRef]
- Maunder, R.G.; Lancee, W.J.; Nolan, R.P.; Hunter, J.J.; Tannenbaum, D.W. The relationship of attachment insecurity to subjective stress and autonomic function during standardized acute stress in healthy adults. J. Psychosom. Res. 2006, 60, 283–290. [Google Scholar] [CrossRef]
- Steptoe, A.; Leigh Gibson, E.; Hamer, M.; Wardle, J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology 2007, 32, 56–64. [Google Scholar] [CrossRef]
- Bostock, S.; Hamer, M.; Wawrzyniak, A.J.; Mitchell, E.S.; Steptoe, A. Positive emotional style and subjective, cardiovascular and cortisol responses to acute laboratory stress. Psychoneuroendocrinology 2011, 36, 1175–1183. [Google Scholar] [CrossRef]
- Papousek, I.; Nauschnegg, K.; Paechter, M.; Lackner, H.K.; Goswami, N.; Schulter, G. Trait and state positive affect and cardiovascular recovery from experimental academic stress. Biol. Psychol. 2010, 83, 108–115. [Google Scholar] [CrossRef]
- Kamarck, T.W.; Lovallo, W.R. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosom. Med. 2003, 65, 9–21. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 8, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Panagi, L.; Poole, L.; Hackett, R.A.; Steptoe, A. Happiness and Inflammatory Responses to Acute Stress in People with Type 2 Diabetes. Ann. Behav. Med. 2019, 53, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Mihara, K.; Okamura, H.; Yajima, J.; Tsuda, A. The differential relations of eudaimonic well-being and hedonic well-being to psychoneuroendocrinoimmunological responses and perceived health in students. Jpn. J. Behav. Med. 2019, 24, 84–96. [Google Scholar] [CrossRef]
- Lewis, G.J.; Kanai, R.; Rees, G.; Bates, T.C. Neural correlates of the “good life”: Eudaimonic well-being is associated with insular cortex volume. Soc. Cogn. Affect. Neurosci. 2014, 9, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Suardi, A.C.; Diano, M.; Cauda, F.; Duca, S.; Rusconi, M.L.; Sotgiu, I. The neural correlates of hedonic and eudaimonic happiness: An fMRI study. Neurosci. Lett. 2019, 712, 134491. [Google Scholar] [CrossRef]
- O’Donnell, K.; Brydon, L.; Wright, C.E.; Steptoe, A. Self-esteem levels and cardiovascular and inflammatory responses to acute stress. Brain Behav. Immun. 2008, 22, 1241–1247. [Google Scholar] [CrossRef]
- Creswell, J.D.; Welch, W.T.; Taylor, S.E.; Sherman, D.K.; Gruenewald, T.L.; Mann, T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol. Sci. 2005, 16, 846–851. [Google Scholar] [CrossRef]
- Fava, G.A.; Ruini, C.; Rafanelli, C.; Finos, L.; Salmaso, L.; Mangelli, L.; Sirigatti, S. Well-being therapy of generalized anxiety disorder. Psychother. Psychosom. 2005, 74, 26–30. [Google Scholar] [CrossRef]
- Cantarella, A.; Borella, E.; Marigo, C.; De Beni, R. Benefits of Well-Being Training in Healthy Older Adults. Appl. Psychol. Health Well-Being 2017, 9, 261–284. [Google Scholar] [CrossRef]
- Friedman, E.M.; Ruini, C.; Foy, C.R.; Jaros, L.; Love, G.; Ryff, C.D. Lighten UP! A Community-Based Group Intervention to Promote Eudaimonic Well-Being in Older Adults: A Multi-Site Replication with 6 Month Follow-Up. Clin. Gerontol. 2019, 42, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. On Happiness and Human Potentials: A Review of Research on Hedonic and Eudaimonic Well-Being. Annu. Rev. Psychol. 2001, 52, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, R.J.; Blanchard, C.M.; Mageau, G.A.; Koestner, R.; Ratelle, C.F.; Leonard, M.; Gagne, M.; Marsolais, J. Les passions de l’Ame: On obsessive and harmonious passion. J. Personal. Soc. Psychol. 2003, 85, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Shrira, A.; Palgi, Y.; Wolf, J.J.; Haber, Y.; Goldray, O.; Shacham-Shmueli, E.; Ben-Ezra, M. The positivity ratio and functioning under stress. Stress Heal 2011, 27, 265–271. [Google Scholar] [CrossRef]
| Personal Growth | ||
|---|---|---|
| Low (n = 11) | High (n = 13) | |
| Women, n (%) | 6 (54.5) | 5 (38.5) |
| Age, years | 21.3 ± 1.6 | 20.1 ± 1.4 |
| BMI, m2/kg | 22.0 ± 3.9 | 20.5 ± 2.0 |
| free-MHPG, ng/ml | 12.7 ± 3.0 | 11.3 ± 3.2 |
| HF, ln ms2 | 6.4 ± 0.4 | 6.6 ± 0.8 |
| HR, bpm | 84.9 ± 10.6 | 74.8 ± 12.4 * |
| EA score | 14.6 ±3.4 | 15.8 ± 3.0 |
| TA score | 14.5 ± 2.5 | 9.9 ± 3.6 ** |
| GHQ-28 total score | 8.2 ± 4.6 | 4.8 ± 3.9 |
| GHQ-physical symptoms | 2.7 ±2.4 | 2.2 ± 1.9 |
| GHQ-anxiety and insomnia | 2.8 ± 1.3 | 1.5 ± 1.6 * |
| GHQ-social dysfunction | 0.9 ± 1.4 | 0.8 ± 1.2 |
| GHQ-depression | 1.7 ± 1.7 | 0.3 ± 0.9 * |
| PANAS-positive affect | 29.0 ± 4.3 | 36.1 ± 11.3 * |
| PANAS-negative affect | 26.0 ± 13.1 | 23.8 ± 10.9 |
| Personal growth score | 32.2 ± 2.4 | 45.2 ± 1.9 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihara, K.; Okamura, H.; Shoji, Y.; Tashiro, K.; Kinoshita, Y.; Tsuda, A. Personal Growth and Psychobiological Stress Responsiveness to the Trier Social Stress Test in Students. Sustainability 2020, 12, 4497. https://doi.org/10.3390/su12114497
Mihara K, Okamura H, Shoji Y, Tashiro K, Kinoshita Y, Tsuda A. Personal Growth and Psychobiological Stress Responsiveness to the Trier Social Stress Test in Students. Sustainability. 2020; 12(11):4497. https://doi.org/10.3390/su12114497
Chicago/Turabian StyleMihara, Kengo, Hisayoshi Okamura, Yoshihisa Shoji, Kyoko Tashiro, Yukie Kinoshita, and Akira Tsuda. 2020. "Personal Growth and Psychobiological Stress Responsiveness to the Trier Social Stress Test in Students" Sustainability 12, no. 11: 4497. https://doi.org/10.3390/su12114497
APA StyleMihara, K., Okamura, H., Shoji, Y., Tashiro, K., Kinoshita, Y., & Tsuda, A. (2020). Personal Growth and Psychobiological Stress Responsiveness to the Trier Social Stress Test in Students. Sustainability, 12(11), 4497. https://doi.org/10.3390/su12114497






