Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals from Waste Biomaterials as Calcium Supplement
Abstract
:1. Introduction
2. Experimental
2.1. Materials
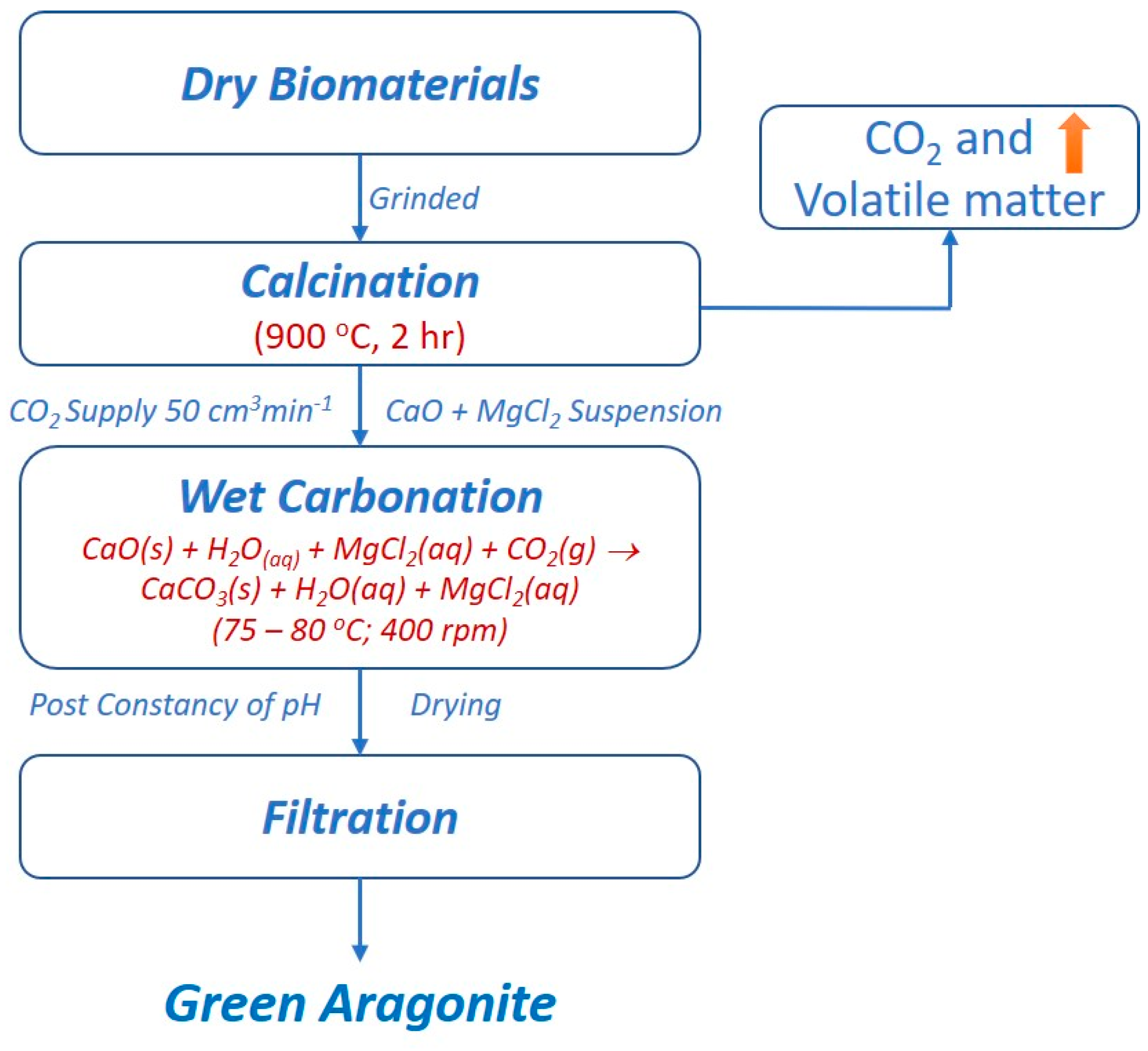
2.2. Synthesis of Green Aragonite
2.3. Characterization
3. Result and Discussion
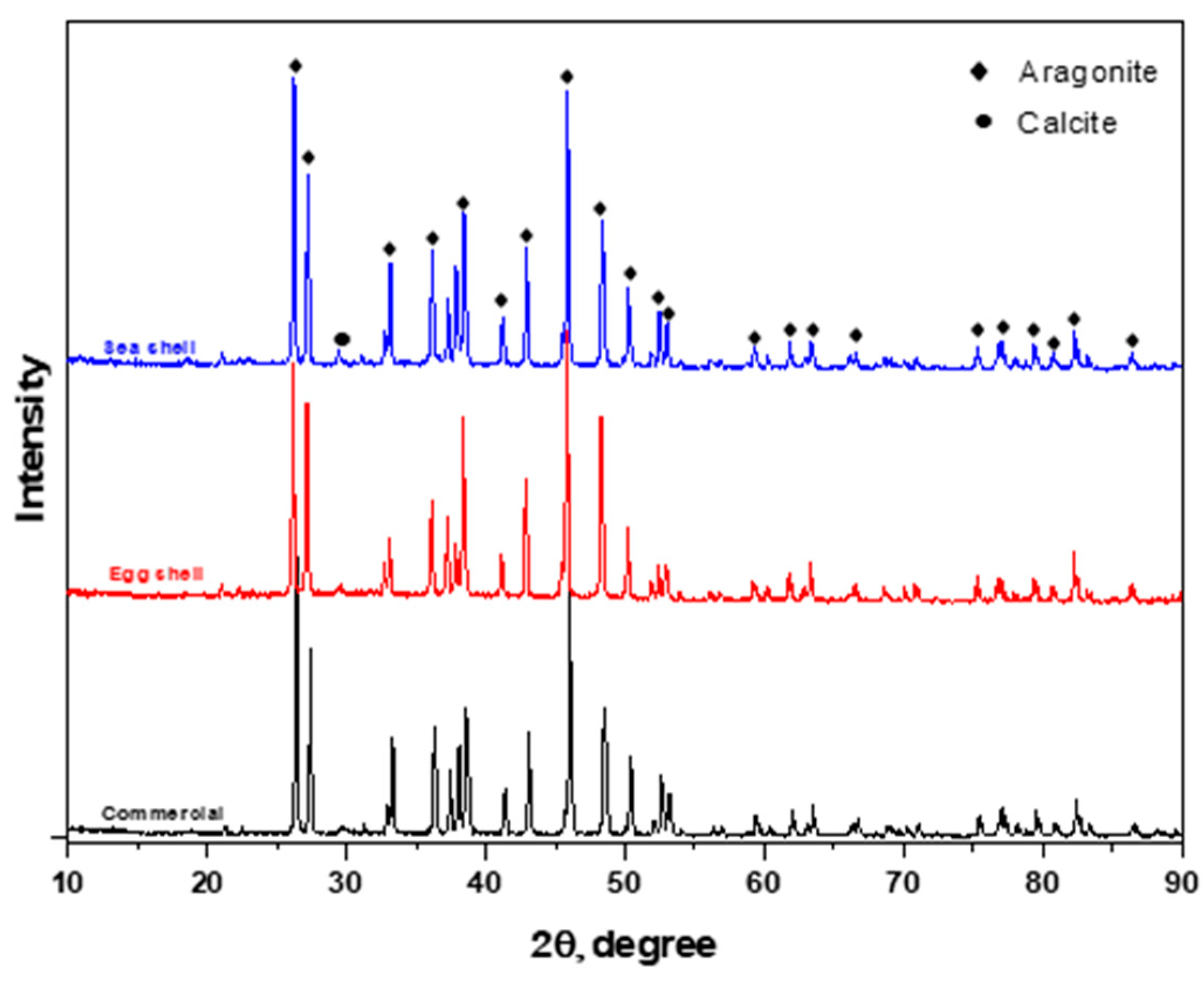
3.1. XRD Analysis
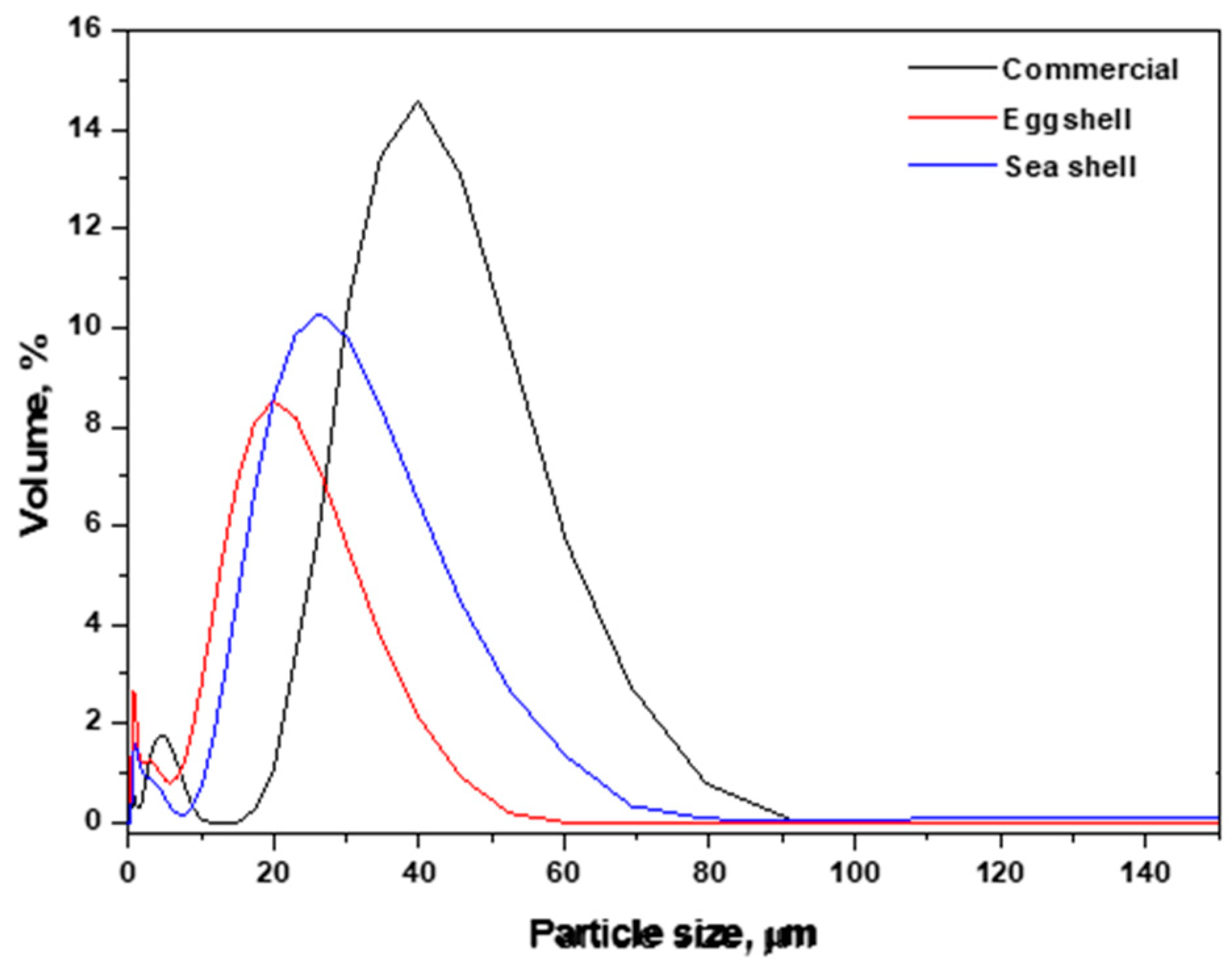
3.2. Particle Size Distribution
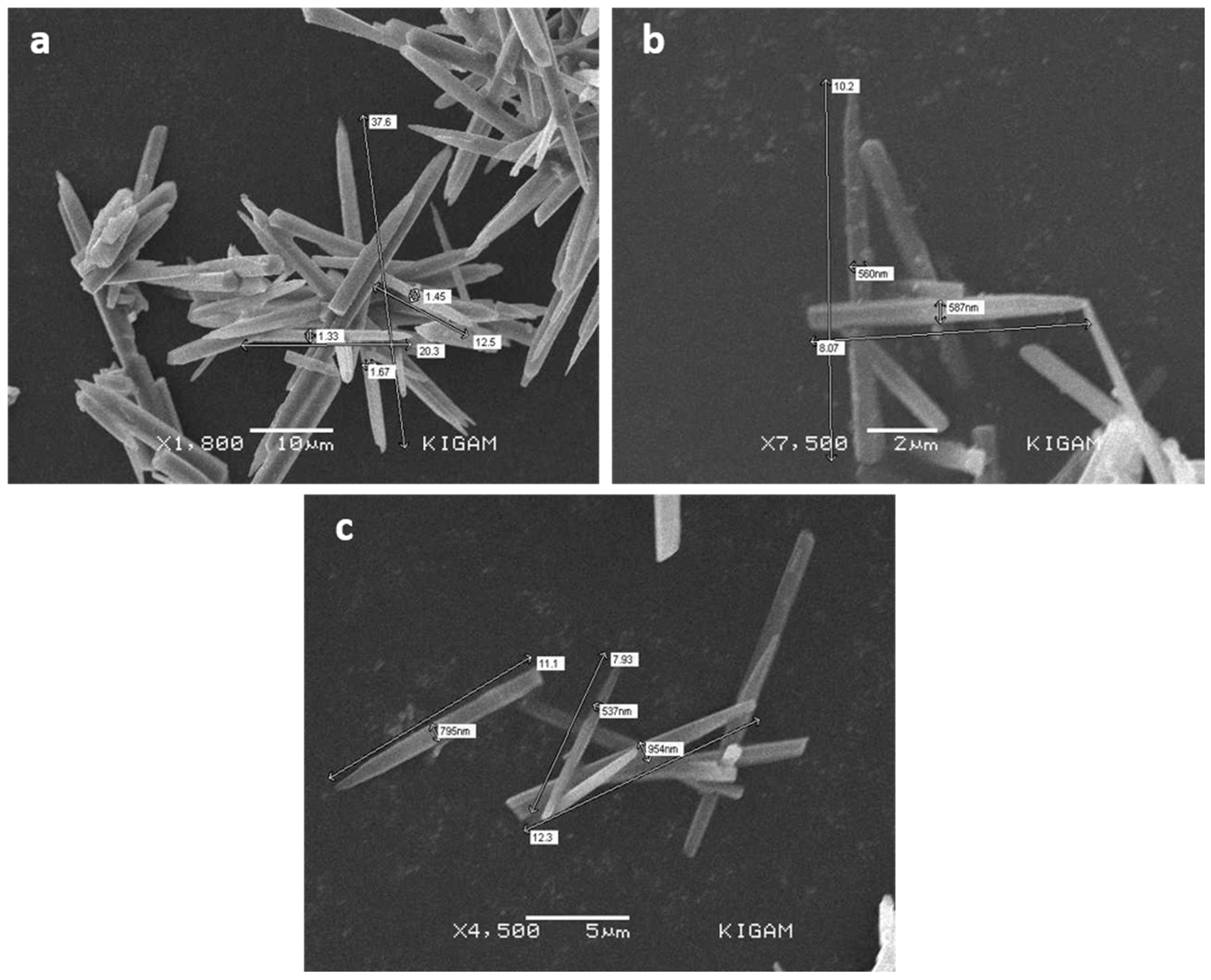
3.3. SEM Analysis
3.4. BET Analysis
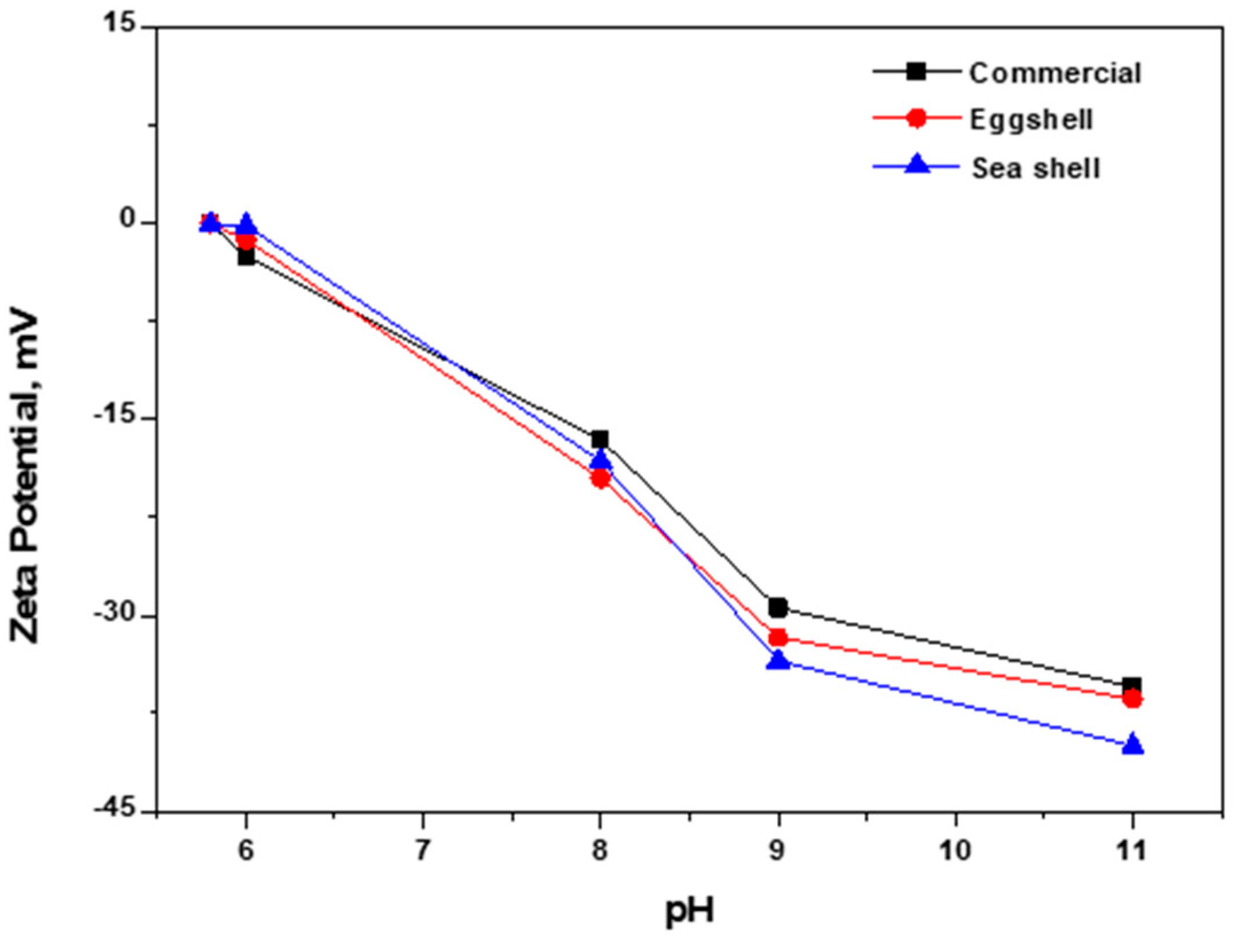
3.5. Zeta Potential Analysis
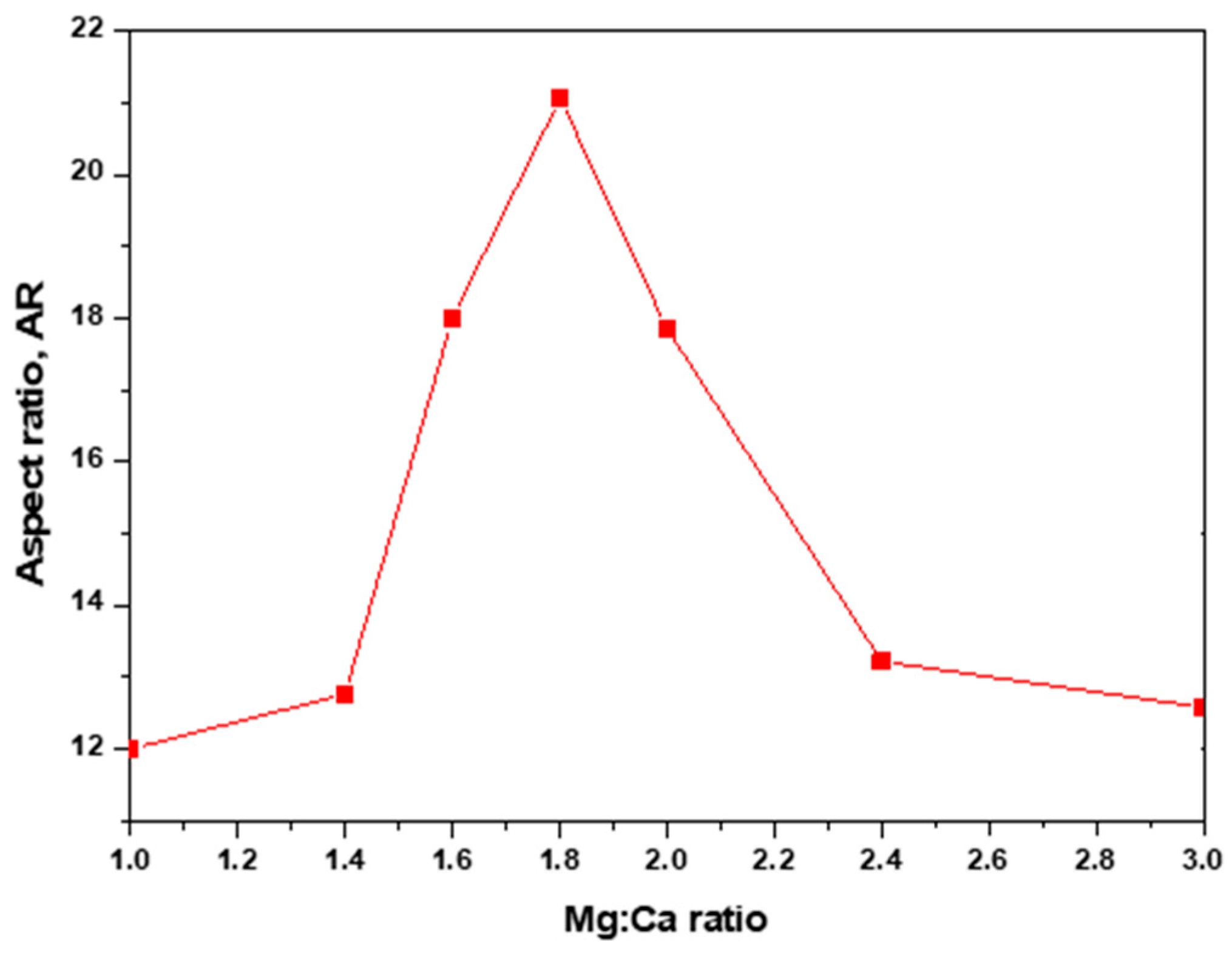
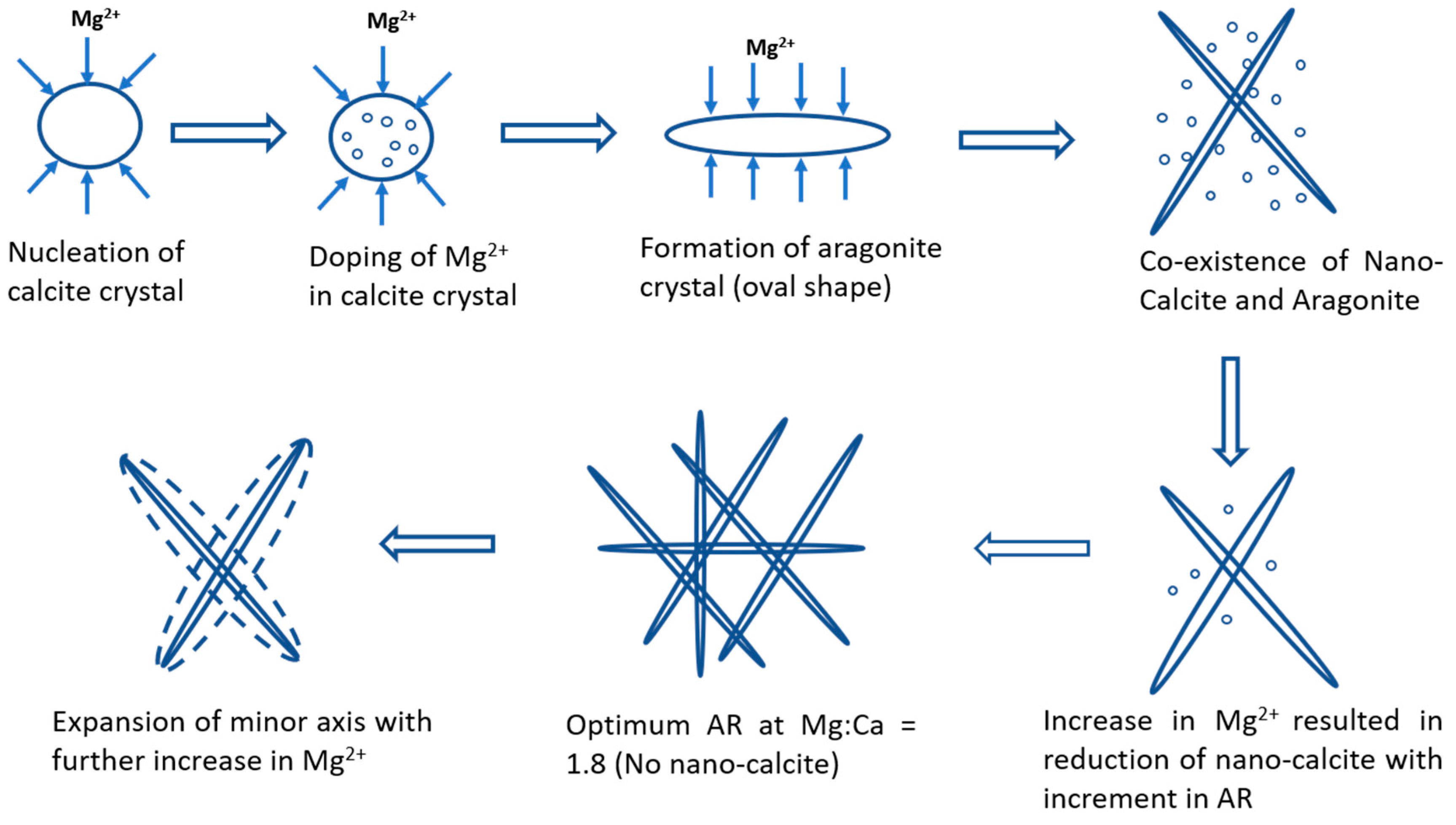
4. Green Aragonite Crystal Growth Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- George, J.; Ishida, H. A review on the very high nanofiller-content nanocomposites: Their preparation methods and properties with high aspect ratio fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Dunne, P.; Rulikowska, A.; Alguacil, V.; Gun’Ko, Y.K. Luminescent calcium carbonate micro ‘bow ties’. Mater. Today Commun. 2019, 20, 100590. [Google Scholar] [CrossRef]
- Li, H.-Y.; Tan, Y.-Q.; Zhang, L.; Zhang, Y.-X.; Song, Y.; Ye, Y.; Xia, M. Bio-filler from waste shellfish shell: Preparation, characterization, and its effect on the mechanical properties on polypropylene composites. J. Hazard. Mater. 2012, 217, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.; Huang, F.; Zhu, X.; Liu, X.; Zhang, J.-Z. Adsorption and Desorption of Phosphate on Calcite and Aragonite in Seawater. Aquat. Geochem. 2001, 7, 33–56. [Google Scholar] [CrossRef]
- Islam, K.N.; Bin Abu Bakar, Z.; Ali, E.; Hussein, M.Z.; Noordin, M.M.; Loqman, M.; Miah, G.; Wahid, M.H.; Hashim, U. A novel method for the synthesis of calcium carbonate (aragonite) nanoparticles from cockle shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Samanta, A.; Podder, S.; Kumarasamy, M.; Ghosh, C.K.; Lahiri, D.; Roy, P.; Bhattacharjee, S.; Ghosh, J.; Mukhopadhyay, A.K. Au nanoparticle-decorated aragonite microdumbbells for enhanced antibacterial and anticancer activities. Mater. Sci. Eng. C 2019, 103, 109734. [Google Scholar] [CrossRef]
- Ota, Y.; Inui, S.; Iwashita, T.; Kasuga, T.; Abe, Y. Preparation of Aragonite Whiskers. J. Am. Ceram. Soc. 1995, 78, 1983–1984. [Google Scholar] [CrossRef]
- Park, W.K.; Ko, S.-J.; Lee, S.W.; Cho, K.-H.; Ahn, J.-W.; Han, C. Effects of magnesium chloride and organic additives on the synthesis of aragonite precipitated calcium carbonate. J. Cryst. Growth 2008, 310, 2593–2601. [Google Scholar] [CrossRef]
- Nancollas, G.; Sawada, K. Formation of Scales of Calcium Carbonate Polymorphs: The Influence of Magnesium Ion and Inhibitors. J. Pet. Technol. 1982, 34, 645–652. [Google Scholar] [CrossRef]
- Hamideh, F.; Akbar, A. Application of eggshell wastes as valuable and utilizable products: A review. Res. Agric. Eng. 2018, 64, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, J. Recovery and Recycling of Waste Tires in Poland. Procedia Eng. 2017, 182, 229–234. [Google Scholar] [CrossRef]
- Khan, M.D.; Ahn, J.W.; Nam, G. Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. J. Environ. Manag. 2018, 223, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Holuszko, M.; Espinosa, D. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thriveni, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Kumar, V.; Bansal, M.C. Utilization of egg shell waste in cellulase production by Neurospora crassa under Wheat bran-based solid state fermentation. Pol. J. Environ. Stud. 2012, 21, 491–497. [Google Scholar]
- Felipe-Sesé, M.A.; Eliche-Quesada, D.; Corpas-Iglesias, F.A. The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceram. Int. 2011, 37, 3019–3028. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Hsien, K.-J.; Hsu, H.-C.; Lin, C.-M.; Lin, K.-Y.; Chiu, C.-H. Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour. Technol. 2008, 99, 1623–1629. [Google Scholar] [CrossRef]
- Dai, L.; Tan, F.-R.; Li, H.; Zhu, N.; He, M.; Zhu, Q.-L.; Hu, G.Q.; Wang, L.; Zhao, J. Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J. Environ. Manag. 2017, 198, 70–74. [Google Scholar] [CrossRef]
- Lu, J.; Cong, X.; Li, Y.; Hao, Y.; Wang, C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2018, 172, 1978–1985. [Google Scholar] [CrossRef]
- Wang, L.; Sondi, I.; Matijević, E. Preparation of Uniform Needle-Like Aragonite Particles by Homogeneous Precipitation. J. Colloid Interface Sci. 1999, 218, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Huang, F.; Li, S.; Shena, Y.; Shena, Y.; Pan, J.; Zhang, Y.; Cai, Y. Biomimetic synthesis of aragonite superstructures using hexamethylenetetramine. J. Solid State Chem. 2011, 184, 2825–2833. [Google Scholar] [CrossRef]
- Correia, L.M.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L.; Vieira, R.S.; Rodrí Guez- Castelló, N.E.; Loureiro, L. Relevance of the Physicochemical Properties of Calcined Quail Eggshell (CaO) as a Catalyst for Biodiesel Production. J. Chem. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J.W. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 2016, 34, 225–230. [Google Scholar] [CrossRef]
- Kezuka, Y.; Kawai, K.; Eguchi, K.; Tajika, M. Fabrication of Single-Crystalline Calcite Needle-Like Particles Using the Aragonite–Calcite Phase Transition. Miner 2017, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Handley-Sidhu, S.; Mullan, T.K.; Grail, Q.; Albadarneh, M.; Ohnuki, T.; Macaskie, L.E. Influence of pH, competing ions and salinity on the sorption of strontium and cobalt onto biogenic hydroxyapatite. Sci. Rep. 2016, 6, 23361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.-W.; Choi, K.-S.; Yoon, S.-H.; Kim, H. Synthesis of Aragonite by the Carbonation Process. J. Am. Ceram. Soc. 2004, 87, 286–288. [Google Scholar] [CrossRef]
- Hosoi, K.; Hashida, T.; Takahashi, H.; Yamasaki, N.; Korenaga, T. Solidification behaviour of calcium carbonate via aragonite-calcite wet transformation with hydrothermal hot pressing. J. Mater. Sci. Lett. 1997, 16, 382–385. [Google Scholar] [CrossRef]
- Synthesis of Aragonite-Type Calcium Carbonate by Overgrowth Technique at Atmospheric Pressure. J. Mater. Sci. Lett. 1998, 17, 905–908. [CrossRef]
- Kezuka, Y.; Tochigi, E.; Murata, H.; Yoshida, M.; Eguchi, K.; Nakahira, A.; Ikuhara, Y.; Tajika, M. Synthesis of Tunable-Aspect-Ratio Calcite Nanoparticles via Mg2+ Doping. Cryst. Growth Des. 2019, 19, 6784–6791. [Google Scholar] [CrossRef]
- Lippmann, F. Versuche zur Aufklärung der Bildungsbedingungen von Calcit und Aragonit, Fortschr. Miner 1960, 38, 156–161. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habte, L.; Khan, M.D.; Shiferaw, N.; Farooq, A.; Lee, M.-h.; Jung, S.-h.; Ahn, J.W. Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals from Waste Biomaterials as Calcium Supplement. Sustainability 2020, 12, 5062. https://doi.org/10.3390/su12125062
Habte L, Khan MD, Shiferaw N, Farooq A, Lee M-h, Jung S-h, Ahn JW. Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals from Waste Biomaterials as Calcium Supplement. Sustainability. 2020; 12(12):5062. https://doi.org/10.3390/su12125062
Chicago/Turabian StyleHabte, Lulit, Mohd Danish Khan, Natnael Shiferaw, Adeeba Farooq, Mee-hye Lee, Seok-ho Jung, and Ji Whan Ahn. 2020. "Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals from Waste Biomaterials as Calcium Supplement" Sustainability 12, no. 12: 5062. https://doi.org/10.3390/su12125062
APA StyleHabte, L., Khan, M. D., Shiferaw, N., Farooq, A., Lee, M.-h., Jung, S.-h., & Ahn, J. W. (2020). Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals from Waste Biomaterials as Calcium Supplement. Sustainability, 12(12), 5062. https://doi.org/10.3390/su12125062



