Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Sample Collection
2.2. Chemical Analysis
2.3. Prescription Analysis and Antibiotic Consumption Evaluation
2.4. Statistical Analysis
2.5. Ecological Risk Assessment of Antibiotics Detected in the Environment
3. Results and Discussion
3.1. Chemical Analysis
3.1.1. Antibiotics in Water Samples
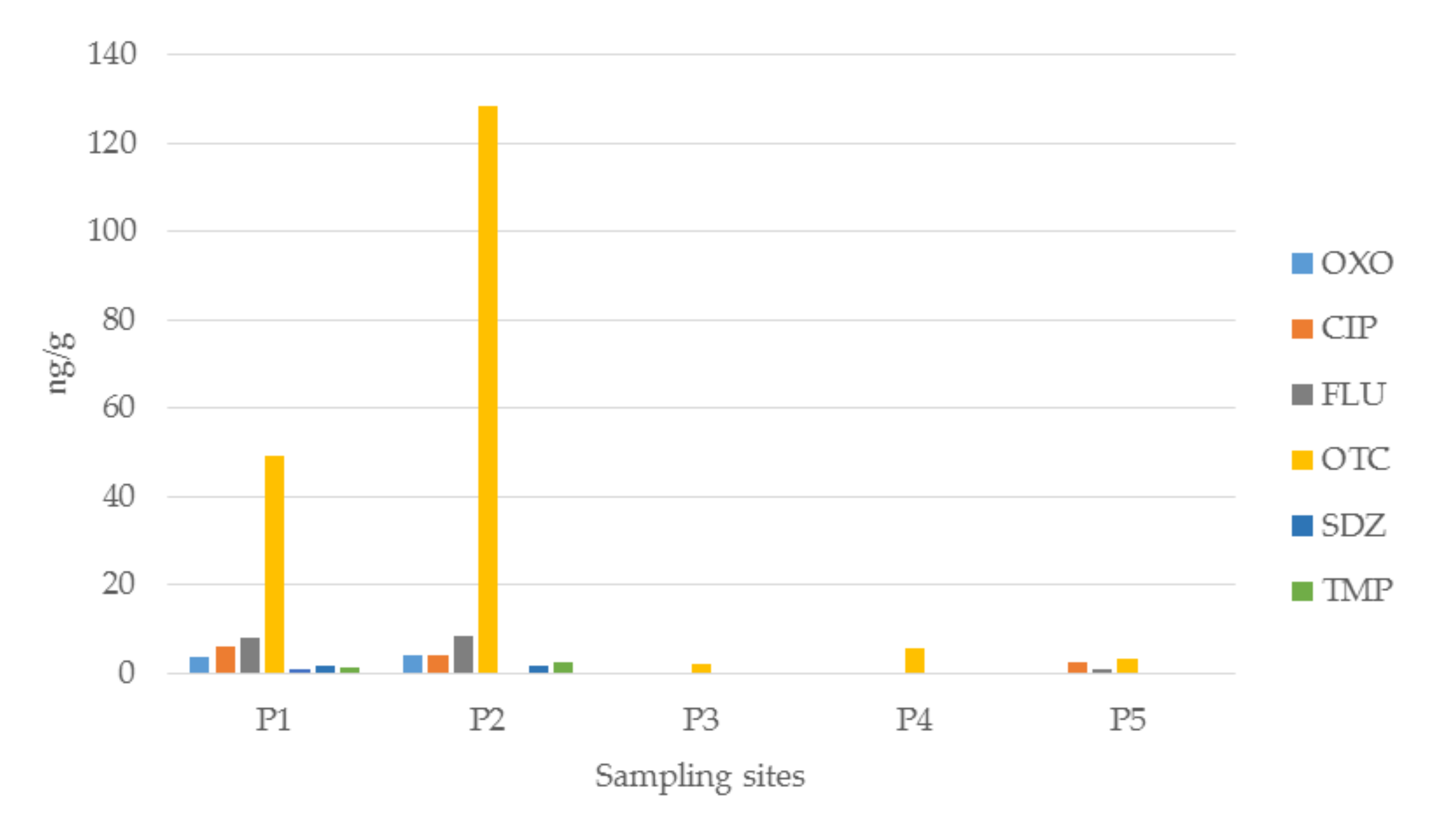
3.1.2. Antibiotics in Sediment Samples
3.2. Analysis of Antibiotic Consumption
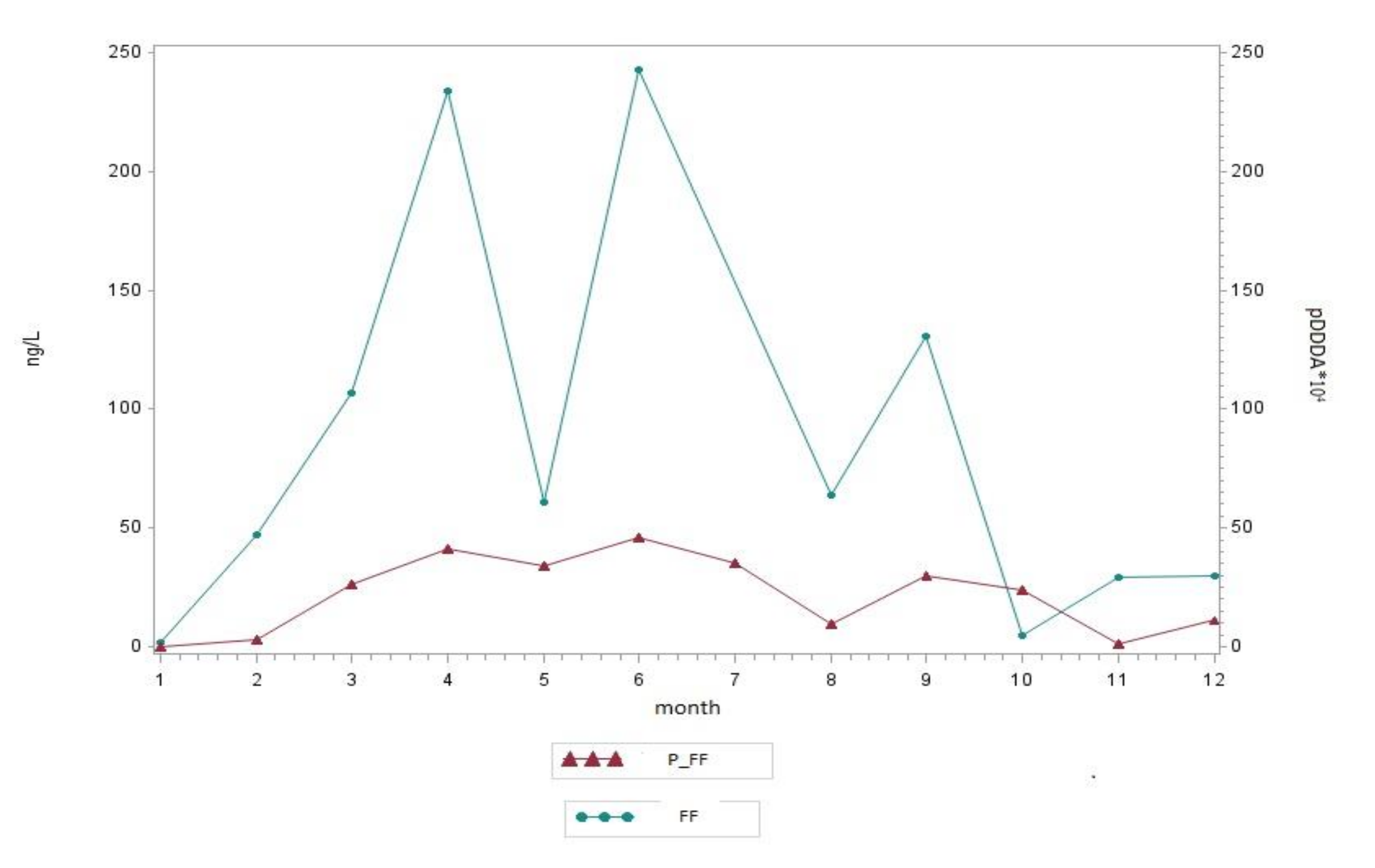
3.3. Correlation Between Antibiotic Consumption and Surface Water Residues
3.4. Ecotoxicological Risk Assessment
4. Conclusions
Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [Green Version]
- Westphal-Settele, K.; Konradi, S.; Balzer, F.; Schönfeld, J.; Schmithausen, R. Die Umwelt als Reservoir für Antibiotikaresistenzen. Bundesgesundheitsblatt Gesundheitsforsch. Gesundh. 2018, 61, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Le Page, G.; Gunnarsson, L.; Snape, J.; Tyler, C.R. Integrating human and environmental health in antibiotic risk assessment: A critical analysis of protection goals, species sensitivity and antimicrobial resistance. Environ. Int. 2017, 109, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Segura, P.A.; François, M.; Gagnon, C.; Sauvé, S. Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters. Environ. Health Perspect. 2009, 117, 675–684. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Edwards, M.; Topp, E.; Metcalfe, C.D.; Li, H.; Gottschall, N.; Bolton, P.; Curnoe, W.; Payne, M.; Beck, A.; Kleywegt, S.; et al. Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field. Sci. Total Environ. 2009, 407, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy; EC Decision 2015/495 Commission Implementing Decision (EU) 2015/495; 2015; Volume 78, pp. 40–42. [Google Scholar]
- Loos, R.; Marinov, D.; Sanseverino, I.; Napierska, D.; Lettieri, T. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List; EUR 29173 EN; Publication Office of the European Union: Luxembourg, 2018; ISBN 9789279818394. JRC111198. [Google Scholar] [CrossRef]
- European Parliament. Directive 2008/105/EC on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council; European Parliament: Brussels, Belgium, 2008; Volume 51, p. 84. [Google Scholar]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, E.; Achene, L.; Beccaloni, E.; Carere, M.; Comba, P.; Crebelli, R.; Pasetto, R.; Eleonora, M.; Soggiu, E.T. Guidelines for health impact assessment (Italian Legislative Decree n. 104/2017).; Istituto Superiore di Sanità: Rome, Italy, 2019; (Rapporti ISTISAN 19/9); Volume VII, p. 72. [Google Scholar]
- Boxall, A.B.A. The environmental side effects of medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Cabello, F. Antibiotics and aquaculture in Chile: Implications for human and animal health. Rev. Med. Chile 2004, 132, 1001–1006. [Google Scholar]
- Naylor, R.; Burke, M.; Wrigley, J. Raising Tigers of the Sea. Annu. Rev. Environ. Resour. 2005, 30, 185–218. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Lalumera, G.M.; Calamari, D.; Galli, P.; Castiglioni, S.; Crosa, G.; Fanelli, R. Preliminary investigation on the environmental occurrence and effects of antibiotics used in aquaculture in Italy. Chemosphere 2004, 54, 661–668. [Google Scholar] [CrossRef]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Romero, J.; Gloria, C.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives. In Health and Environment in Aquaculture; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.-C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [Green Version]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Peterson, R.K.D. Comparing ecological risks of pesticides: The utility of a risk quotient ranking approach across refinements of exposure. Pest Manag. Sci. 2006, 62, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Thomaidi, V.S.; Matsoukas, C.; Stasinakis, A.S. Risk assessment of triclosan released from sewage treatment plants in European rivers using a combination of risk quotient methodology and Monte Carlo simulation. Sci. Total Environ. 2017, 603, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Dusi, G.; Giusepponi, D.; Pellicciotti, S.; Rossi, R.; Saluti, G.; Cruciani, G.; Galarini, R. Screening and confirmatory method for multiclass determination of 62 antibiotics in meat. J. Chromatogr. A 2016, 1429, 175–188. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EMA-ESVAC Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet); European Medicines Agency—Veterinary Medicines Division: London, UK, 2015; Volume 44. [Google Scholar]
- Veterinary Medicines Handbook—Online Database. Available online: https://www.vetinfo.it/j6_prontuario/public/ (accessed on 9 April 2020).
- Kruskal, W.H. A Nonparametric test for the Several Sample Problem. Ann. Math. Stat. 1952, 23, 525–540. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Technical Guidance Document on Risk Assessment: Part II; European Chemicals Bureau: Luxembourg, 2003.
- Gökçe, D. Algae as an Indicator of Water Quality. In Algae—Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; InTech: Rijeka, Croatia, 2016; pp. 81–101. [Google Scholar]
- Maul, J.D.; Schuler, L.J.; Belden, J.B.; Whiles, M.R.; Lydy, M.J. Effects of the antibiotic ciprofloxacin on stream microbial communities and detritivorous macroinvertebrates. Environ. Toxicol. Chem. 2006, 25, 1598–1606. [Google Scholar] [CrossRef]
- EMEA. EMEA Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; European Medicine Agency: Amsterdam, The Netherlands, 2006; p. 12. [Google Scholar]
- Agnihotri, V.K. Anabaena flos-aquae. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1995–2037. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Heijerick, D.G.; De Schamphelaere, K.A.C.; Janssen, C.R. Biotic ligand model development predicting Zn toxicity to the alga Pseudokirchneriella subcapitata: Possibilities and limitations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 207–218. [Google Scholar] [CrossRef]
- Ciucă, V. Environmental assessment for Florfenicol FP 10% Evaluarea riscului pentru mediu a produsului Florfenicol FP 10%. In Medicamentul Veterinar/Veterinary Drug; Romanian National Association of the Veterinary Products Manufactures: Bucharest, Romania, 2018. [Google Scholar]
- Gallagher, S.P.; Kendall, T.Z.; Krueger, H.O. Florfenicol: A 96-Hour Toxicity Test with the Freshwater Alga (Anabaena Flos-Aquae); Schering-Plough Research Institute: Kenilworth, NJ, USA, 2008; No.: 07119. [Google Scholar]
- Tell, J.; Caldwell, D.J.; Häner, A.; Hellstern, J.; Hoeger, B.; Journel, R.; Mastrocco, F.; Ryan, J.J.; Snape, J.; Straub, J.O.; et al. Science-based Targets for Antibiotics in Receiving Waters from Pharmaceutical Manufacturing Operations. Integr. Environ. Assess. Manag. 2019, 15, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, L.; Qin, S.; Cui, J.; Liu, Y. Quinolones antibiotics in the Baiyangdian Lake, China: Occurrence, distribution, predicted no-effect concentrations (PNECs) and ecological risks by three methods. Environ. Pollut. 2020, 256, 113458. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Baskaran, S.; Kennedy, I.R. Ecological relative risk (EcoRR): Another approach for risk assessment of pesticides in agriculture. Agric. Ecosyst. Environ. 2002, 91, 37–57. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence, distribution, ecological and resistance risks of antibiotics in surface water of finfish and shellfish aquaculture in Bangladesh. Chemosphere 2017, 188, 329–336. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef]
- Han, Q.F.; Zhao, S.; Zhang, X.R.; Wang, X.L.; Song, C.; Wang, S.G. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ. Int. 2020, 138, 105551. [Google Scholar] [CrossRef]
- Deng, W.J.; Li, N.; Ying, G.G. Antibiotic distribution, risk assessment, and microbial diversity in river water and sediment in Hong Kong. Environ. Geochem. Health 2018, 40, 2191–2203. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, L.; Luo, Z.; Yan, C.; Zhang, X. Occurrence of selected antibiotics in Jiulongjiang River in various seasons{,} South China. J. Environ. Monit. 2011, 13, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Database. Sulfadiazine, CID=5215. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulfadiazine (accessed on 2 April 2020).
- National Center for Biotechnology Information. PubChem Database. Oxytetracycline, CID=54675779. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oxytetracycline (accessed on 2 April 2020).
- Menanteau-ledouble, S.; Kumar, G.; Saleh, M.; El-matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. Dis. Aquat. Org. 2016, 120, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.; Menanteau-Ledouble, S.; Saleh, M.; El-Matbouli, M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet. Res. 2015, 46, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OsMed. National Report on Antibiotics Use in Italy. Year 2018; The Medicines Utilisation Monitoring Centre: Rome, Italy, 2019. [Google Scholar]
- EMA. Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. Eur. Med. Agency Sci. Med. Agency 2018, 31, 1–3. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment. European Commission: Brussels, Belgium, 2019. [Google Scholar]




| Variable | r (%) | p-Value |
|---|---|---|
| ERY | 51 | 0.0882 |
| OTC | 50 | 0.1006 |
| SDZ | 40 | 0.2028 |
| TMP | 54 | 0.0709 |
| AS | pDDDA × 104 |
|---|---|
| ERY | 5.3 |
| FF | 260.7 |
| FLU | 53 |
| OTC | 191 |
| SDZ | 1049.3 |
| TMP | 1049.3 |
| Tot. | 2608.5 |
| Variable | r (%) | p-Value |
|---|---|---|
| ERY | 42 | 0.1746 |
| FLU | 72 | 0.0085 |
| FF | 83 | 0.0008 |
| OTC | 43 | 0.1646 |
| SDZ | 41 | 0.1858 |
| TMP | 46 | 0.1369 |
| AS | MECMAX (µg/L) | PNECENV (µg/L) | Species | References | RQENV |
|---|---|---|---|---|---|
| Fluvial Water | Fluvial Water | ||||
| ERY | 0.018 | 0.5 | Anabaena flos-aquae | [45] | 0.036 |
| FLU | 1.083 | 18.7 | Green Chlorophyta | [46] | 0.058 |
| FF | 0.192 | 23 | Anabaena flos-aquae | [44] | 0.008 |
| OTC | 1.139 | 18 | Raphidocelis subcapitata | [45] | 0.063 |
| SDZ | 0.571 | 13 | Raphidocelis subcapitata | [45] | 0.044 |
| TMP | 0.18 | 100 | Anabaena flos-aquae | [45] | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargenti, M.; Bartolacci, S.; Luciani, A.; Di Biagio, K.; Baldini, M.; Galarini, R.; Giusepponi, D.; Capuccella, M. Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy. Sustainability 2020, 12, 5176. https://doi.org/10.3390/su12125176
Sargenti M, Bartolacci S, Luciani A, Di Biagio K, Baldini M, Galarini R, Giusepponi D, Capuccella M. Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy. Sustainability. 2020; 12(12):5176. https://doi.org/10.3390/su12125176
Chicago/Turabian StyleSargenti, Marta, Silvia Bartolacci, Aurora Luciani, Katiuscia Di Biagio, Marco Baldini, Roberta Galarini, Danilo Giusepponi, and Marinella Capuccella. 2020. "Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy" Sustainability 12, no. 12: 5176. https://doi.org/10.3390/su12125176






