The Hydrochemical and Isotopic Evolution of the Surface Water and Groundwater for Impoundment in the Xiluodu Reservoir, Jinsha River, China
Abstract
:1. Introduction
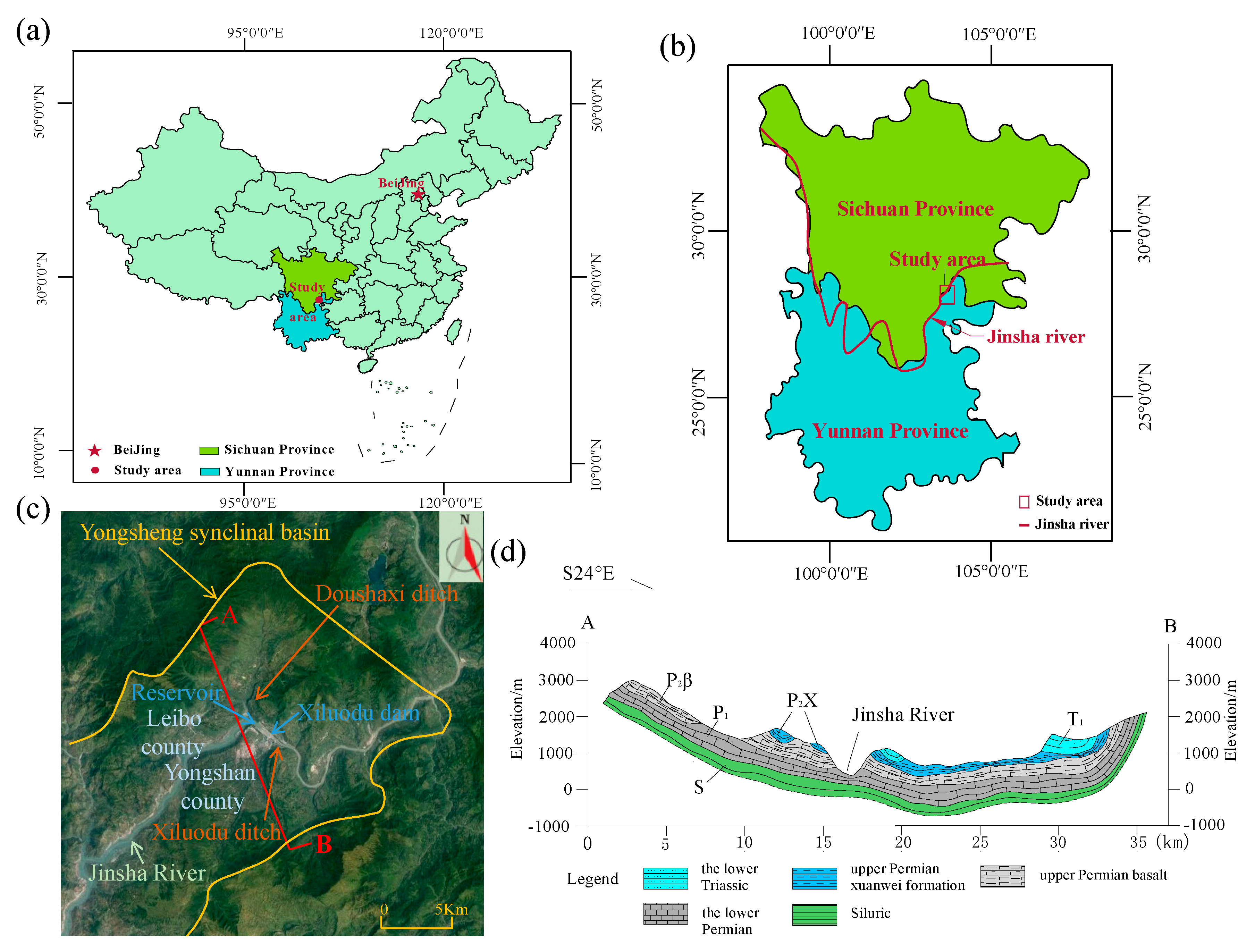
2. Study Area
2.1. Physical Geography
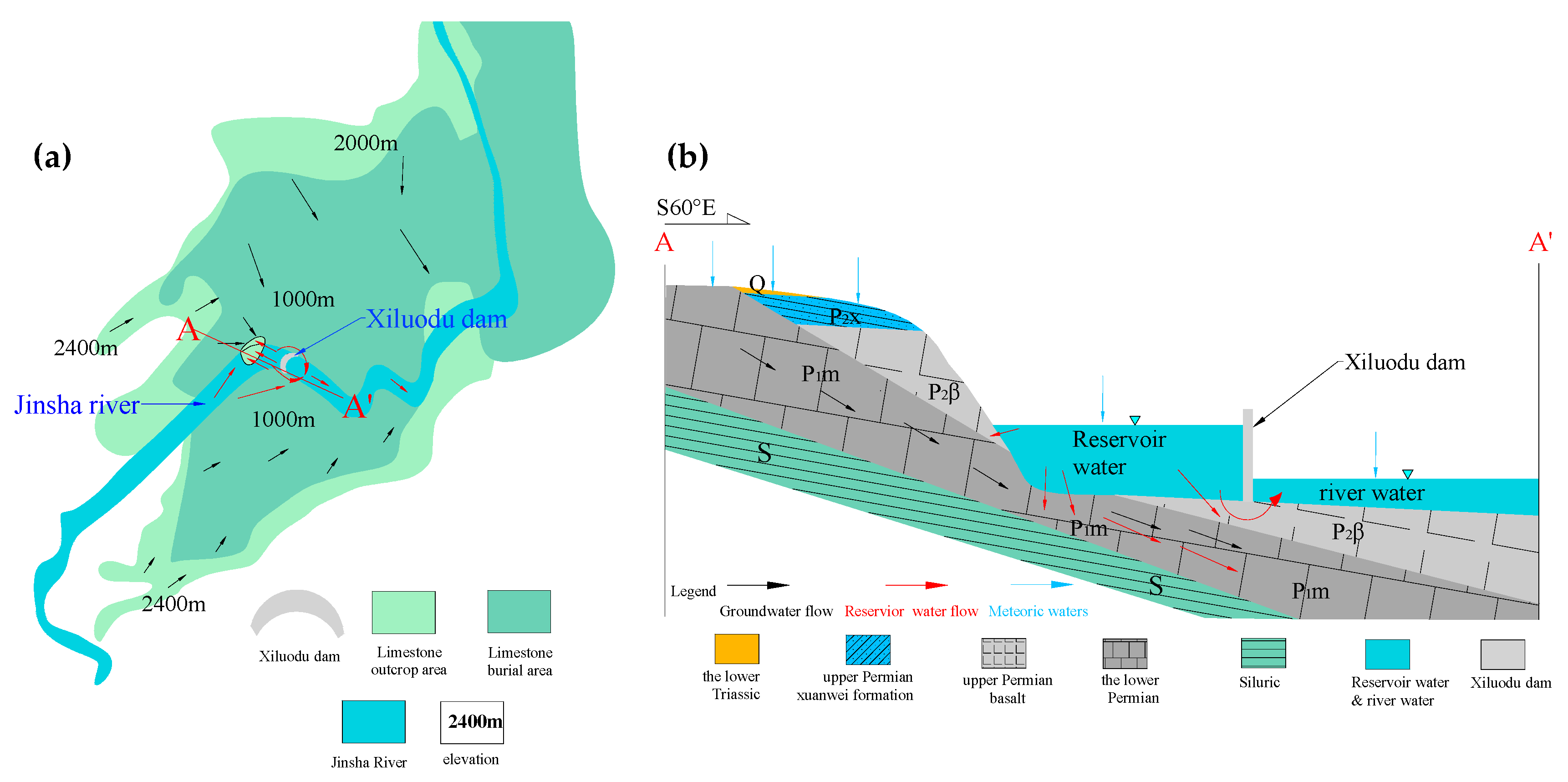
2.2. Geology and Hydrogeology
3. Methods
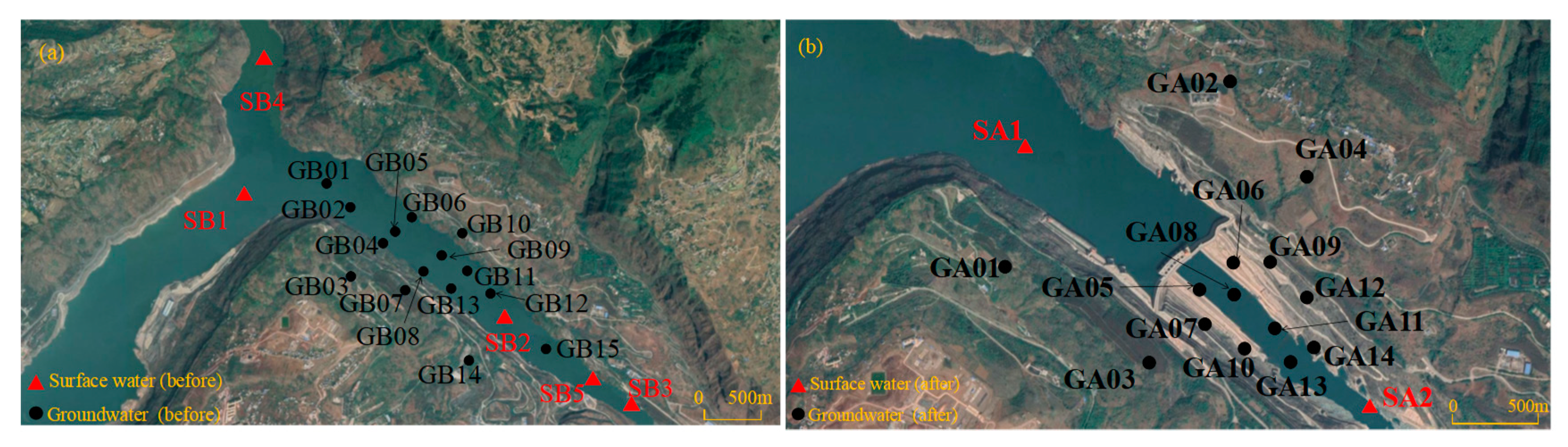
3.1. Sampling and Analysis
3.2. Data Treatment for Multivariate Analysis
4. Results
4.1. The Characteristics of the Surface Water and Groundwater before Impoundment
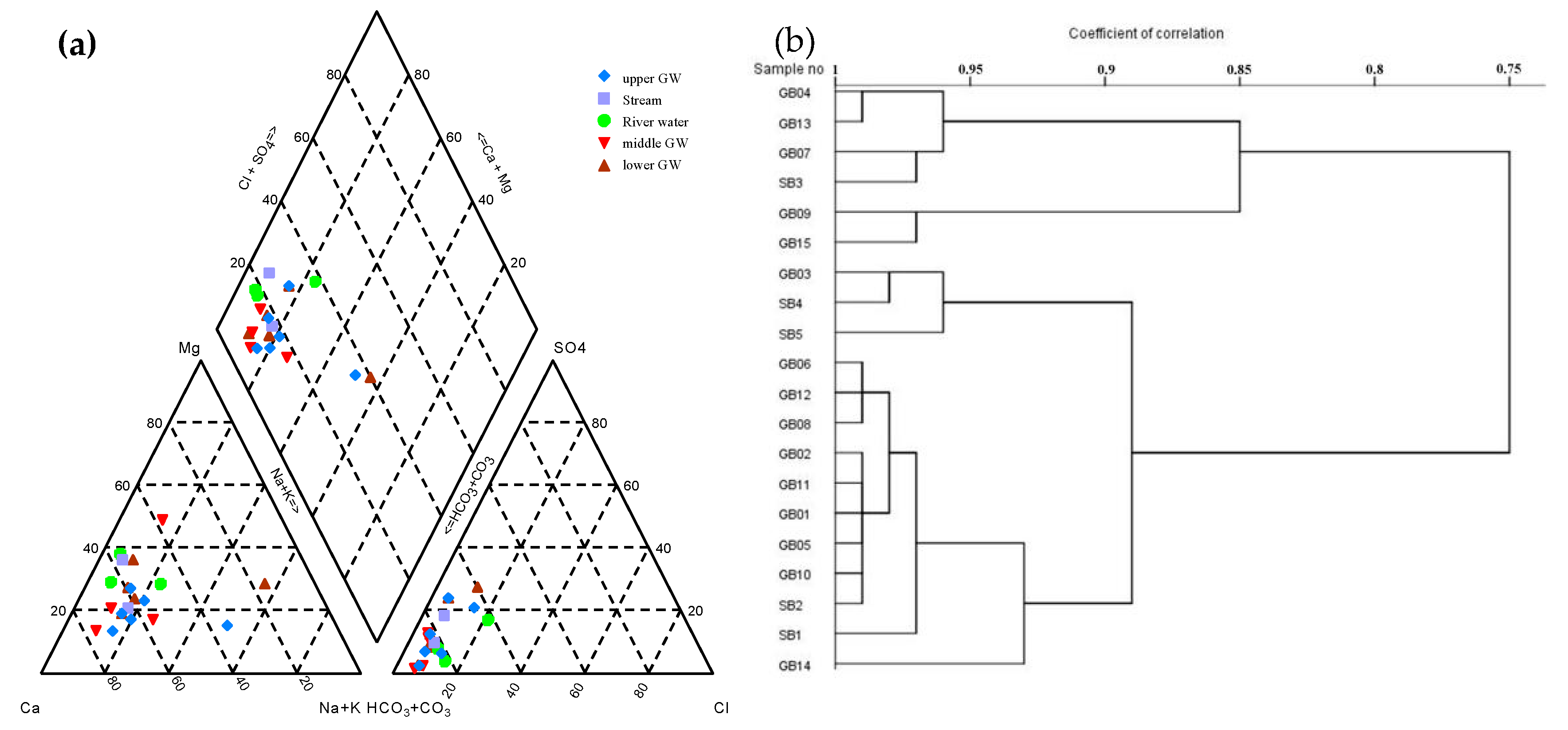
4.1.1. Hydrochemical Features
4.1.2. Isotopic Characteristics
4.2. The Characteristics of the Surface Water and Groundwater after Impoundment
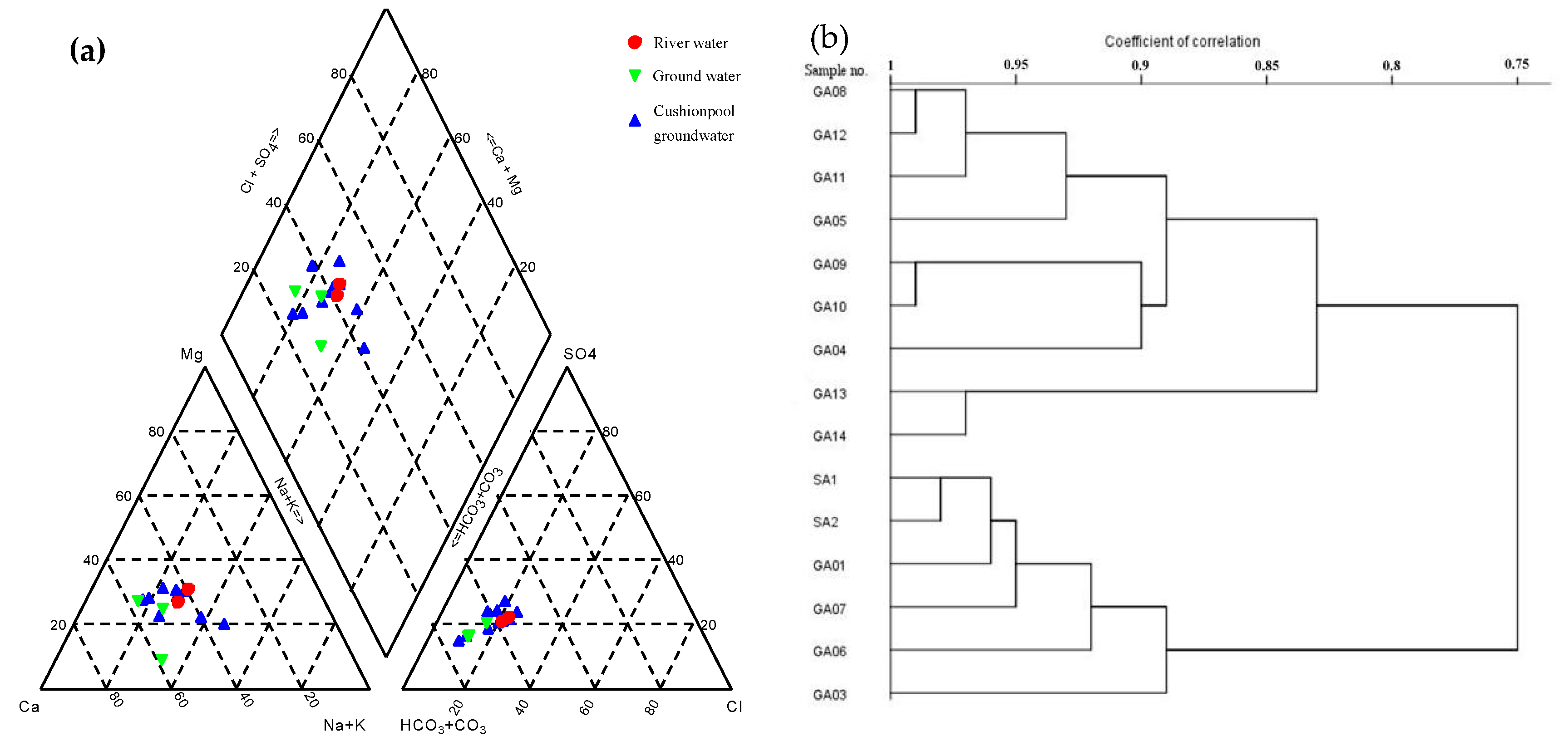
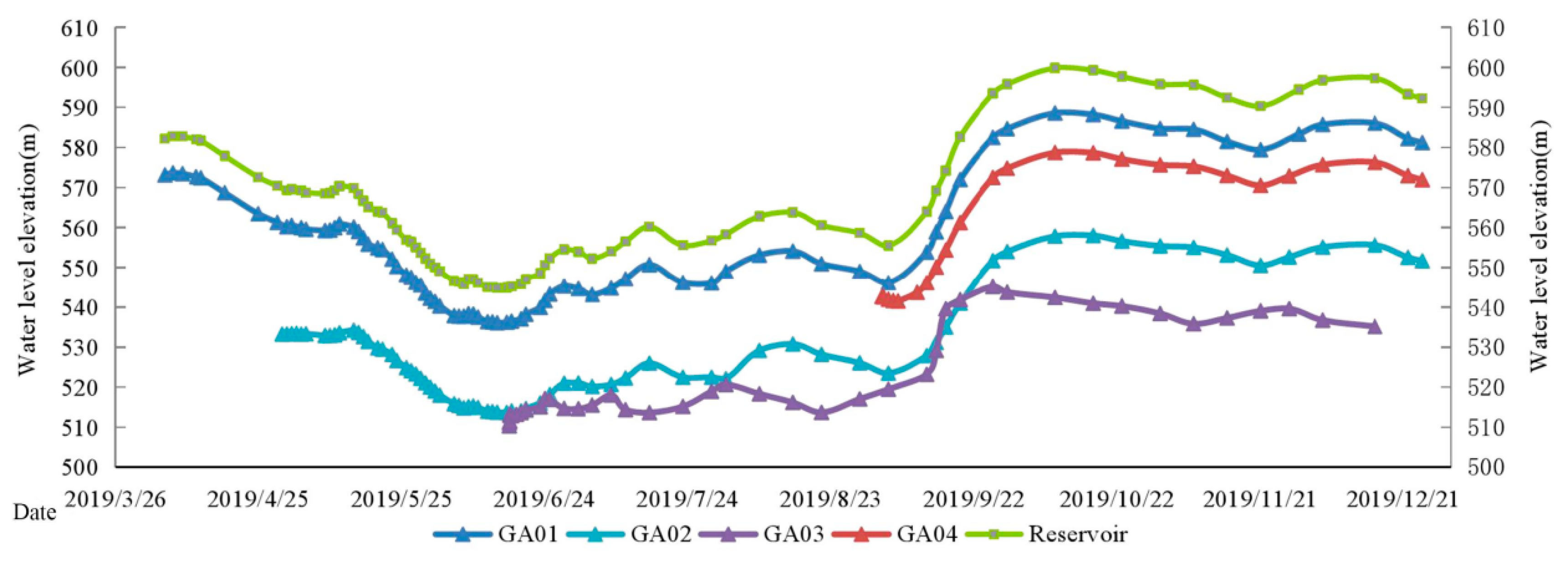
4.2.1. Hydrochemical Features
4.2.2. Isotopic Characteristics
5. Discussion
5.1. The Interaction between Surface Water and Groundwater before Impoundment
5.1.1. Hydrochemical Characteristics
5.1.2. Stable Isotope Ratios
5.2. The Interaction between the Surface Water and Groundwater after Impoundment
5.2.1. Hydrochemical Characteristics
5.2.2. Stable Isotope Ratios
5.3. The Evolution Laws, Reasons and Influences of the Interaction Between the Surface Water and Groundwater
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, W.; Bai, J.H.; Yan, M.H. Problems and countermeasures of water resources for sustainable utilization in China. Chin. Geogr. Sci. 2002, 12, 289–293. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J. Facing the challenge: Barriers to sustainable water resources development in China. Hydrol. Sci. J. 1999, 44, 507–516. [Google Scholar]
- Jiang, Y. China’s water scarcity. J. Environ. Manag. 2009, 90, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, G.; Yu, Y. Review of river basinwater resource management in China. Water 2018, 10, 425. [Google Scholar]
- Chang, J.; Leung, D.Y.C.; Wu, C.Z.; Yuan, Z.H. A review on the energy production, consumption, and prospect of renewable energy in China. Renew. Sustain. Energy Rev. 2003, 7, 453–468. [Google Scholar] [CrossRef]
- Huang, H.; Yan, Z. Present situation and future prospect of hydropower in China. Renew. Sustain. Energy Rev. 2009, 13, 1652–1656. [Google Scholar] [CrossRef]
- Ma, H.; Oxley, L.; Gibson, J.; Li, W. A survey of China’s renewable energy economy. Renew. Sustain. Energy Rev. 2010, 14, 438–445. [Google Scholar] [CrossRef]
- Liu, W.; Lund, H.; Mathiesen, B.V.; Zhang, X. Potential of renewable energy systems in China. Appl. Energy 2011, 88, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, Y. Status and outlook of China’s free-carbon electricity. Renew. Sustain. Energy Rev. 2010, 14, 1014–1025. [Google Scholar] [CrossRef]
- Haile, E.; Fryar, A.E. Evolution chimique de l’eau souterraine dans l’aquifère de Wilcox du Nord de la Plaine Cotière du Golf, Etats-Unis d’Amérique. Hydrogeol. J. 2017, 25, 2403–2418. [Google Scholar] [CrossRef]
- Han, D.M.; Song, X.F.; Currell, M.J.; Yang, J.L.; Xiao, G.Q. Chemical and isotopic constraints on evolution of groundwater salinization in the coastal plain aquifer of Laizhou Bay, China. J. Hydrol. 2014, 508, 12–27. [Google Scholar] [CrossRef]
- Klassen, J.; Allen, D.M. Assessing the risk of saltwater intrusion in coastal aquifers. J. Hydrol. 2017, 551, 730–745. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Rao, V.V.S.G. Hydrogeochemical, seawater intrusion and oxygen isotope studies on a coastal region in the Puri District of Odisha, India. Catena 2019, 172, 558–571. [Google Scholar] [CrossRef]
- Werner, A.D. On the classification of seawater intrusion. J. Hydrol. 2017, 551, 619–631. [Google Scholar] [CrossRef]
- Matiatos, I.; Paraskevopoulou, V.; Lazogiannis, K.; Botsou, F.; Dassenakis, M.; Ghionis, G.; Alexopoulos, J.D.; Poulos, S.E. Surface–ground water interactions and hydrogeochemical evolution in a fluvio-deltaic setting: The case study of the Pinios River delta. J. Hydrol. 2018, 561, 236–249. [Google Scholar] [CrossRef]
- Abdou Babaye, M.S.; Orban, P.; Ousmane, B.; Favreau, G.; Brouyère, S.; Dassargues, A. Characterization of recharge mechanisms in a Precambrian basement aquifer in semi-arid south-west Niger. Hydrogeol. J. 2019, 27, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.K.; Ajeena, A.R. Assessment of interconnection between surface water and groundwater in Sawa Lake area, southern Iraq, using stable isotope technique. Arab. J. Geosci. 2016, 9, 648. [Google Scholar] [CrossRef]
- Cao, W.G.; Yang, H.F.; Liu, C.L.; Li, Y.J.; Bai, H. Hydrogeochemical characteristics and evolution of the aquifer systems of Gonghe Basin, Northern China. Geosci. Front. 2018, 9, 907–916. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, Z.; Wang, S. The source, flow rates, and hydrochemical evolution of groundwater in an alluvial fan of Qilian Mountain, Northwest China. Water (Switzerland) 2017, 9, 912. [Google Scholar] [CrossRef] [Green Version]
- Chitsazan, M.; Aghazadeh, N.; Mirzaee, Y.; Golestan, Y. Hydrochemical characteristics and the impact of anthropogenic activity on groundwater quality in suburban area of Urmia city, Iran. Environ. Dev. Sustain. 2019, 21, 331–351. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, Z.; Huang, G.; Dou, Z. Variations of groundwater quality in the multi-layered aquifer system near the Luanhe river, China. Sustainability 2019, 11, 994. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Song, X.; Yang, L.; Zhang, Y.; Han, D.; Ma, Y.; Bu, H. Identifying the origin and geochemical evolution of groundwater using hydrochemistry and stable isotopes in the Subei Lake basin, Ordos energy base, Northwestern China. Hydrol. Earth Syst. Sci. 2015, 19, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.; Penna, D.; Bertoldi, G.; Vignoli, G.; Tirler, W.; Comiti, F. Controls on spatial and temporal variability in streamflow and hydrochemistry in a glacierized catchment. Hydrol. Earth Syst. Sci. 2019, 23, 2041–2063. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, Z.; Wang, J.; Dou, Z.; Guo, Q. Spatial and temporal variability of the chemistry of the shallow groundwater in the alluvial fan area of the Luanhe river, North China. Environ. Earth Sci. 2014, 72, 5123–5137. [Google Scholar] [CrossRef]
- Ledesma-Ruiz, R.; Pastén-Zapata, E.; Parra, R.; Harter, T.; Mahlknecht, J. Investigation of the geochemical evolution of groundwater under agricultural land: A case study in northeastern Mexico. J. Hydrol. 2015, 521, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.; Li, H.; Shananan, M.; Zhang, X.; Wang, X.; Zhang, Y.; Zhang, X.; Liu, H. Coastal water quality assessment and groundwater transport in a subtropical mangrove swamp in Daya Bay, China. Sci. Total Environ. 2019, 646, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Dansgaard, W. stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Luz, B.; Barkan, E. Variations of 17O/16O and 18O/16O in meteoric waters. Geochim. Cosmochim. Acta 2010, 74, 6276–6286. [Google Scholar]
- Han, D.; Currell, M.J. Delineating multiple salinization processes in a coastal plain aquifer, northern China: Hydrochemical and isotopic evidence. Hydrol. Earth Syst. Sci. 2018, 22, 3473–3491. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yamanaka, T.; Zhou, X.; Tian, F.; Ma, W. Combined use of tracer approach and numerical simulation to estimate groundwater recharge in an alluvial aquifer system: A case study of Nasunogahara area, central Japan. J. Hydrol. 2014, 519, 833–847. [Google Scholar] [CrossRef]
- Peng, T.R.; Lu, W.C.; Chen, K.Y.; Zhan, W.J.; Liu, T.K. Groundwater-recharge connectivity between a hills-and-plains’ area of western Taiwan using water isotopes and electrical conductivity. J. Hydrol. 2014, 517, 226–235. [Google Scholar] [CrossRef]
- Adomako, D.; Gibrilla, A.; Maloszewski, P.; Ganyaglo, S.Y.; Rai, S.P. Tracing stable isotopes (δ2H and δ18O) from meteoric water to groundwater in the Densu River basin of Ghana. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, F.; Li, Y.; Gu, C.; Zhang, Q.; Qiao, Y.; Jiao, L.; Zhu, N. stable isotope evidence for identifying the recharge mechanisms of precipitation, surface water, and groundwater in the Ebinur Lake basin. Sci. Total Environ. 2019, 657, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F. Isotope evidence for quantifying river evaporation and recharge processes in the lower reaches of the Yellow River. Environ. Earth Sci. 2017, 76, 1–15. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J. Comprehensive evaluation of environmental hydrogeology of the Jinshajiang Xiluodu reservoir. Geol. J. China Univ. 2002, 8, 227–235. [Google Scholar]
- Wang, J.; Zhou, Z. Study on environmental groundwater quality in Xiluodu reservoir. Geol. J. China Univ. 2000, 2, 11–15, (In Chinese with English abstract). [Google Scholar]
- Zhuang, C.; Zhou, Z.F.; Li, M.W.; Wang, J.G. Prediction of valley shrinkage deformation in Xiluodu Hydropower Plant based on the hydraulic responses of a confined aquifer. Yantu Gongcheng Xuebao/Chinese J. Geotech. Eng. 2019, 41, 1472–1480, (In Chinese with English abstract). [Google Scholar]
- Zhang, G.; Hu, W.J.; Li, Q.; Liu, J.J.; Wang, F.; Zhou, L. Groundwater chemical characteristics of the qiaojia district in the Jinshajiang river valley, Yunnan, China. Carsol. Sin. 2017, 36, 340–345. (In Chinese) [Google Scholar]
- Li, S.X.; Yan, Z.W.; Lao, W.K.; Yi, W. Chemical characteristics of qiaojia county buckwheat river basin and the Jinsha River right bank karst water. Water Conserv. Sci. Technol. Econ. 2014, 20, 5–9. (In Chinese) [Google Scholar]
- Luo, Z.J.; Zhang, H.; Li, H.Z.; Wang, T.L. Analysis of origin and distribution characteristics of SO42- in dam site area of wudong hydropower station. Hydrogeol. Eng. Geol. 2011, 38, 32–37. (In Chinese) [Google Scholar]
- Tao, Z.; Zhao, Z.; Zhang, D.; Li, X.; Wang, B.; Wu, Q.; Zhang, W.; Liu, C. Chemical weathering processes in the sanjiang (the Jinshajiang, lancangjiang and nujinag) basins in southwest China. J. Ecol. 2015, 34, 2297–2308, (In Chinese with English abstract). [Google Scholar]
- Huh, Y.; Tsoi, M.Y.; Zaitsev, A.; Edmond, J.M. The fluvial geochemistry of the rivers of eastern Siberia: I. Tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim. Cosmochim. Acta 1998, 62, 1657–1676. [Google Scholar] [CrossRef]
- Coetsiers, M.; Walraevens, K. Chemical characterization of the Neogene Aquifer, Belgium. Hydrogeol. J. 2006, 14, 1556–1568. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Hu, Z.; Zhou, Z. Hydrochemical and Isotopic Evolution of Groundwater Flowing Downstream of the Daqing River (Liaodong Bay, China). J. Coast. Res. 2020, 36, 608–618. [Google Scholar] [CrossRef]
- Gan, Y.; Zhao, K.; Deng, Y.; Liang, X.; Ma, T.; Wang, Y. Groundwater flow and hydrogeochemical evolution in the Jianghan Plain, central China. Hydrogeol. J. 2018, 26, 1609–1623. [Google Scholar] [CrossRef]
- Güler, C.; Thyne, G.D. Delineation of hydrochemical facies distribution in a regional groundwater system by means of fuzzy c-means clustering. Water Resour. Res. 2004, 40, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zheng, Y.; Zhang, J.; Wu, B.; Wang, S.; Tian, Y.; Li, J.; Meng, X. Investigating Hydrochemical Groundwater Processes in an Inland Agricultural Area with Limited Data: A Clustering Approach. Water 2017, 9, 723. [Google Scholar] [CrossRef] [Green Version]
- Plummer, L.N.; Busby, J.F.; Lee, R.W.; Hanshaw, B.B. Geochemical Modeling of the Madison Aquifer in Parts of Montana. Water Resour. Res. 1990, 26, 1981–2014. [Google Scholar] [CrossRef]
- Global Network of Isotopes in Precipitation. Available online: http://www-naweb.iaea.org/napc/ih/IHS_resources_gnip.html (accessed on 21 October 2018).
- Walton-Day, K.; Poeter, E. Investigating hydraulic connections and the origin of water in a mine tunnel using stable isotopes and hydrographs. Appl. Geochem. 2009, 24, 2266–2282. [Google Scholar] [CrossRef]
- Yeh, H.F.; Lee, C.H.; Hsu, K.C. Oxygen and hydrogen isotopes for the characteristics of groundwater recharge: A case study from the Chih-Pen Creek basin, Taiwan. Environ. Earth Sci. 2011, 62, 393–402. [Google Scholar] [CrossRef]
- Su, C.; Wang, Y.; Pan, Y. Hydrogeochemical and isotopic evidences of the groundwater regime in Datong Basin, Northern China. Environ. Earth Sci. 2013, 70, 877–885. [Google Scholar] [CrossRef]
- Kattan, Z. Characterization of surface water and groundwater in the Damascus Ghotta basin: Hydrochemical and environmental isotopes approaches. Environ. Geol. 2006, 51, 173–201. [Google Scholar] [CrossRef]

| Water Type | Sample No. | Longitude | Latitude | pH | TDS (mg/L) | Main Ion Content (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl- | ||||||||
| Ground water previous | GB01 | 103°38′30″ | 28°15′43″ | 7.0 | 223 | 3.7 | 11.1 | 31.1 | 7.7 | 12.1 | 8.2 | 139.7 |
| GB02 | 103°38′43″ | 28°15′34″ | 7.8 | 206 | 3.0 | 9.2 | 30.7 | 5.0 | 6.0 | 8.2 | 134.2 | |
| GB03 | 103°38′41″ | 28°15′14″ | 8.4 | 144 | 7.1 | 21.4 | 15.0 | 4.0 | 8.5 | 15.8 | 62.2 | |
| GB04 | 103°38′52″ | 28°15′22″ | 7.1 | 306 | 3.9 | 11.8 | 50.9 | 8.9 | 7.5 | 44.7 | 168.4 | |
| GB05 | 103°38′59″ | 28°15′28″ | 6.9 | 196 | 2.4 | 7.1 | 33.4 | 3.8 | 6.0 | 2.5 | 130.9 | |
| GB06 | 103°39′05″ | 28°15′32″ | 7.1 | 191 | 2.2 | 6.6 | 27.7 | 7.7 | 4.6 | 13.9 | 118.4 | |
| GB07 | 103°39′00″ | 28°15′10″ | 7.2 | 300 | 6.3 | 18.8 | 41.8 | 7.6 | 6.0 | 22.5 | 186.7 | |
| GB08 | 103°39′04″ | 28°15′14″ | 6.9 | 191 | 1.6 | 4.7 | 35.5 | 3.8 | 4.5 | 12.5 | 118.4 | |
| GB09 | 103°39′12″ | 28°15′21″ | 7.4 | 284 | 2.2 | 6.7 | 19.2 | 15.1 | 8.5 | 2.4 | 219.7 | |
| GB10 | 103°39′18″ | 28°15′27″ | 7.0 | 187 | 1.7 | 5.1 | 30.9 | 5.7 | 6.7 | 2.4 | 124.5 | |
| GB11 | 103°39′24″ | 28°15′16″ | 7.7 | 209 | 2.9 | 8.7 | 30.5 | 7.4 | 6.4 | 10.1 | 133.0 | |
| GB12 | 103°41′21″ | 28°15′07″ | 7.1 | 190 | 1.2 | 6.6 | 27.7 | 7.7 | 4.6 | 13.9 | 118.4 | |
| GB13 | 103°39′13″ | 28°15′08″ | 7.1 | 306 | 3.9 | 11.8 | 50.9 | 8.9 | 7.5 | 44.7 | 168.4 | |
| GB14 | 103°39′24″ | 24°14′44″ | 7.9 | 222 | 8.0 | 24.3 | 7.2 | 7.8 | 13.8 | 39.9 | 111.1 | |
| GB15 | 103°39′50″ | 28°14′50″ | 6.5 | 330 | 2.9 | 8.6 | 44.3 | 18.2 | 9.9 | 4.8 | 231.3 | |
| SD | - | - | 0.5 | 57.0 | 2.1 | 6.0 | 12.2 | 4.0 | 2.7 | 14.9 | 43.8 | |
| Surface water previous | SB1 | 103°38′05″ | 28°15′42″ | 8.1 | 247 | 5.1 | 15.6 | 33.7 | 11.9 | 24.3 | 25.6 | 120.7 |
| SB2 | 103°39′36″ | 28°14′57″ | 8.0 | 202 | 1.0 | 3.1 | 30.9 | 12.6 | 13.1 | 4.3 | 126.7 | |
| SB3 | 103°40′24″ | 28°14′27″ | 7.9 | 257 | 1.6 | 4.8 | 42.7 | 11.7 | 11.7 | 12.0 | 162.9 | |
| SB4 | 103°38′07″ | 28°16′22″ | 7.9 | 115 | 1.5 | 4.4 | 17.0 | 3.4 | 3.9 | 6.2 | 68.3 | |
| SB5 | 103°40′07″ | 28°14′39″ | 7.5 | 161 | 1.0 | 2.9 | 23.0 | 8.9 | 5.0 | 17.8 | 92.7 | |
| SD | - | - | 0.2 | 59.5 | 1.7 | 5.3 | 9.9 | 3.8 | 8.2 | 8.7 | 35.8 | |
| Ground water late | GA01 | 103°38′31″ | 28°15′14″ | 8.2 | 339 | 1.3 | 26.1 | 47.1 | 14.0 | 26.1 | 43.3 | 171.0 |
| GA02 | 103°39′15″ | 28°15′44″ | 10.7 | 558 | 1.5 | 79.0 | 4.0 | 1.2 | 12.2 | 41.0 | 0.0 | |
| GA03 | 103°38′58″ | 28°15′03″ | 8.1 | 317 | 0.8 | 15.3 | 48.1 | 14.0 | 19.0 | 32.1 | 174.0 | |
| GA04 | 103°39′27″ | 28°15′32″ | 8.0 | 239 | 1.8 | 20.2 | 33.1 | 3.0 | 13.3 | 22.3 | 125.0 | |
| GA05 | 103°39′07″ | 28°15′15″ | 8.2 | 292 | 1.5 | 22.6 | 43.0 | 11.1 | 33.3 | 44.2 | 123.0 | |
| GA06 | 103°39′13″ | 28°15′20″ | 8.0 | 318 | 1.0 | 16.9 | 48.0 | 14.6 | 22.0 | 47.5 | 154.0 | |
| GA07 | 103°39′08″ | 28°15′09″ | 8.4 | 335 | 2.2 | 30.2 | 39.0 | 17.5 | 36.9 | 46.4 | 153.0 | |
| GA08 | 103°39′14″ | 28°15′14″ | 8.2 | 285 | 1.9 | 23.8 | 35.0 | 13.8 | 27.0 | 37.3 | 137.0 | |
| GA09 | 103°39′20″ | 28°15′19″ | 8.1 | 268 | 1.4 | 17.3 | 34.0 | 13.7 | 15.0 | 27.1 | 150.0 | |
| GA10 | 103°39′16″ | 28°15′05″ | 8.1 | 259 | 1.1 | 14.2 | 36.0 | 11.6 | 12.7 | 23.6 | 151.0 | |
| GA11 | 103°39′20″ | 28°15′09″ | 8.1 | 292 | 1.3 | 23.5 | 35.0 | 15.0 | 24.2 | 33.9 | 148.0 | |
| GA12 | 103°39′27″ | 28°15′14″ | 8.1 | 288 | 1.3 | 24.7 | 35.0 | 14.6 | 28.8 | 38.2 | 135.0 | |
| GA13 | 103°39′23″ | 20°15′03″ | 8.6 | 241 | 1.7 | 33.5 | 22.0 | 7.9 | 19.5 | 35.9 | 109.0 | |
| GA14 | 103°39′28″ | 28°15′05″ | 8.1 | 264 | 1.5 | 30.9 | 29.0 | 9.8 | 23.0 | 44.9 | 114.0 | |
| SD | - | - | 0.7 | 79.0 | 0.4 | 16.0 | 11.5 | 4.7 | 7.7 | 8.3 | 42.6 | |
| Surface water late | SA1 | 103°38′37″ | 28°15′36″ | 8.3 | 358 | 2.5 | 33.2 | 40.0 | 18.9 | 35.9 | 47.9 | 171.0 |
| SA2 | 103°39′42″ | 28°14′54″ | 8.1 | 360 | 2.2 | 32.7 | 46.0 | 16.7 | 38.9 | 51.3 | 165.0 | |
| Sample No. | Anhydrite | Aragonite | Calcite | pCO2 | Dolomite | Gypsum | Halite |
|---|---|---|---|---|---|---|---|
| GB01 | −3.59 | −0.90 | −0.76 | −1.85 | −1.77 | −3.37 | −8.42 |
| GB02 | −3.58 | −0.12 | 0.02 | −2.67 | −0.40 | −3.36 | −8.81 |
| GB09 | −4.35 | −0.53 | −0.38 | −2.06 | −0.52 | −4.13 | −8.80 |
| GB12 | −3.39 | −0.91 | −0.77 | −2.02 | −1.75 | −3.17 | −9.07 |
| GB15 | −3.75 | −1.07 | −0.92 | −1.14 | −1.88 | −3.53 | −8.63 |
| GA01 | −2.77 | 0.50 | 0.65 | −2.99 | 1.12 | −2.55 | −7.73 |
| GA02 | −2.88 | 0.43 | 0.57 | −2.88 | 0.96 | −2.66 | −8.10 |
| GA03 | −3.12 | 0.07 | 0.21 | −2.91 | −0.27 | −2.90 | −8.12 |
| GA07 | −2.82 | 0.56 | 0.7 | −3.25 | 1.41 | −2.6 | −7.52 |
| GA08 | −2.93 | 0.30 | 0.44 | −3.08 | 0.83 | −2.71 | −7.75 |
| GA09 | −3.07 | 0.23 | 0.38 | −2.94 | 0.71 | −2.85 | −8.14 |
| GA11 | −2.98 | 0.23 | 0.38 | −2.95 | 0.73 | −2.76 | −7.81 |
| Water Type | Sample No. | Elevation (m) | δ18O (‰) | δD (‰) | d-Excess (‰) | Recharge Elevation (m) |
|---|---|---|---|---|---|---|
| Groundwater previous | GB01 | 424.3 | −8.9 | −61.4 | 9.8 | 911.5 |
| GB11 | 404.3 | −7.9 | −55.1 | 8.1 | 670.5 | |
| GB15 | 405.7 | −8.5 | −58.3 | 9.7 | 788.5 | |
| Surface water previous | SB03 | 380.0 | −11.4 | −97.4 | −6.2 | 2207.5 |
| SB04 | 380.0 | −8.6 | −60.5 | 8.3 | 854.9 | |
| Groundwater late | GA03 | 389.9 | −12.6 | −94.8 | 6.0 | 2119.3 |
| GA04 | 387.8 | −11.8 | −86.9 | 7.5 | 1829.7 | |
| GA07 | 335.0 | −13.9 | −101.5 | 9.7 | 2337.2 | |
| GA09 | 335.0 | −12.2 | −85.1 | 12.5 | 1735.8 | |
| GA10 | 335.0 | −11.9 | −82.1 | 13.1 | 1625.8 | |
| GA11 | 335.0 | −13.3 | −92.5 | 13.9 | 2007.2 | |
| SD | - | 0.83 | 7.16 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhou, Z.; Xu, H.; Li, M. The Hydrochemical and Isotopic Evolution of the Surface Water and Groundwater for Impoundment in the Xiluodu Reservoir, Jinsha River, China. Sustainability 2020, 12, 5805. https://doi.org/10.3390/su12145805
Zhou Z, Zhou Z, Xu H, Li M. The Hydrochemical and Isotopic Evolution of the Surface Water and Groundwater for Impoundment in the Xiluodu Reservoir, Jinsha River, China. Sustainability. 2020; 12(14):5805. https://doi.org/10.3390/su12145805
Chicago/Turabian StyleZhou, Ziwen, Zhifang Zhou, Haiyang Xu, and Mingwei Li. 2020. "The Hydrochemical and Isotopic Evolution of the Surface Water and Groundwater for Impoundment in the Xiluodu Reservoir, Jinsha River, China" Sustainability 12, no. 14: 5805. https://doi.org/10.3390/su12145805
APA StyleZhou, Z., Zhou, Z., Xu, H., & Li, M. (2020). The Hydrochemical and Isotopic Evolution of the Surface Water and Groundwater for Impoundment in the Xiluodu Reservoir, Jinsha River, China. Sustainability, 12(14), 5805. https://doi.org/10.3390/su12145805





