Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China
Abstract
:1. Introduction
2. Methodology
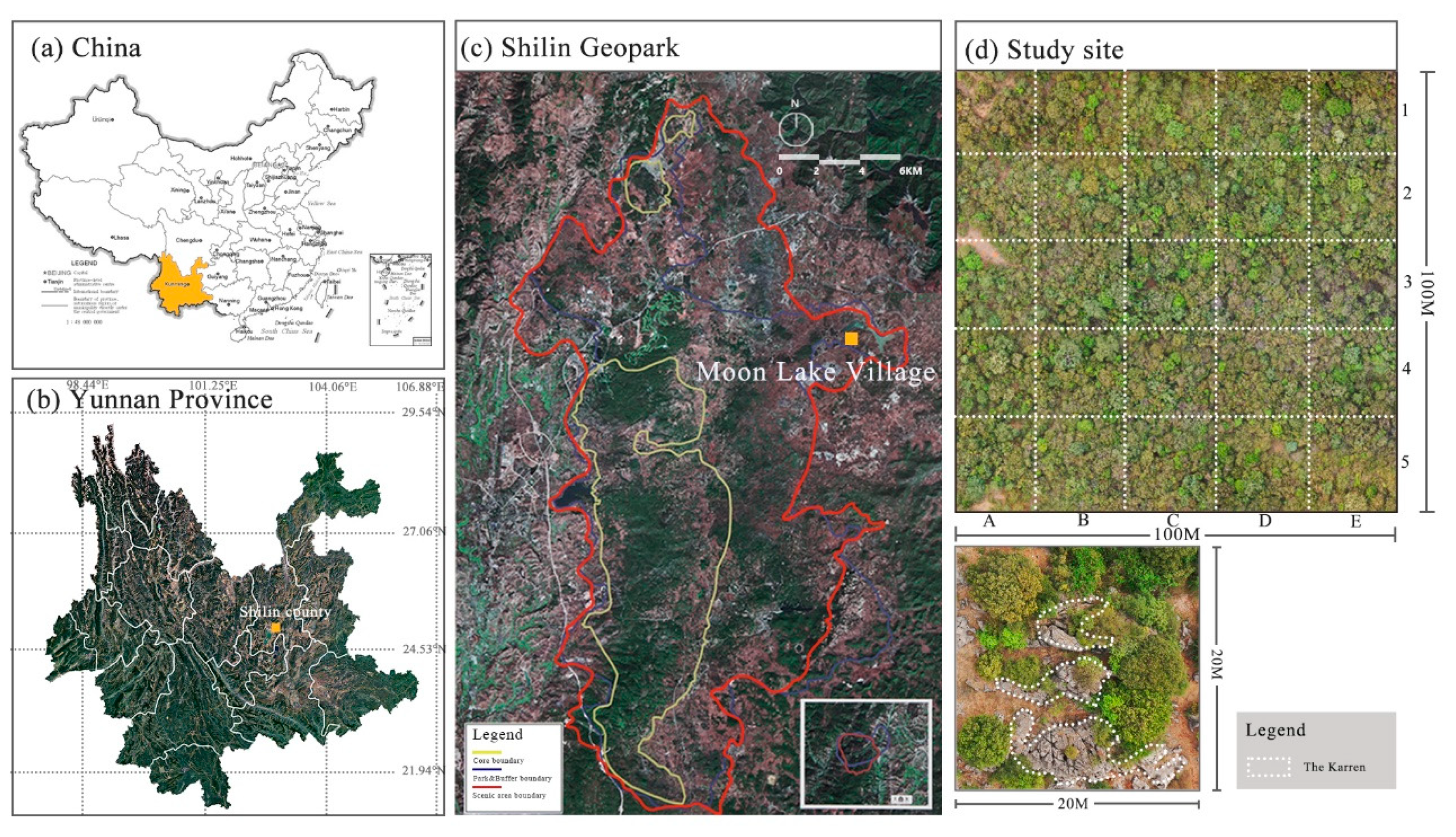
2.1. Study Area and Site Description
2.2. Sampling Design
2.3. Data Measurements
2.4. Data Analyses
- The α diversity of plants was computed using the Patrick index, the Shannon–Wiener index, and the Pielou index.
- One-way analysis of variance (ANOVA) was performed to compare plant α diversity between karren habitat types if the data of response variables complied with normal distribution. However, when the data did not show a trend of normal distribution, we performed variation analysis using non-parametric Kruskal–Wallis ANOVA (kruskal test and dunn test in R).
- Canonical correspondence analysis (CCA) was calculated with the R package vegan [41] to analyze the relationship between plant species distribution and environmental factors. A data matrix was constructed consisting of species and environmental variables. To investigate whether specific karren habitat types (KHT) differed in species distribution, these types were pooled into the environmental variables as factor constraints. Occasional plant species with a frequency below 3% have been checked for whether they belong to the category of conservation species. If not, occasional species were omitted to eliminate the excessive effect. The statistical significance of species–environmental correlations was examined using 1000 distribution-free Monte Carlo permutations [41].
- Pearson correlation between all influencing factors and species α diversity was calculated by using the R package Hmisc [42].
- Stepwise linear regression was performed to further demonstrate the effects of dominant environmental variables on plant α diversity and examine the extent of effects.
3. Results
3.1. Plant Taxonomic Composition in Different Karren Habitats
3.2. α Diversity Distribution of Plants in Different Karren Habitats
3.3. Effect of Environmental Factors on Woody and Herbaceous Species Distribution
3.4. Environmental Influence on Plant α Diversity
3.5. Dominant Environmental Factors on α Diversity of Plants
4. Discussion
4.1. Plant Diversity Maintenance Mechanism in Karren Habitats
4.2. Environmental Interpretation for the Plant Species Distribution in Karren Habitats
4.3. Implications for Planting Design and Management in the Karren Habitats of KRD Restoration Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ford, D.; Williams, P.W. Karst Hydrogeology and Geomorphology; John Wiely & Sons: Chichester, UK, 2007. [Google Scholar]
- Wang, S.J.; Liu, Q.M.; Zhang, D.F. Karst rocky desertification in southwestern china: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Wang, K.; Zhang, C.; Yue, Y.; Tian, R.; Fan, F. Spatio-temporal evolution of rocky desertification and its driving forces in karst areas of Northwestern Guangxi, China. Environ. Earth Sci. 2011, 64, 383–393. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, H.; Li, M. Mining spatial information to investigate the evolution of karst rocky desertification and its human driving forces in Changshun, China. Sci. Total Environ. 2013, 458–460, 419–426. [Google Scholar] [CrossRef]
- Liao, C.; Yue, Y.; Wang, K.; Fensholt, R.; Tong, X.; Brandt, M. Ecological restoration enhances ecosystem health in the karst regions of southwest China. Ecol. Indic. 2018, 90, 416–425. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- Stas, S.M.; Rutishauser, E.; Chave, J.; Anten, N.P.R.; Laumonier, Y. Estimating the aboveground biomass in an old secondary forest on limestone in the Moluccas, Indonesia: Comparing locally developed versus existing allometric models. For. Ecol. Manag. 2017, 389, 27–34. [Google Scholar] [CrossRef]
- Kiernan, K. Environmental degradation in karst areas of Cambodia: A legacy of war? Land Degrad. Dev. 2010, 21, 503–519. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Zhang, J.; Yang, W.; Zhang, L.; Hull, V.; Wang, Z.; Zheng, H.; Liu, J.; Polasky, S.; et al. Strengthening protected areas for biodiversity and ecosystem services in China. Proc. Natl. Acad. Sci. USA 2017, 114, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dai, H.; Wang, C.; Zeng, C.; Su, W. The challenge and future of rocky desertification control in karst areas in southwest China. Solid Earth Discuss. 2016, 7, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; He, X.; Wang, K.; Su, Y.; Wu, J. Interactions of vegetation succession, soil bio-chemical properties and microbial communities in a Karst ecosystem. Eur. J. Soil Biol. 2012, 51, 1–7. [Google Scholar] [CrossRef]
- Li, K.; Xing, X.; Li, Y.; Li, X.; Li, R.; Fan, S.; Dong, L. Effect of different artificial restoration methods of karst rocky desertification on community composition and niche characteristics of woody populations in Shilin scenic area. Acta Ecol. Sin. 2020, 40. (In Chinese) [Google Scholar] [CrossRef]
- Brandt, M.; Yue, Y.; Wigneron, J.P.; Tong, X.; Tian, F.; Jepsen, M.R.; Xiao, X.; Verger, A.; Mialon, A.; Al Yaari, A.; et al. Satellite-observed major greening and biomass increase in south China karst during recent decade. Earth’s Future 2018, 6, 1017–1028. [Google Scholar] [CrossRef]
- Xiao, K.; He, T.; Chen, H.; Peng, W.; Song, T.; Wang, K.; Li, D. Impacts of vegetation restoration strategies on soil organic carbon and nitrogen dynamics in a karst area. Ecol. Eng. 2017, 101, 247–254. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y. Characteristics of woody plant regeneration in Karren-habitats successional plant communities in Yunnan Shilin karst area of China. Chin. J. Plant Ecol. 2010, 34, 889–897. (In Chinese) [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Ma, Z. A preliminary study on flora diversity of karst microhabitat in Shilin Park, Yunnan, China. J. Mt. Sci. 2007, 25, 438–447. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, S.; He, J.; Wei, L. Ecological Research on Karst Forest (III): A Study on Microhabitats to Karst Forest in Maolan; Guizhou Science and Technology Press: Guiyang, China, 2003; pp. 38–48. (In Chinese) [Google Scholar]
- Sweeting, M. Tectonics and fluvial denudation in the formation of cone karst, with particular reference to South China. Tübinger Geogr. Stud. 1992, 109, 45–56. [Google Scholar]
- Liu, F.; Wang, S.; Luo, H.; Liu, Y.; LIu, H. Micro-habitats in karst forest ecosystem and variablity of soils. Acta Pedol. Sin. 2008, 45, 1055–1062. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Q.; Long, J.; Liao, H.; Liu, L.; Li, J.; Wu, J.; Xiao, X. Soil bacterial community characteristics under different microhabitat types on Maolan karst forest, Guizhou, Southwest China. Chin. J. Appl. Ecol. 2019, 30, 108–116. (In Chinese) [Google Scholar] [CrossRef]
- Yu, G.; Wang, S.; Rong, L. Microclimate characteristics of differnt microhabitats in successional stages of Maolan karst forest. Earth Environ. 2011, 39, 469–477. (In Chinese) [Google Scholar]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Taberlet, P.; Cheddadi, R. Quaternary Refugia and Persistence of Biodiversity. Science 2002, 5589, 2009–2010. [Google Scholar] [CrossRef] [PubMed]
- Tapper, S.L.; Byrne, M.; Yates, C.J.; Keppel, G.; Hopper, S.D.; Niel, K.V.; Shut, A.G.T.; Mucina, L.; Wardell-Johnson, G.W. Isolated with persistence or dynamically connected? Genetic patterns in a common granite outcrop endemic. Divers. Distrib. 2014, 20, 987–1001. [Google Scholar] [CrossRef]
- Miyawaki, A. Creative Ecology: Restoration of Native Forests by Native Trees. Plant Biotechnol. 1999, 16, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.; Heckman, J.R. Restor. Ecology: The State of an Emerging Field. Annu. Rev. Energy Environ. 1996, 21, 167–189. [Google Scholar] [CrossRef] [Green Version]
- National Bureau of Statistics of China (Yunnan). Yunnan Statistical Yearbook 2019; China Statistics Press: Beijing, China, 2019. [Google Scholar]
- Shen, Y.; Yang, G.; Huang, J. Comparison of tree sprouting in three regeneration stages of an evergreen broadleaved forest in a karst landscape, SW China. Acta Ecol. Sin. 2011, 31, 126–132. [Google Scholar] [CrossRef]
- Tang, C.Q.; Li, Y.; Zhang, Z.; Hou, X.; Hara, K.; Tomita, M.; He, L.; Li, X. Effects of management on vegetation dynamics and associated nutrient cycling in a karst area, Yunnan, SW China. Landsc. Ecol. Eng. 2015, 11, 177–188. [Google Scholar] [CrossRef]
- Veress, M. The KARREN and KARREN formation of bare slopes. Earth-Sci. Rev. 2019, 188, 272–290. [Google Scholar] [CrossRef]
- Zhang, F.; Geng, H.; Li, Y.; Liang, Y.; Yang, Y.; Ren, J.; Wang, F.; Tao, H.; Li, Z. Study on the Lunan Stone Forest Karst; Yunnan Science and Technology Press: Kunming, China, 1997. (In Chinese) [Google Scholar]
- Shen, Y.X.; Liu, W.Y.; Cao, M.; Li, Y.H. Seasonal variation in density and species richness of soil seed-banks in karst forests and degraded vegetation in central Yunnan, SW China. Seed Sci. Res. 2007, 17, 99–107. [Google Scholar] [CrossRef]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Du, H.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Song, M.; Zhang, H. Spatial pattern of woody plants and their environmental interpretation in the karst forest of southwest China. Plant Biosyst. 2015, 149, 121–130. [Google Scholar] [CrossRef]
- Compilation Committee of the Flora of China; Flora of China; Science Press: Beijing, China, 2004. (In Chinese)
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Development Core Team: 2019. R Foundation for Statistical Computing, Auckland, New Zealand. Available online: http://www.r-project.org/ (accessed on 10 July 2019).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Zhang, J. Quantitative Ecology; The Science Publishing Company: Beijing, China, 2004; pp. 123–125. (In Chinese) [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975; p. 165. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.4-4. Available online: http://github.com/vegandevs/vegan (accessed on 24 August 2017).
- Harrell, F. With contributions from Charles Dupont and many others. In Hmisc: Harrell Miscellaneous; 405 R Package Version 4.1-1; Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 3 January 2018).
- Sandel, B.; Svenning, J.C. Human impacts drive a global topographic signature in tree cover. Nat. Commun. 2013, 4, 2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, K.; Condit, R.; Hubbell, S.; Foster, R. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Kalajnxhiu, A.; Tsiripidis, I.; Bergmeier, E. The diversity of woodland vegetation in Central Albania along an altitudinal gradient of 1300 m. Plant Biosyst. 2012, 146, 954–969. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Z.; Dai, J.; Wu, X.; Peng, J.; Wang, H.; Meersmans, J.; Green, S.M.; Quine, T.A. Rock crevices determine woody and herbaceous plant cover in the karst critical zone. Sci. China Earth Sci. 2019, 62, 1756–1763. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 2005, 126, 129–140. [Google Scholar] [CrossRef]
- Takyu, M.; Aiba, S. Effects of topography on tropical lower montane forests under different geological conditions on Mount Kinabalu, Borneo. Plant Ecol. 2002, 159, 35–49. [Google Scholar] [CrossRef]
- Mitchell, A.K. Growth limitations for conifer regeneration under alternative silvicultural systems in a coastal montane forest in British Columbia, Canada. For. Ecol. Manag. 2001, 145, 129–136. [Google Scholar] [CrossRef]
- Shirley, H. Light as an Ecological Factor and Its Measurement. Bot. Rev. 1935, 1, 355–381. [Google Scholar] [CrossRef]
- Barrette, M.; Bélanger, L.; De Grandpré, L.; Royo, A.A. Demographic disequilibrium caused by canopy gap expansion and recruitment failure triggers forest cover loss. For. Ecol. Manag. 2017, 401, 117–124. [Google Scholar] [CrossRef]
- Scariot, A. Seedling Mortality by Litterfall in Amazonian Forest Fragments. Biotropica 2000, 32, 662–669. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, Z.; Mallik, A.; Zhou, A.; Mo, L.; He, C.; Huang, Y. Seasonal water use strategy of cyclobalanopsis glauca in a karst area of southern China. Environ. Earth Sci. 2015, 74, 1007–1014. [Google Scholar] [CrossRef]
- Richter, D.D.; Billings, S.A. ‘One physical system’: Tansley’s ecosystem as Earth’s critical zone. New Phytol. 2015, 206, 900–912. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, H.; Wang, K.; Yang, J. Water source utilization by woody plants growing on dolomite outcrops and nearby soils during dry seasons in karst region of Southwest China. J. Hydrol. 2012, 420–421, 264–274. [Google Scholar] [CrossRef]
- Kümmerling, M.; Müller, N. The relationship between landscape design style and the conservation value of parks: A case study of a historical park in Weimar, Germany. Landsc. Urban Plan. 2012, 107, 111–117. [Google Scholar] [CrossRef]
- Li, X.; Fan, S.; Guan, J.; Zhao, F.; Dong, L. Diversity and influencing factors on spontaneous plant distribution in Beijing Olympic Forest Park. Landsc. Urban Plan. 2019, 181, 157–168. [Google Scholar] [CrossRef]
- Kaligarič, M.; Ivajnšič, D. Vanishing landscape of the “classic” Karst: Changed landscape identity and projections for the future. Landsc. Urban Plan. 2014, 132, 148–158. [Google Scholar] [CrossRef]
- Macdonald, E.; King, E.G. Novel ecosystems: A bridging concept for the consilience of cultural landscape conservation and ecological restoration. Landsc. Urban Plan. 2018, 177, 148–159. [Google Scholar] [CrossRef]




| Karren Habitat Types | Formation | Shape | Characteristics of Soil and Litter | |||
|---|---|---|---|---|---|---|
| Length/m | Width/m | Soil Proportion/% | Surface Soil Thickness/cm | Litter Thickness/cm | ||
| Grike (GR) | Structural fractures | 0.20–20.00 | 0.10–0.20 | 20–60 | 1.00–52.00 | 0.00–5.00 |
| Deep solution pit (DSP) | Dissolution | 0.40–5.00 | 0.10–0.30 | ≥40 | 9.00–38.50 | 0.00–5.00 |
| Solution corridor (SC) | Structural fractures | 1.35–10.00 | 0.60–10.00 | ≥70 | 10.00–65.30 | 1.00–5.00 |
| Solution rock debris (SRD) | Collapse of the clint | 1.15–10.00 | 0.25–10.00 | ≤15 | 15.00–46.00 | 0.00–5.00 |
| Solution well (SW) | Dissolution | 0.65–10.00 | 0.30–2.30 | ≥40 | 19.00–64.00 | 1.00–3.00 |
| Karren Habitat Type | No. of Karren Habitat | Total No. of Species/Family Number | Mean No. of Species Number ± SE a | No. of Woody Species/Family Number | No. of Herbaceous Species/Family Number |
|---|---|---|---|---|---|
| GR | 62 | 60/28 | 7 ± 2.69 b | 44/22 | 16/8 |
| DSP | 54 | 52/25 | 6 ± 2.64 c | 36/19 | 16/8 |
| SC | 39 | 48/26 | 9 ± 3.57 a | 35/21 | 13/6 |
| SRD | 23 | 50/26 | 7 ± 2.32 b | 28/20 | 22/11 |
| SW | 11 | 11/10 | 2 ± 0.69 d | 9/8 | 2/2 |
| Woody Plant (p = 0.004 **) | Herbaceous Plant (p = 0.033 *) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CCA1 | CCA2 | ChiSquare | p-Value | CCA1 | CCA2 | ChiSquare | p-Value | ||
| Karren habitat features | Karren habitat type (KHT) | —— | —— | 0.062 | 0.037 * | —— | —— | 0.103 | 0.181 |
| Karren habitat width (KHW) | −0.965 | 0.264 | 0.051 | 0.040 * | 0.959 | 0.285 | 0.046 | 0.394 | |
| Karren habitat length (KHL) | −0.870 | −0.493 | 0.016 | 0.357 | 0.954 | 0.298 | 0.075 | 0.155 | |
| Karren habitat height (KHH) | 0.581 | −0.814 | 0.045 | 0.043 * | 0.992 | −0.123 | 0.073 | 0.394 | |
| Canopy density (CD) | −1.000 | −0.015 | 0.352 | 0.001 *** | 0.530 | −0.848 | 0.269 | 0.002 ** | |
| Soil thickness (ST) | −0.612 | 0.791 | 0.041 | 0.096 | 1.000 | 0.023 | 0.023 | 0.628 | |
| Litter thickness (LT) | —— | —— | —— | —— | −0.030 | −1.000 | 0.158 | 0.037 * | |
| Topographic factors | Slope gradient (SG) | −0.021 | −1.000 | 0.251 | 0.001 *** | −0.951 | −0.308 | 0.202 | 0.020 * |
| Slope Aspect (SAS) | −0.036 | −0.999 | 0.105 | 0.002 ** | −0.883 | −0.470 | 0.096 | 0.141 | |
| Arbor Layer | Shrub Layer | Herb Layer | |||||
|---|---|---|---|---|---|---|---|
| Shannon–Wiener | Pielou | Shannon–Wiener | Pielou | Shannon–Wiener | Pielou | ||
| Karren habitat features | SA | 0.520 *** | 0.109 | 0.327 *** | 0.147 | 0.197 ** | −0.084 |
| ST | 0.848 *** | 0.274 * | 0.199 * | 0.094 | 0.303 *** | 0.021 | |
| KHH | 0.391 *** | 0.013 | 0.157 | 0.116 | −0.011 | 0.030 | |
| LT | 0.041 | −0.100 | 0.155 | 0.006 | −0.028 | 0.173 * | |
| CD | −0.043 | −0.018 | −0.038 | 0.234 * | −0.134 | −0.061 | |
| Topographic factors | SAS | −0.212 * | −0.062 | −0.083 | 0.068 | −0.157 * | 0.003 |
| SG | −0.124 | −0.063 | 0.025 | −0.034 | −0.151 * | −0.146 | |
| Arbor Layer | ||||
|---|---|---|---|---|
| Shannon–Wiener a | Pielou b | |||
| β | p-Value | β | p-Value | |
| ST | 0.784 | 0.000 *** | 0.371 | 0.021 * |
| SA | 0.149 | 0.005 ** | —— | —— |
| CD | −0.125 | 0.007 ** | —— | —— |
| LT | —— | —— | −0.171 | 0.138 |
| Shrub Layer | ||||
|---|---|---|---|---|
| Shannon–Wiener a | Pielou b | |||
| β | p-Value | β | p-Value | |
| SA | 0.327 | 0.000 *** | —— | —— |
| CD | —— | —— | 0.228 | 0.028 * |
| Herb Layer | ||||
|---|---|---|---|---|
| Shannon–Wiener a | Pielou b | |||
| β | p-Value | β | p-Value | |
| KHH | −0.160 | 0.045 * | —— | —— |
| SA | 0.162 | 0.058 | −0.149 | 0.063 |
| ST | 0.289 | 0.000 *** | —— | —— |
| CD | −0.180 | 0.014 * | —— | —— |
| LT | —— | —— | 0.257 | 0.002 ** |
| SG | —— | —— | −0.216 | 0.008 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhang, M.; Li, Y.; Xing, X.; Fan, S.; Cao, Y.; Dong, L.; Chen, D. Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China. Sustainability 2020, 12, 5808. https://doi.org/10.3390/su12145808
Li K, Zhang M, Li Y, Xing X, Fan S, Cao Y, Dong L, Chen D. Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China. Sustainability. 2020; 12(14):5808. https://doi.org/10.3390/su12145808
Chicago/Turabian StyleLi, Kun, Mengyuan Zhang, Yilun Li, Xiaoyi Xing, Shuxin Fan, Yu Cao, Li Dong, and Desheng Chen. 2020. "Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China" Sustainability 12, no. 14: 5808. https://doi.org/10.3390/su12145808
APA StyleLi, K., Zhang, M., Li, Y., Xing, X., Fan, S., Cao, Y., Dong, L., & Chen, D. (2020). Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China. Sustainability, 12(14), 5808. https://doi.org/10.3390/su12145808




