The Response of Water and Nutrient Dynamics and of Crop Yield to Conservation Agriculture in the Ethiopian Highlands
Abstract
:1. Introduction
2. Materials and Methods
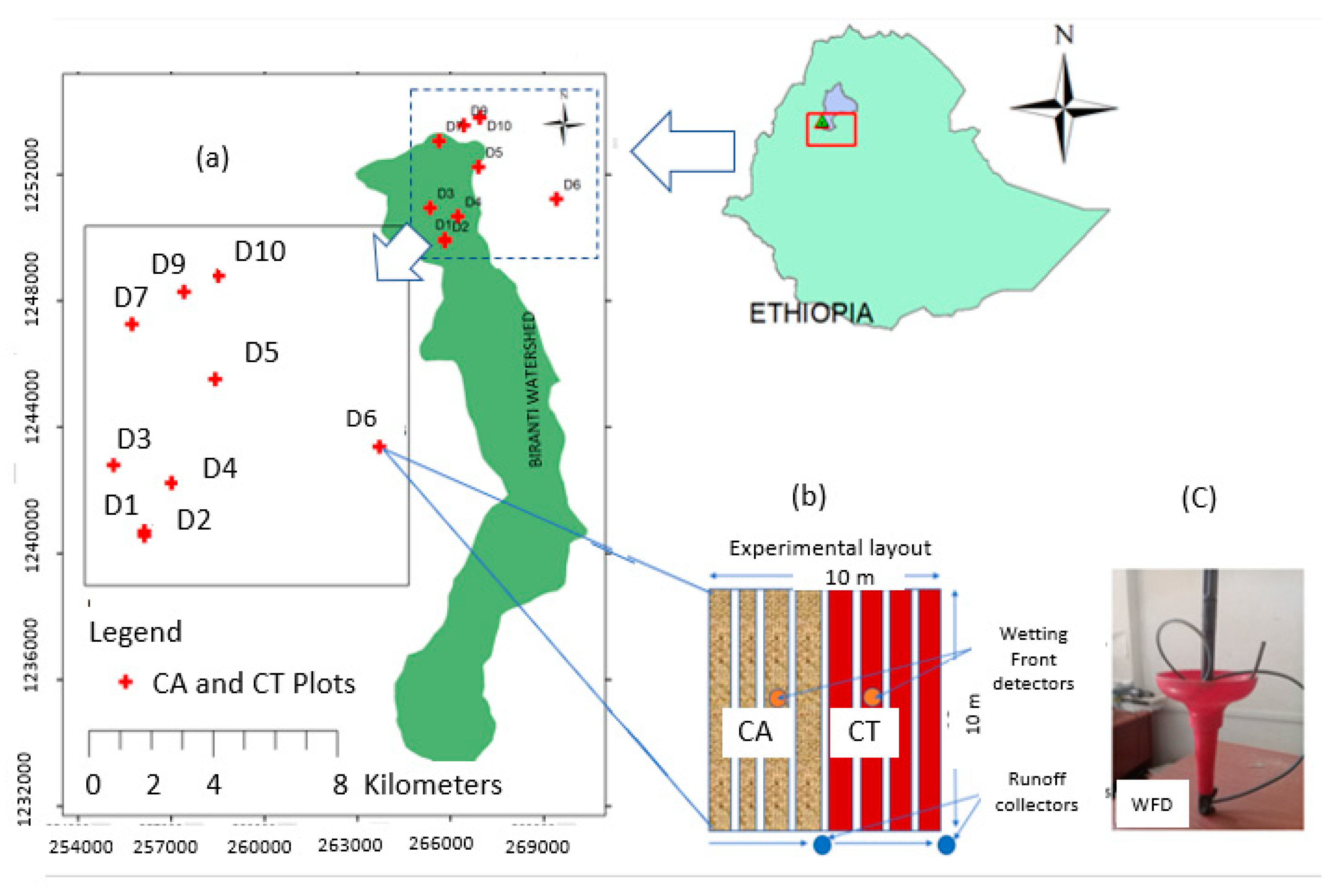
2.1. Description of the Study Area
2.2. Experimental Design and Layout
2.3. Crop Management Practices
2.4. Data Collection
2.5. Data Analysis
3. Results
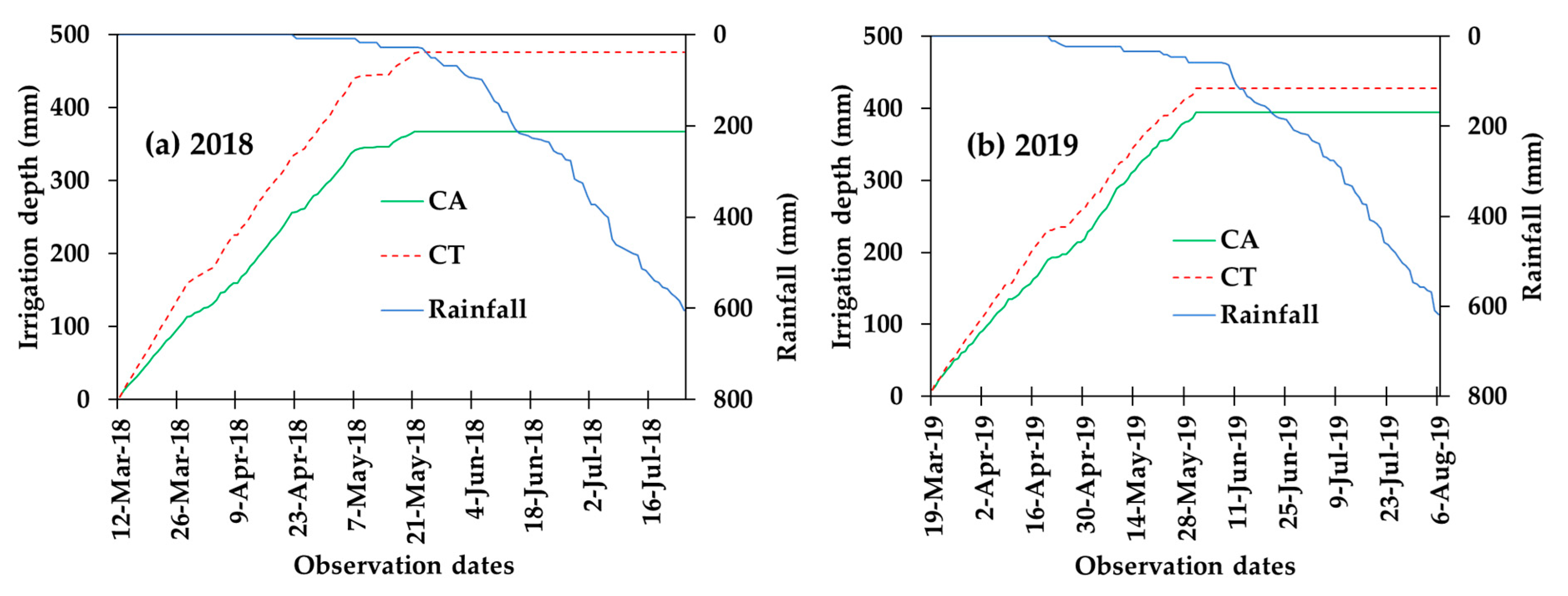
3.1. Irrigation and Rainfall Contributions in the Pepper Growing Period
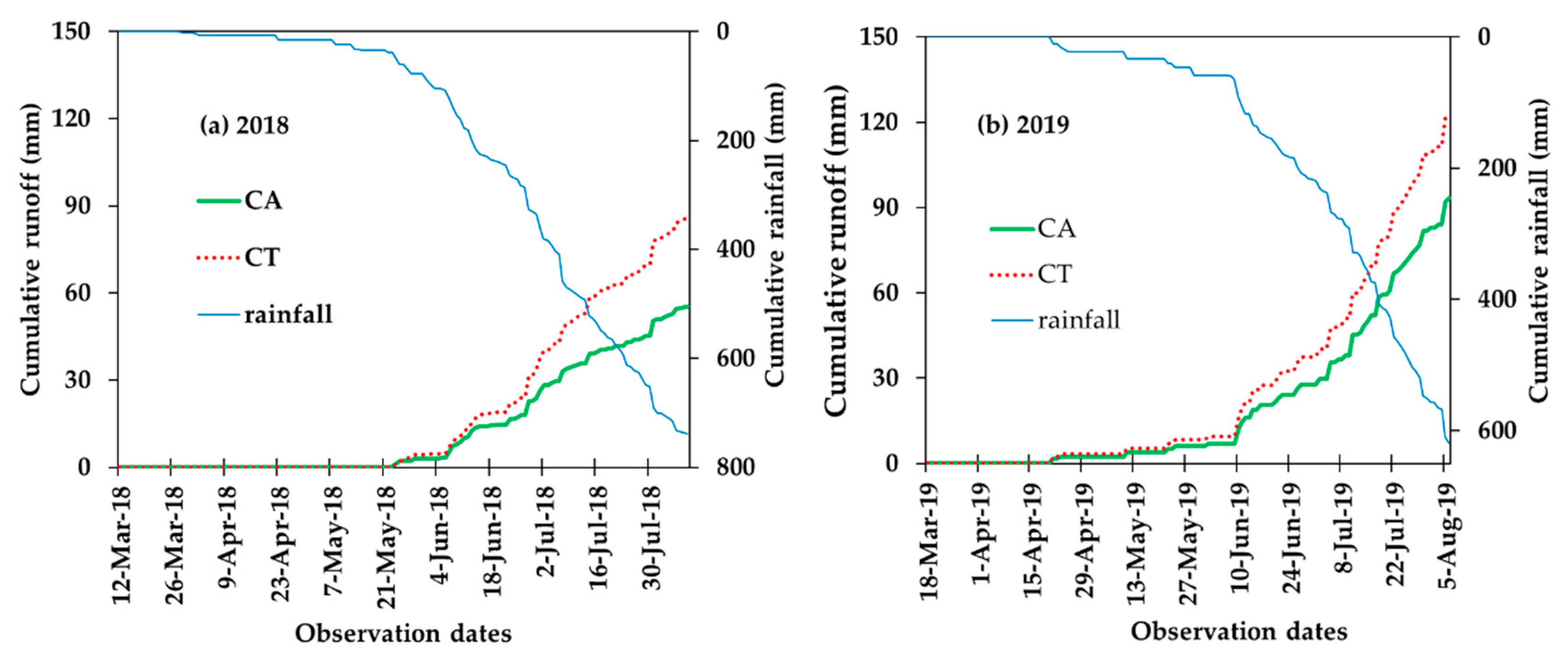
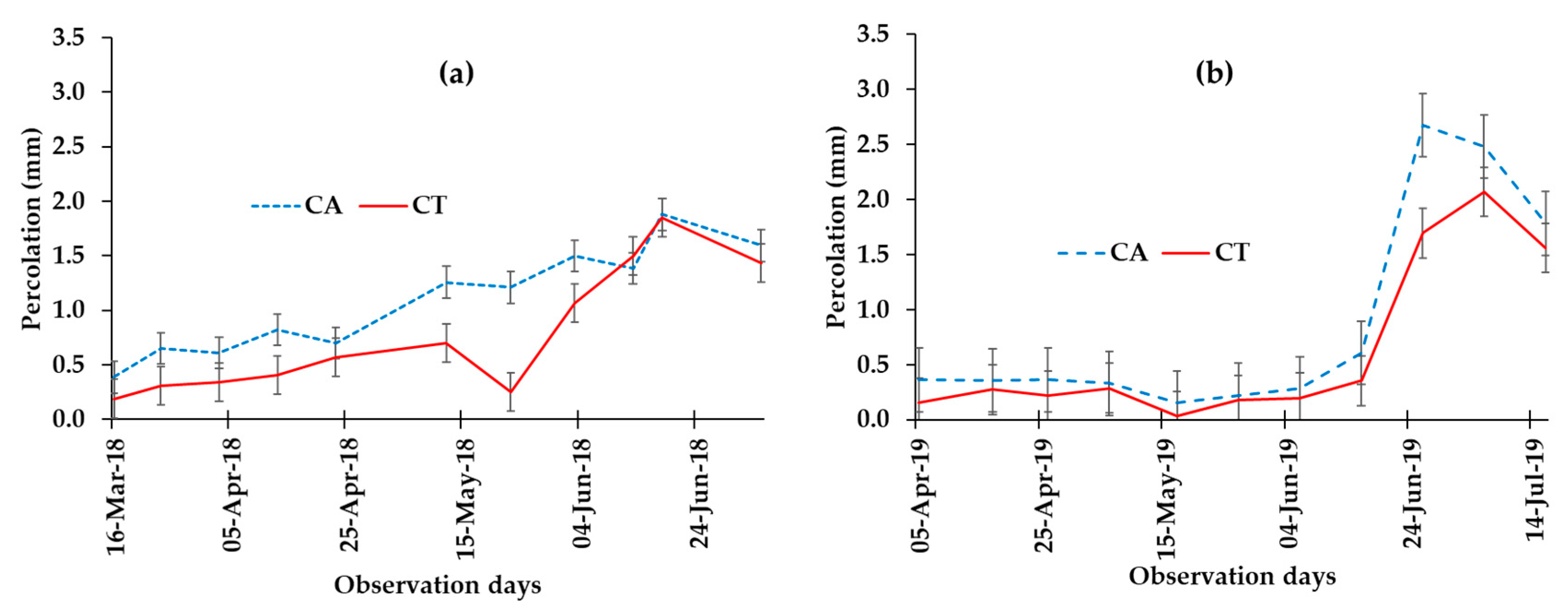
3.2. Effect of Conservation Agriculture on Water Dynamics (Runoff and Percolation/Leaching)
3.3. Consumptive Water Use of Pepper (ETc) under Supplementary Irrigation
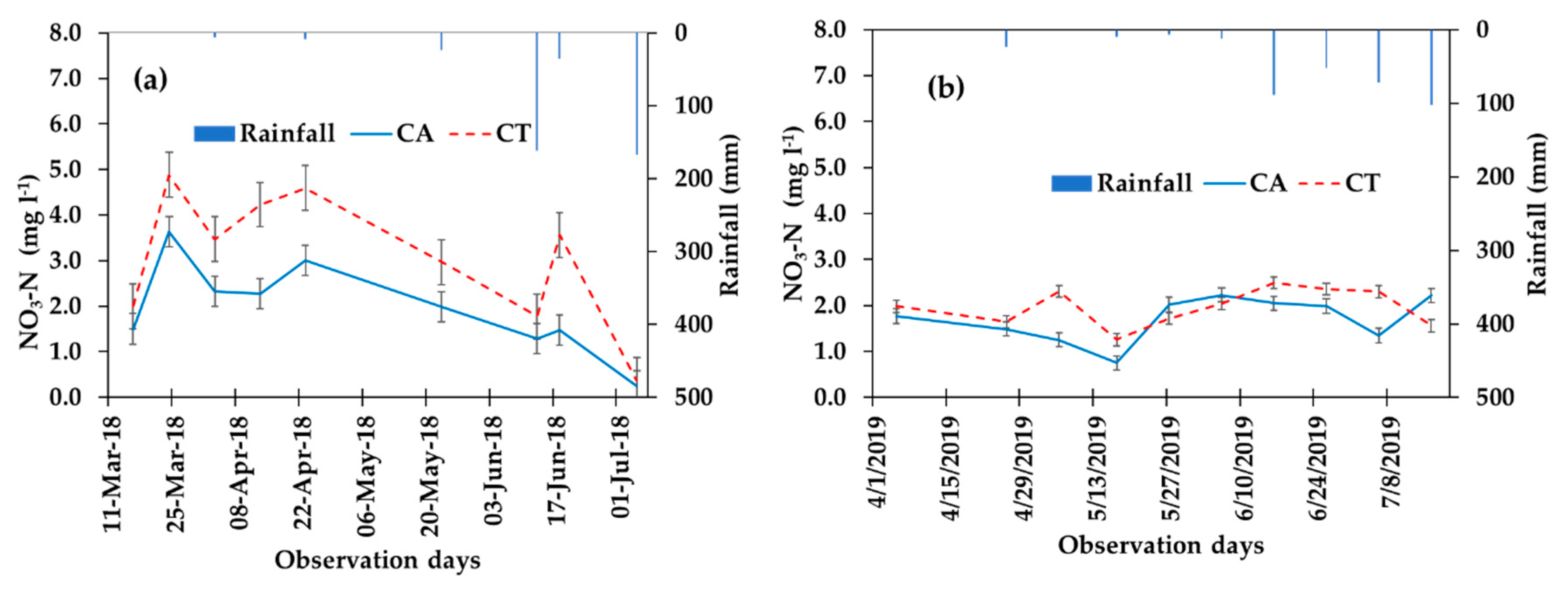
3.4. Nitrogen (NO3-N) Dynamics
3.5. Phosphorus (PO4-P) Dynamics
4. Discussion
4.1. Effects of CA on Agricultural Water Management
4.2. Effects of CA on the Nitrogen Movement
4.3. Effects of CA on Phosphorus Movement
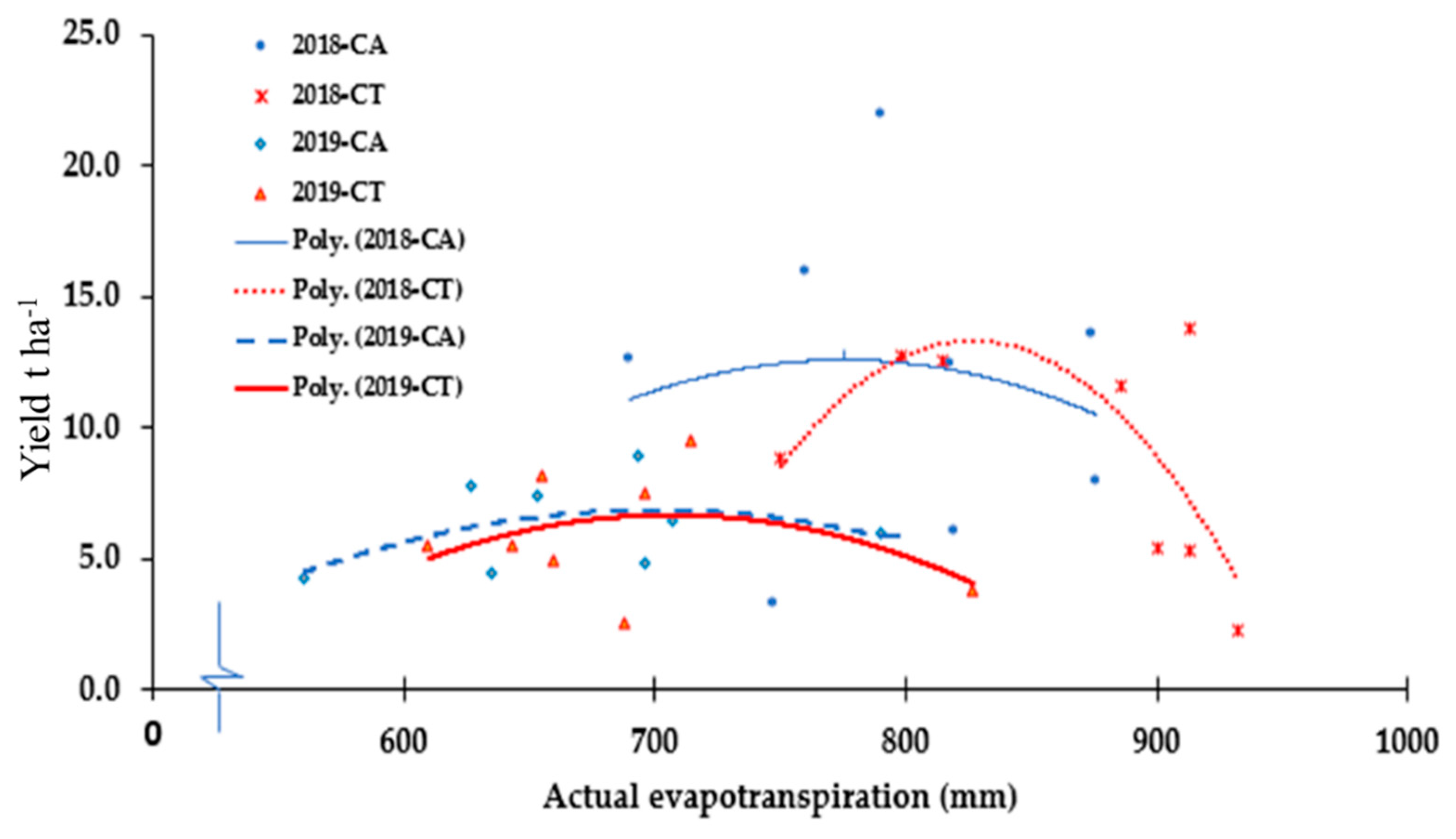
4.4. Effects of CA on Pepper Yield
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rockström, J.; Kaumbutho, P.; Mwalley, J.; Nzabi, A.; Temesgen, M.; Mawenya, L.; Barron, J.; Mutua, J.; Damgaard-Larsen, S. Conservation farming strategies in East and Southern Africa: Yields and rain water productivity from on-farm action research. Soil Tillage Res. 2009, 103, 23–32. [Google Scholar] [CrossRef]
- Bekele, S.; Tilahun, K. Regulated deficit irrigation scheduling of onion in a semiarid region of Ethiopia. Agric. Water Manag. 2007, 89, 148–152. [Google Scholar] [CrossRef]
- Rockström, J. On-farm green water estimates as a tool for increased food production in water scarce regions. Phys. Chem. Earth Part B Hydrol. Oceans Atmos. 1999, 24, 375–383. [Google Scholar] [CrossRef]
- Lanckriet, S.; Araya, T.; Cornelis, W.; Verfaillie, E.; Poesen, J.; Govaerts, B.; Bauer, H.; Deckers, J.; Haile, M.; Nyssen, J. Impact of conservation agriculture on catchment runoff and soil loss under changing climate conditions in May Zeg-zeg (Ethiopia). J. Hydrol. 2012, 475, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Bosch, D.D.; Potter, T.L.; Truman, C.C.; Bednarz, C.W.; Strickland, T.C. Surface runoff and lateral subsurface flow as a response to conservation tillage and soil-water conditions. Trans. ASAE 2005, 48, 2137–2144. [Google Scholar] [CrossRef]
- Tomer, M.; Moorman, T.; Kovar, J.; Cole, K.; Nichols, D. Eleven years of runoff and phosphorus losses from two fields with and without manure application, Iowa, USA. Agric. Water Manag. 2016, 168, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Grandy, A.S.; Loecke, T.D.; Parr, S.; Robertson, G.P. Long-term trends in nitrous oxide emissions, soil nitrogen, and crop yields of till and no-till cropping systems. J. Environ. Qual. 2006, 35, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Assefa, T.; Jha, M.; Reyes, M.; Worqlul, A. Modeling the impacts of conservation agriculture with a drip irrigation system on the hydrology and water management in Sub-Saharan Africa. Sustainability 2018, 10, 4763. [Google Scholar] [CrossRef] [Green Version]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Logan, T.J. Agricultural best management practices for water pollution control: Current issues. Agric. Ecosyst. Environ. 1993, 46, 223–231. [Google Scholar] [CrossRef]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Heathwaite, A.L.; Johnes, P.J. Contribution of nitrogen species and phosphorus fractions to stream water quality in agricultural catchments. Hydrol. Process. 1996, 10, 971–983. [Google Scholar] [CrossRef]
- Assefa, T.; Jha, M.; Reyes, M.; Srinivasan, R.; Worqlul, A.W. Assessment of suitable areas for home gardens for irrigation potential, water availability, and water-lifting technologies. Water 2018, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Assefa, T.T. Experimental and Modeling Evaluation of Conservation Agriculture with Drip Irrigation for Small-Scale Agriculture in Sub-Saharan Africa. North. Ph.D. Thesis, Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2018. [Google Scholar]
- Lal Bhardwaj, R. Effect of mulching on crop production under rainfed condition—A review. Agric. Rev. 2013, 34, 188–197. [Google Scholar] [CrossRef]
- Giller, K.E.; Corbeels, M.; Nyamangara, J.; Triomphe, B.; Affholder, F.; Scopel, E.; Tittonell, P. A research agenda to explore the role of conservation agriculture in African smallholder farming systems. Field Crops Res. 2011, 124, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Araya, T.; Cornelis, W.M.; Nyssen, J.; Govaerts, B.; Gebregziabher, T.; Oicha, T.; Getnet, F.; Raes, D.; Haile, M.; Sayre, K.D.; et al. Impact of Conservation Agriculture on Runoff, Soil Loss and Crop Yield on a Vertisol in the Northern Ethiopian Highlands. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Tesfaye, A.; Cornelis, W.M.; Nyssen, J.; Govaerts, B.; Bauer, H.; Gebregziabher, T.; Oicha, T.; Rates, D.; Sayre, K.D.; Haile, M.; et al. Effects of conservation agriculture on runoff, soil loss and crop yield under rainfed conditions in Tigray, Northern Ethiopia. Soil Use Manag. 2011, 27, 404–414. [Google Scholar]
- Romic, D.; Romic, M.; Borosic, J.; Poljak, M. Mulching decreases nitrate leaching in bell pepper (Capsicum annuum L.) cultivation. Agric. Water Manag. 2003, 60, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Thierfelder, C.; Wall, P.C. Effects of conservation agriculture techniques on infiltration and soil water content in Zambia and Zimbabwe. Soil Tillage Res. 2009, 105, 217–227. [Google Scholar] [CrossRef]
- Radford, B.J.; Thornton, C.M. Effects of 27 years of reduced tillage practices on soil properties and crop performance in the semi-arid subtropics of Australia. Int. J. Energy Environ. Econ. 2011, 19, 565. [Google Scholar]
- Blanco-Canqui, H.; Lal, R. Regional assessment of soil compaction and structural properties under no-tillage farming. Soil Sci. Soc. Am. J. 2007, 71, 1770–1778. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.N.; Dogra, P.; Sharma, N.K.; Bhattacharyya, R.; Mishra, P.K. Conservation agriculture impact for soil conservation in maize-wheat cropping system in the Indian sub-Himalayas. Int. Soil Water Conserv. Res. 2015, 3, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Assefa, T.; Jha, M.; Reyes, M.; Tilahun, S.; Worqlul, A.W. Experimental evaluation of conservation agriculture with drip irrigation for water productivity in Sub-Saharan Africa. Water 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- Belay, S.A.; Schmitter, P.; Worqlul, A.W.; Steenhuis, T.S.; Reyes, M.R.; Tilahun, S.A. Conservation agriculture saves irrigation water in the dry monsoon phase in the Ethiopian highlands. Water 2019, 11, 2103. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.; Pereira, L.; Raes, D.; Smith, M. Guidelines for Computing Crop Water Requirements. FAO Irrigation and Drainage Paper 56. Available online: http://www.fao.org/docrep (accessed on 16 March 2020).
- Kresović, B.; Tapanarova, A.; Tomić, Z.; Životić, L.; Vujović, D.; Sredojević, Z.; Gajić, B. Grain yield and water use efficiency of maize as influenced by different irrigation regimes through sprinkler irrigation under temperate climate. Agric. Water Manag. 2016, 169, 34–43. [Google Scholar] [CrossRef]
- Jarque, C.M.; Bera, A.K. Efficient tests for normality, homoscedasticity and serial independence of regression residuals. Econ. Lett. 1980, 6, 255–259. [Google Scholar] [CrossRef]
- Aliyu, L. Effect of organic and mineral fertilizers on growth, yield and composition of pepper (Capsicum annuum L.). Biol. Agric. Hortic. 2000, 18, 29–36. [Google Scholar] [CrossRef]
- Diaz, F.; Jimenez, C.C.; Tejedor, M. Influence of the thickness and grain size of tephra mulch on soil water evaporation. Agric. Water Manag. 2005, 74, 47–55. [Google Scholar] [CrossRef]
- Mohammad, A.G.; Adam, M.A. The impact of vegetative cover type on runoff and soil erosion under different land uses. Catena 2010, 81, 97–103. [Google Scholar] [CrossRef]
- Kabir, Z. Tillage or no-tillage: Impact on mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Stroosnijder, L. Modifying land management in order to improve efficiency of rainwater use in the African highlands. Soil Tillage Res. 2009, 103, 247–256. [Google Scholar] [CrossRef]
- Babalola, O.; Oshunsanya, S.; Are, K. Effects of vetiver grass (Vetiveria nigritana) strips, vetiver grass mulch and an organomineral fertilizer on soil, water and nutrient losses and maize (Zea mays L) yields. Soil Tillage Res. 2007, 96, 6–18. [Google Scholar] [CrossRef]
- Erenstein, O. Crop residue mulching in tropical and semi-tropical countries: An evaluation of residue availability and other technological implications. Soil Tillage Res. 2002, 67, 115–133. [Google Scholar] [CrossRef]
- Edwards, W.M.; Norton, L.D.; Redmond, C.E. Characterizing macropores that affect infiltration into non-tilled soil. Soil Sci. Soc. Am. J. 1988, 52, 483–487. [Google Scholar] [CrossRef]
- Drees, L.R.; Karathanasis, A.D.; Wilding, L.P.; Blevins, R.L. Micromorphological characteristics of long-term no-till and conventionally tilled soils. Soil Sci. Soc. Am. J. 1994, 58, 508–517. [Google Scholar] [CrossRef]
- Pagliai, M.; Raglione, M.; Panini, T.; Maletta, M.; La Marca, M. The structure of two alluvial soils in Italy after 10 years of conventional and minimum tillage. Soil Tillage Res. 1994, 34, 209–223. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, Z.; Wu, Y.; Mwiya, R. Response of vertical migration and leaching of nitrogen in percolation water of paddy fields under water-saving irrigation and straw return conditions. Water 2019, 11, 868. [Google Scholar] [CrossRef] [Green Version]
- Govaerts, B.; Sayre, K.D.; Goudeseune, B.; De Corte, P.; Lichter, K.; Dendooven, L.; Deckers, J. Conservation agriculture as a sustainable option for the central Mexican highlands. Soil Tillage Res. 2009, 103, 222–230. [Google Scholar] [CrossRef]
- Yadav, S.N. Formulation and estimation of nitrate-nitrogen leaching from corn cultivation. J. Environ. Qual. 1997, 26, 808–814. [Google Scholar] [CrossRef]
- Larsson, A. The Effect of Agricultural Intensification on Nitrate Concentrations in Shallow Groundwater in Two Watersheds in Ethiopia. Master’s Thesis, Uppsala Universitet, Uppsala, Sweden, June 2019. [Google Scholar]
- Ben-Gal, A.; Dudley, L.M. Phosphorus availability under continuous point source irrigation. Soil Sci. Soc. Am. J. 2003, 67, 1449–1456. [Google Scholar] [CrossRef]
- Bolliger, A.; Magid, J.; Amado, J.C.T.; Neto, F.S.; dos Santos Ribeiro, M.D.F.; Calegari, A.; Ralisch, R.; de Neergaard, A. Taking stock of the Brazilian “zero-till revolution”: A review of landmark research and farmers’ practice. Adv. Agron. 2006, 91, 47–110. [Google Scholar]
- Tiecher, T.; dos Santos, D.R.; Calegari, A. Soil organic phosphorus forms under different soil management systems and winter crops, in a long-term experiment. Soil Tillage Res. 2012, 124, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Redel, Y.D.; Rubio, R.; Rouanet, J.L.; Borie, F. Phosphorus bioavailability affected by tillage and crop rotation on a Chilean volcanic derived Ultisol. Geoderma 2007, 139, 388–396. [Google Scholar] [CrossRef]
- dos Santos Rheinheimer, D.; Anghinoni, I. Accumulation of soil organic phosphorus by soil tillage and cropping systems under subtropical conditions. Commun. Soil Sci. Plant Anal. 2003, 34, 2339–2354. [Google Scholar] [CrossRef]
- Ravinderkumar, S. Influence of different mulches on flowering an fruit setting of winter tomato. Crop Res. 1998, 12, 174–176. [Google Scholar]
- Jaimez, R.; Vielma, O.; Rada, F.; García-Núñez, C. Effects of water deficit on the dynamics of flowering and fruit production in Capsicum chinense Jacq in a tropical semiarid region of Venezuela. J. Agron. Crop Sci. 2000, 185, 113–119. [Google Scholar] [CrossRef]
- Wale, A.; Girmay, G. Determination of irrigation regime for hot pepper (Capsicum annum L.) in dry-land areas of Wag-Himra, North Eastern Amhara, Ethiopia. Arch. Agric. Environ. Sci. 2019, 4, 101–108. [Google Scholar] [CrossRef]

| Year | Crop | Management Activities | Date | Methods and Tools |
|---|---|---|---|---|
| 2018 | Local variety pepper (Capsicum annuum L.) | Seedling | 20 January 2018 | Watering-can |
| Cow dung application | 5 February 2018 | Manual | ||
| Tillage | 10–20 February 2018 | Draught animal | ||
| Planting | 13 March 2018 | Manual | ||
| Mulch application | 12 March 2018 | Manual | ||
| Irrigation | 13 March 2018–12 May 2018 | Drip irrigation | ||
| Weeding/hoeing | 20 April 2018, 5 May 2018, 10 July 2018 | Handpick | ||
| Harvesting | 1 June 2018–25 August2018 | Handpick | ||
| 2019 | Local variety pepper (Capsicum annuum L.) | Seedling | 9 January 2018 | Watering-can |
| Cow dung application | 5 February 2018 | Manual | ||
| Tillage | 15–25 February 2019 | Draught animal | ||
| Planting | 19 March 2019 | Manual | ||
| Mulch application | 12 March 2019 | Manual | ||
| Irrigation | 19 March 2019–18 May 2019 | Drip irrigation | ||
| Weeding/hoeing | 25 April 2018, 15 May 2018, 15 July 2019 | Handpick | ||
| Harvesting | 10 June 2019–29 August 2019 | Handpick |
| Variables | 2018 | 2019 | ||
|---|---|---|---|---|
| CA | CT | CA | CT | |
| Applied irrigation (mm) | 367.3 ± 55.4 b* | 475.4 ± 68 a | 254.8 ± 42.9 b | 287.8 ± 57.7 a |
| Rainfall (mm) ** | 594.0 | 594.0 | 618.0 | 618.0 |
| Runoff (mm) | 53.2 ± 8.0 b | 80.1 ± 22.3 a | 95.5 ± 18.4 b | 123.6 ± 19.2 a |
| Percolation / leaching (mm) | 7.5 ± 2.4 a | 5.9 ± 1.9 b | 6.9 ± 1.6 a | 4.6 ± 2.4 b |
| Crop water used (ETc) (mm) | 796.7 ± 65.3 b | 855.6 ± 83.1 a | 678.7 ± 55.0 a | 686.6 ± 69.1 a |
| Fresh yield (t ha−1) | 11.7 ± 5.9 | 9.1 ± 4.3 | 6.2 ± 1.7 | 5.9 ± 2.3 |
| Contribution of irrigation | 46% | 56% | 37% | 42% |
| Variables | 2018 | 2019 | ||
|---|---|---|---|---|
| CA | CT | CA | CT | |
| NO3-N (leachate), mg L−1 | 2.8 ± 0.9 b | 3.2 ± 1.3 a | 1.8 ± 0.7 b | 2.6 ± 1.2 a |
| NO3-N (leachate), g ha−1 | 20.1 ± 7.8 b | 21.6 ± 9.1 a | 15.1 ± 12.8 b | 16.6 ± 16.2 a |
| NO3-N (runoff), mg L−1 | 0.3 ± 0.1 b | 0.6 ± 0.15 a | 0.4 ± 0.1 b | 0.8 ± 0.3 a |
| NO3-N (runoff), g ha−1 | 148.8 ± 66.2 b | 384.0 ± 75 a | 333.7 ± 122 b | 866 ± 359 a |
| Variables | 2018 | 2019 | ||
|---|---|---|---|---|
| CA | CT | CA | CT | |
| PO4-P (leachate), mg L−1 | 1.2 ± 0.7 a | 0.80 ± 0.4 b | 0.80 ± 0.5 a | 0.6 ± 0.3 b |
| PO4-P (leachate), g ha−1 | 8.4 ± 4.0 a | 5.6 ± 2.6 b | 15.1 ± 4.2 a | 16.6 ± 2.6 b |
| PO4-P (runoff), mg L−1 | 0.55 ± 0.1 a | 0.64 ± 0.15 b | 0.6 ± 0.2 a | 0.7 ± 0.3 b |
| PO4-P (runoff), g ha−1 | 243 ± 66.2 a | 389 ± 75 b | 500.8 ± 215 a | 702.6 ± 312 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belay, S.A.; Assefa, T.T.; Prasad, P.V.V.; Schmitter, P.; Worqlul, A.W.; Steenhuis, T.S.; Reyes, M.R.; Tilahun, S.A. The Response of Water and Nutrient Dynamics and of Crop Yield to Conservation Agriculture in the Ethiopian Highlands. Sustainability 2020, 12, 5989. https://doi.org/10.3390/su12155989
Belay SA, Assefa TT, Prasad PVV, Schmitter P, Worqlul AW, Steenhuis TS, Reyes MR, Tilahun SA. The Response of Water and Nutrient Dynamics and of Crop Yield to Conservation Agriculture in the Ethiopian Highlands. Sustainability. 2020; 12(15):5989. https://doi.org/10.3390/su12155989
Chicago/Turabian StyleBelay, Sisay A., Tewodros T. Assefa, P. V. Vara Prasad, Petra Schmitter, Abeyou W. Worqlul, Tammo S. Steenhuis, Manuel R. Reyes, and Seifu A. Tilahun. 2020. "The Response of Water and Nutrient Dynamics and of Crop Yield to Conservation Agriculture in the Ethiopian Highlands" Sustainability 12, no. 15: 5989. https://doi.org/10.3390/su12155989
APA StyleBelay, S. A., Assefa, T. T., Prasad, P. V. V., Schmitter, P., Worqlul, A. W., Steenhuis, T. S., Reyes, M. R., & Tilahun, S. A. (2020). The Response of Water and Nutrient Dynamics and of Crop Yield to Conservation Agriculture in the Ethiopian Highlands. Sustainability, 12(15), 5989. https://doi.org/10.3390/su12155989







