Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review
Abstract
:1. Introduction
2. Bioplastics
2.1. Classification of Bioplastics
- Chemical synthesis of finite fossil resources (e.g., petroleum);
- Chemical synthesis of biotechnologically manufactured polymer feedstocks;
- Direct biosynthesis of biogenic raw materials including organic waste;
- Production of blends and co-/terpolymers from the previously mentioned groups.
2.2. Bioplastics’ Utilization and Economical Aspects
2.3. Properties of Bioplastics
2.4. Degradation of Bioplastics in the Environment
2.5. Indices of Biodegradability
2.5.1. Anaerobic Biodegradation Indices
- is the total CO2 and CH4 volume from the sample vessel under standard conditions in L;
- is the total CO2 and CH4 volume from the blank vessel under standard conditions in L;
- is the CO2 volume dissolved in the sludge in excess of the blank value under standard conditions in L;
- is the maximum theoretical volume of the biogas (CO2 + CH4) evolved after complete biodegradation of the test material under standard conditions in L.
2.5.2. Aerobic Biodegradation Indices
2.6. Bioplastic-Degrading Microorganisms
3. Waste Management Options for Bioplastics
3.1. Mechanical and Chemical Recycling
- Removal of contaminants, such as food waste [36];
- Grinding/shredding/crushing or milling, to obtain a material as much homogeneous as possible.
- Further processing, such as extrusion, injection molding, or drawing.
3.2. Biological Treatment
3.3. Incineration
3.4. Landfilling
4. Biodegradation under Different Environments
4.1. Aerobic Biodegradation of Bioplastics
4.1.1. Biodegradation of Bioplastics in Soil
4.1.2. Biodegradation of Bioplastics in Compost
4.2. Anaerobic Biodegradation of Bioplastics
4.3. Biodegradation of Bioplastics in Aquatic Environments
4.4. Comparison of Different Degradation Environments
5. Standardization and Certification of Bioplastics
- Industrial composting, such as EN 13432, EN 14995, ISO 18606 and ISO 17088;
- Home composting, such as the Australian Norm AS 5810 and the French Norm NF T 51-800;
- Biodegradability in anaerobic environment, such as ISO 11734, ISO 14853, ISO 15985, ASTM D5210-92, ASTM D5511-02, ASTM D5526-94D;
- Biodegradability in soils, such as EN 17033 and ASTM D5988-03;
- Biodegradability in marine environments ASTM D708, ASTM D6691, ASTM D6692, ASTM D7473, OECD 306, and ISO 16221.
6. Concluding Remarks
- Biodegradation under aerobic conditions such as composting, soil, and some aquatic habitats was more rapid compared to that under anaerobic environments, such as anaerobic digestion plants, landfills, and a few aquatic habitats.
- Eighty three percent (83%) of the experiments cited here were performed in aerobic conditions and over 40% of them exhibited a biodegradation extent of over 60% but different times were required. Specifically, from 14 days to 1 year in soil environments, from 28 to 110 days during aerobic composting, and from 14 to 270 days in aquatic environments.
- Only 17% of the published experiments have taken place under anaerobic conditions and the extent of biodegradation ranged from 2 to 91%; about 50% of the experiments showed a biodegradability over 70% in a period from 9 to 75 days; therefore, there is a need to increase the research on the biodegradability of bioplastics in anaerobic conditions.
- The different extent of biodegradation can be attributed to a variety of factors depending on both the nature of the bio-polymers (such as their chemical and physical structure), as well as the environmental conditions to which they are exposed to (such as temperature, moisture, pH, sunlight, nutrient and oxygen availability, and the involved microbial communities).
- Compost was the most investigated environment (accounting for 39% of the aerobically performed experiments) and that in which almost all biodegradable bioplastics were able to biodegrade.
- The most suitable environment for biodegradation to occur is compost, followed by soil, fresh and marine water, and landfill.
- Almost all the experiments in all environments were conducted at a laboratory scale, thus highlighting the necessity to investigate the biodegradation of bioplastics at real scale, though some of them were carried out in natural environments (for instance, by burying the samples in field soils).
- The majority of the experiments aimed at the optimization of the process by changing the environmental conditions (such as moisture content, temperature, or pH) rather than by modifying the physico-chemical structure of the biopolymer (for instance, by pretreatment prior to the biodegradation process); on the other hand, some researchers have tried to enhance the biodegradability of the bioplastics by producing raw materials easier to degrade.
- Only a few biodegradable polymers decompose under all environmental conditions, such as PHA that biodegrades completely in 49 days under composting conditions; on the other hand, its biodegradation in aquatic environments is slower but still complete.
- Cellulose-based bioplastics completely degraded in less than five months in soil and compost conditions, but their degradation has not been investigated under anaerobic and aquatic environments.
- PHA-based bioplastics were degraded (biodegradability over 80%) in compost and anaerobic conditions after less than four months and two weeks, respectively; on the contrary, they observed less than 10% of biodegradability over a period of one year in aquatic environments, while biodegradation was in general below 50% after one year in soil environment.
- PLA-based bioplastics showed a similar biodegradability of PHAs, as quickly degraded under composting and anaerobic digestion while they were less degraded under the other environments.
- Starch-based bioplastics were degraded in soil and composting, while anaerobic digestion and aquatic environments were not suitable for their degradation.
- Petroleum-based bioplastics (such as PBS and PCL) were degraded by composting (90% in three months) but anaerobic, soil, and aquatic environments are not suitable for their degradation.
- The actual standards assessing the compost quality do not consider particle size smaller than 2 mm, like micro-plastics, thus the introduction of new criteria in ecotoxicity and disintegration tests are highly suggested.
- Anaerobic biodegradation standards for biodegradable bioplastics are lacking and should be developed.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3HB | 3-hydroxybutyrate |
| 3HHx | 3-hydroxyhexanoate |
| 3HV | 3-hydroxyvalerate |
| AD | Anaerobic Digestion |
| ASTM | American Society for Testing and Materials |
| Bio-PE | Bio-Polyethylene |
| Bio-PET | Bio-Polyethylene Terephthalate |
| Bio-PU | Bio-Polyurethane |
| Bio-PVC | Bio-Polyvinyl Chloride |
| BOD | Biological Oxygen Demand |
| CA | Cellulose Acetate |
| CEN | European Committee for Standardization |
| EOL | End of Life |
| EFB | Empty fruit bunch |
| EPA | Environmental Protection Agency |
| GHG | Greenhouse Gases |
| HRT | Hydraulic Retention Time |
| ISO | International Standards Organization |
| LCA | Life Cycle Assessment (or Analysis) |
| NPK fertilizer | Nitrogen-Phosphorus-Potassium fertilizer |
| OECD | Organization for Economic Cooperation and Development |
| P(3HB-co-3HV-co-3HHx) | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) |
| PA | Polyamide (or nylon) |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| PBS | Polybutylene Succinate |
| PBSA | Poly(butylene succinate-co-adipate) |
| PCL | Polycaprolactone |
| PGA | Polyglycolide |
| PHA | Polyhydroxyalkanoates |
| PHB | Polyhydroxybutyrate |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic) Acid |
| PVA | Polyvinyl Alcohol |
| PU | Polyurethane |
| SSF | Solid-State Fermentation |
| ThOD | Theoretical Oxygen Demand |
| TPS | Thermoplastic Starch |
| VFA | Volatile Fatty Acid |
References
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and biodegradation rate of poly(caprolactone)-starch blend and poly(butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Pilla, S. (Ed.) Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118203699. [Google Scholar]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Degli Innocenti, F.; Briones, A.; Innocenti, F.D. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javierre, C.; Sarasa, J.; Claveria, I.; Fernandez, A. Study of the Biodisintegration on a Painted Bioplastic Material Waste. Mater. Plast. 2015, 52, 116–121. [Google Scholar]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Koch, D.; Mihalyi, B. Assessing the Change in Environmental Impact Categories when Replacing Conventional Plastic with Bioplastic in Chosen Application Fields. Chem. Eng. Trans. 2018, 70, 853–858. [Google Scholar]
- European Bioplastics Report-Bioplastics market data 2019—Global production capacities of bioplastics 2019–2024. Available online: https://www.european-bioplastics.org/market/ (accessed on 21 May 2020).
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Tachibana, K.; Urano, Y.; Numata, K. Biodegradability of nylon 4 film in a marine environment. Polym. Degrad. Stab. 2013, 98, 1847–1851. [Google Scholar] [CrossRef]
- Harmaen, A.S.; Khalina, A.; Azowa, I.; Hassan, M.A.; Tarmian, A.; Jawaid, M. Thermal and biodegradation properties of poly(lactic acid)/fertilizer/oil palm fibers blends biocomposites. Polym. Compos. 2015, 36, 576–583. [Google Scholar] [CrossRef]
- Zamakhaeva, S.A.; Fedorov, D.N.; Trotsenko, Y.A. Methylotrophic Producers of Bioplastics (Review). Appl. Biochem. Microbiol. 2017, 53, 389–400. [Google Scholar] [CrossRef]
- Jafari-Sales, A. Bioplastics and the Environment. Electron. J. Biol. 2017, 13, 274–279. [Google Scholar]
- Sarasa, J.; Gracia, J.M.; Javierre, C. Study of the biodisintegration of a bioplastic material waste. Bioresour. Technol. 2009, 100, 3764–3768. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Grosso, M. Bioplastics and waste management. Waste Manag. 2018, 78, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Tachibana, Y.; Oba, K.; Takizawa, R.; Kasuya, K. ichi Microbial degradation of poly(ε-caprolactone) in a coastal environment. Polym. Degrad. Stab. 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Accinelli, C.; Saccà, M.L.; Mencarelli, M.; Vicari, A. Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 2012, 89, 136–143. [Google Scholar] [CrossRef]
- Wahyuningtyas, N.; Suryanto, H. Analysis of Biodegradation of Bioplastics Made of Cassava Starch. J. Mech. Eng. Sci. Technol. 2017, 1, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Urbanek, A.K.; Rymowicz, W.; Strzelecki, M.C.; Kociuba, W.; Franczak, Ł.; Mirończuk, A.M. Isolation and characterization of Arctic microorganisms decomposing bioplastics. AMB Express 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers. A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Mohee, R.; Unmar, G.D.; Mudhoo, A.; Khadoo, P. Biodegradability of biodegradable/degradable plastic materials under aerobic and anaerobic conditions. Waste Manag. 2008, 28, 1624–1629. [Google Scholar] [CrossRef]
- Prosperi, M.; Sisto, R.; Lombardi, M.; Zhu, X. Production of bioplastics for agricultural purposes: A supply chain study. Riv. di Studi Sulla Sostenibilita 2018, 119–136. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- Endres, H.J. Bioplastics. In Biorefineries. Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 166, pp. 427–468. ISBN 978-3-319-97119-3. [Google Scholar]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Ullah, A.; Vasanthan, T.; Bressler, D.; Elias, A.L.; Wu, J. Bioplastics from Feather Quill. Biomacromolecules 2011, 12, 3826–3832. [Google Scholar] [CrossRef]
- Wu, C.-S. Preparation, characterization, and biodegradability of renewable resource-based composites from recycled polylactide bioplastic and sisal fibers. J. Appl. Polym. Sci. 2012, 123, 347–355. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Henschen, C. Biplastiche (biosynthetische) Korrekturoperationen von Zehendeformitäten. Schweiz. Med. Wochenschr. 1952, 82, 168–173. [Google Scholar] [PubMed]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(butylene succinate) (PBS) Bioplastic and Biofuel Co-products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Kawashima, N.; Yagi, T.; Kojima, K. How Do Bioplastics and Fossil-Based Plastics Play in a Circular Economy? Macromol. Mater. Eng. 2019, 304, 1–14. [Google Scholar] [CrossRef]
- Penkhrue, W.; Khanongnuch, C.; Masaki, K.; Pathom-aree, W.; Punyodom, W.; Lumyong, S. Isolation and screening of biopolymer-degrading microorganisms from northern Thailand. World J. Microbiol. Biotechnol. 2015, 31, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.A.; Billington, S.L.; Criddle, C.S. Assessment of models for anaerobic biodegradation of a model bioplastic: Poly(hydroxybutyrate-co-hydroxyvalerate). Bioresour. Technol. 2017, 227, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Mihai, M.; Legros, N.; Alemdar, A. Formulation-properties versatility of wood fiber biocomposites based on polylactide and polylactide/thermoplastic starch blends. Polym. Eng. Sci. 2014, 54, 1325–1340. [Google Scholar] [CrossRef]
- Rahman, A.; Syamsu, K. Biodegradability of oil palm cellulose-based bioplastics. IOP Publ. IOP Conf. Ser. Earth Environ. Sci. 2018, 183, 12012. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Bastos Lima, M.G. Toward multipurpose agriculture: Food, fuels, flex crops, and prospects for a bioeconomy. Glob. Environ. Polit. 2018, 18, 143–150. [Google Scholar] [CrossRef]
- Morone, P. The times they are a-changing: Making the transition toward a sustainable economy. Biofuels Bioprod. Biorefining 2016, 10, 369–377. [Google Scholar] [CrossRef]
- Kartika, T.; Harahap, M.B.; S Ginting, M.H. Utilization of mango seed starch in manufacture of bioplastic reinforced with microparticle clay using glycerol as plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012068. [Google Scholar]
- Shellikeri, A.; Kaulgud, V.; Yaradoddi, J.; Ganachari, S.; Banapurmath, N.; Shettar, A. Development of Neem Based Bioplastic for Food Packaging Application. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012052. [Google Scholar] [CrossRef] [Green Version]
- Adamcová, D.; Elbl, J.; Zloch, J.; Vaverková, M.D.; Kintl, A.; Juřička, D.; Hladký, J.; Brtnický, M. Study in the (bio)degradation process of bioplastic materials under industrial composting conditions. Acta Univ. Agric. Silvic. Mendelianae Brun. 2017, 65, 791–798. [Google Scholar]
- European Bioplastics Bioplastics–European Bioplastics e.V. Available online: https://www.european-bioplastics.org/bioplastics/ (accessed on 20 January 2020).
- Ashok, A.; Rejeesh, C.R. Investigations in to Biodegradability and Physical Properties of Starch Derived Bioplastic Films Reinforced with Nanosilica. Int. J. Nanosci. 2019, 18, 1850037. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers (Basel) 2016, 8, 262. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Beucker, S.; Marscheider-Weidemann, F. Potentials and Challenges of Bioplastics-Insights from a German Survey on “Green” Future Markets. In Proceedings of the R’07 World Congress; Hilty, L.M., Edelmann, X., Ruf, A., Eds.; EMPA: St. Gallen, Davos, Switzerland, 2007. [Google Scholar]
- Plastics Industry Association Plastics Market Watch|Plastics Industry Association. Available online: https://www.plasticsindustry.org/data/plastics-market-watch (accessed on 2 February 2020).
- Hong, J.W.; Song, H.S.; Moon, Y.M.; Hong, Y.G.; Bhatia, S.K.; Jung, H.R.; Choi, T.R.; Yang, S.Y.; Park, H.Y.; Choi, Y.K.; et al. Polyhydroxybutyrate production in halophilic marine bacteria Vibrio proteolyticus isolated from the Korean peninsula. Bioprocess Biosyst. Eng. 2019, 42, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Shim, Y.H.; Jeon, J.M.; Brigham, C.J.; Kim, Y.H.; Kim, H.J.; Seo, H.M.; Lee, J.H.; Kim, J.H.; Yi, D.H.; et al. Starch based polyhydroxybutyrate production in engineered escherichia coli. Bioprocess Biosyst. Eng. 2015, 38, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Jeon, J.M.; Kim, J.S.; Lee, Y.K.; Yang, Y.H. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. Int. J. Biol. Macromol. 2019, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.R.; Jeon, J.M.; Yi, D.H.; Song, H.S.; Yang, S.Y.; Choi, T.R.; Bhatia, S.K.; Yoon, J.J.; Kim, Y.G.; Brigham, C.J.; et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolymer production from volatile fatty acids using engineered Ralstonia eutropha. Int. J. Biol. Macromol. 2019, 138, 370–378. [Google Scholar] [CrossRef]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.M.; Tayeb, A.M. Production of biodegradable plastic from agricultural wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Biopolymer production and end of life comparisons using life cycle assessment. Resour. Conserv. Recycl. 2017, 122, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Elvers, D.; Song, C.H.; Steinbüchel, A.; Leker, J. Technology Trends in Biodegradable Polymers: Evidence from Patent Analysis. Polym. Rev. 2016, 56, 584–606. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Suárez-Ojeda, M.E. Bioplastic recovery from wastewater: A new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour. Technol. 2019, 282, 361–369. [Google Scholar] [CrossRef]
- Nwinyi, O.C.; Owolabi, T.A. Scanning electron microscopy and Fourier transmission analysis of polyhydroxyalkanoates isolated from bacteria species from abattoir in Ota, Nigeria. J. King Saud Univ. -Sci. 2019, 31, 285–298. [Google Scholar] [CrossRef]
- Ciesielski, S.; Mozejko-Ciesielska, J.; Ciesielski, S. Molecular Identification of Polyhydroxyalkanoates-Producing Bacteria Isolated from Enriched Microbial Community. Pol. J. Microbiol. 2013, 62, 45–50. [Google Scholar] [CrossRef]
- Akinmulewo, A.B.; Nwinyi, O.C. Polyhydroxyalkanoate: A biodegradable polymer (a mini review). J. Phys. Conf. Ser. 2019, 1378, 042007. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, J.H.; Kim, M.S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.J.; Jeon, J.M.; Kim, S.H.; Ahn, J.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef]
- Rivero, C.P.; Hu, Y.; Kwan, T.H.; Webb, C.; Theodoropoulos, C.; Daoud, W.; Lin, C.S.K. Bioplastics From Solid Waste. In Current Developments in Biotechnology and Bioengineering: Solid Waste Management; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–26. ISBN 9780444636751. [Google Scholar]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative Production of Bioproducts From Organic Waste Through Solid-State Fermentation. Front. Sustain. Food Syst. 2019, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vaverková, M.D.; Adamcová, D. Biodegrability of bioplastic materials in a controlled composting environment. J. Ecol. Eng. 2015, 16, 155–160. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Rincones, J.; Zeidler, A.F.; Grassi, M.C.B.; Carazzolle, M.F.; Pereira, G.A.G. The Golden Bridge for Nature: The New Biology Applied to Bioplastics. Polym. Rev. 2009, 49, 85–106. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Shier, W.T.; Vicari, A.; Little, N.S.; Aloise, M.R.; Giacomini, S. Degradation of microplastic seed film-coating fragments in soil. Chemosphere 2019, 226, 645–650. [Google Scholar] [CrossRef]
- Calabro’, P.S.; Folino, A.; Fazzino, F.; Komilis, D. Preliminary evaluation of the anaerobic biodegradability of three biobased materials used for the production of disposable plastics. J. Hazard. Mater. 2020, 390, 121653. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Springs, B.; O’Connor, R.P.; Kolstad, J.; Gruber, P.R. The Sustainability of NatureWorks™ Polylactide Polymers and Ingeo™ Polylactide Fibers: An Update of the Future. Macromol. Biosci. 2004, 4, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Sandeep, K.; Singh, M.; Singh, G.P.; Lee, J.K.; Bhatia, S.K.; Kalia, V.C. Biotechnological application of polyhydroxyalkanoates and their composites as anti-microbials agents. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 207–225. ISBN 9789811337598. [Google Scholar]
- Bhatia, S.K.; Wadhwa, P.; Bhatia, R.K.; Patel, S.K.S.; Yang, Y.H. Strategy for biosynthesis of polyhydroxyalkonates polymers/copolymers and their application in drug delivery. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 13–34. ISBN 9789811337598. [Google Scholar]
- Bhatia, S.K.; Wadhwa, P.; Hong, J.W.; Hong, Y.G.; Jeon, J.M.; Lee, E.S.; Yang, Y.H. Lipase mediated functionalization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 2019, 123, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Åkesson, D.; Taherzadeh, M.J.; Ferreira, J.A. Recycling strategies for polyhydroxyalkanoate-based waste materials: An overview. Bioresour. Technol. 2020, 298, 1–9. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Pagliaro, M. Biodegradable and Compostable Plastics: A Critical Perspective on the Dawn of their Global Adoption. ChemistryOpen 2020, 9, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Saiful; Helwati, H.; Saleha, S.; Iqbalsyah, T.M. Development of bioplastic from wheat Janeng starch for food packaging. In Proceedings of the The 8th Annual International Conference (AIC) 2018 on Science and Engineering, Aceh, Indonesia, 12–14 September 2018. [Google Scholar]
- Saxena, A.; Tiwari, A. Polyhydroxyalkonates: Green plastics of the future. Int. J. Biomed. Adv. Res. 2011, 2, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energy Sources, Part A Recover. Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Leong, Y.K.; Show, P.L.; Lan, J.C.W.; Loh, H.S.; Lam, H.L.; Ling, T.C. Economic and environmental analysis of PHAs production process. Clean Technol. Environ. Policy 2017, 19, 1941–1953. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Heal. Sci. Eng. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.-D.; Eberiel, D.T.; Mccarthy, S.P.; Gross, R.A. Cellulose Acetate Biodegradability upon Exposure to Simulated Aerobic Composting and Anaerobic Bioreactor Environments 1. J. Environ. Polym. Degrad. 1993, 1, 143–153. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; McDonald, A.G. Accelerated weathering studies on the bioplastic, poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Degrad. Stab. 2016, 126, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Ochi, S. Development of high strength biodegradable composites using Manila hemp fiber and starch-based biodegradable resin. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1879–1883. [Google Scholar] [CrossRef]
- Stevens, E.S.; Klamczynski, A.; Glenn, G.M. Starch-lignin foams. Express Polym. Lett. 2010, 4, 311–320. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers (Basel) 2019, 11, 751. [Google Scholar] [CrossRef] [Green Version]
- Ching, K.Y.; Chee, C.Y.; Afzan, M.; Kang, L.Z.; Eng, C.K. Mechanical and thermal properties of Chemical treated oil palm empty fruit bunches fiber reinforced polyvinyl alcohol composite. J. Biobased Mater. Bioenergy 2015, 9, 231–235. [Google Scholar] [CrossRef]
- Ali, M.E.; Yong, C.K.; Ching, Y.C.; Chuah, C.H.; Liou, N.S. Effect of single and double stage chemically treated kenaf fibers on mechanical properties of polyvinyl alcohol film. BioResources 2015, 10, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.K.; Ching, Y.C.; Chuah, C.H.; Liou, N.S. Effect of fiber orientation on mechanical properties of kenaf-reinforced polymer composite. BioResources 2015, 10, 2597–2608. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, A.; Roslan, A.; Sanusi, S.N.A.; Shahimi, M.Q.; Nazari, N.Z. Mechanical properties of bioplastic form cellulose nanocrystal (CNC) mangosteen peel using glycerol as plasticizer. J. Phys. Conf. Ser. 2019, 1349, 012099. [Google Scholar] [CrossRef]
- Jangong, O.S.; Gareso, P.L.; Mutmainna, I.; Tahir, D. Fabrication and characterization starch/chitosan reinforced polypropylene as biodegradable. J. Phys. Conf. Ser. 2019, 1341, 082022. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.; Lu, X.; He, C. Biodegradable and renewable poly(lactide)-lignin composites: Synthesis, interface and toughening mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. [Google Scholar] [CrossRef]
- Suryanto, H.; Kharismawan, F.A.; Solichin; Rahmawan, A.W.; Sahana, R.T.; Muhajir, M.; Yanuhar, U. Influence of Nanoclay on Thermal Decomposition of Biocomposite Matrix Starch/Carrageenan Blend. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012077. [Google Scholar] [CrossRef]
- Jafar, A.; Mostafa, R.-T.; Esmaeil, B.; Heidari, K.S.; Rahim, J. Mechanical properties of chitosan-starch composite filled hydroxyapatite micro-and nanopowders. J. Nanomater. 2011, 2011, 1–16. [Google Scholar]
- Masruri, M.; Azhar, A.Z.; Rosyada, I.; Febrianto, A. The effect of kaffir lime (Citrus hystrix DC) essential oil on bioplastic derived from cassava peel waste. J. Phys. Conf. Ser. 2019, 1374, 012015. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.H.D.; Fikriyyah, A.K.; Putri, O.D.; Puspa Asri, P.P. Fabrication and Characterization of Poly Lactic Acid (PLA)-Starch Based Bioplastic Composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012052. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Maulida, M.; Siagian, P. Tarigan Production of Starch Based Bioplastic from Cassava Peel Reinforced with Microcrystalline Celllulose Avicel PH101 Using Sorbitol as Plasticizer Related content. J. Phys. Conf. Ser. 2016, 710, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Niaounakis, M. Recycling of biopolymers–The patent perspective. Eur. Polym. J. 2019, 114, 464–475. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Nakasaki, K.; Matsuura, H.; Tanaka, H.; Sakai, T. Synergy of two thermophiles enables decomposition of poly-e-caprolactone under composting conditions. Fed. Eur. Microbiol. Soc. 2006, 58, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Billington, S.L.; Criddle, C.S. Biocomposite Fiber-Matrix Treatments that Enhance In-Service Performance Can Also Accelerate End-of-Life Fragmentation and Anaerobic Biodegradation to Methane. J. Polym. Environ. 2018, 26, 1715–1726. [Google Scholar] [CrossRef]
- Menicagli, V.; Balestri, E.; Lardicci, C. Exposure of coastal dune vegetation to plastic bag leachates: A neglected impact of plastic litter. Sci. Total Environ. 2019, 683, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Witt, T.; Xie, F.; Warren, F.J.; Halley, P.J.; Gilbert, R.G. Biodegradation of starch films: The roles of molecular and crystalline structure. Carbohydr. Polym. 2015, 122, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Arcos-Hernandez, M.V.; Laycock, B.; Pratt, S.; Donose, B.C.; Nikolič, M.A.L.; Luckman, P.; Werker, A.; Lant, P.A. Biodegradation in a soil environment of activated sludge derived polyhydroxyalkanoate (PHBV). Polym. Degrad. Stab. 2012, 97, 2301–2312. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Evangelou, A.; Calabrò, P.S.; Greco, R.; Sánchez, A.; Komilis, D. Biodegradation Activity of Eight Organic Substrates: A Correlation Study of Different Test Methods. Waste Biomass Valorization 2016, 7, 1067–1080. [Google Scholar] [CrossRef] [Green Version]
- Nishide, H.; Toyota, K.; Kimura, M. Effects of soil temperature and anaerobiosis on degradation of biodegradable plastics in soil and their degrading microorganisms. Soil Sci. Plant Nutr. 1999, 45, 963–972. [Google Scholar] [CrossRef]
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Degradation of polyhydroxyalkanoates in eutrophic reservoir. Polym. Degrad. Stab. 2007, 92, 580–586. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Bioplastic biodegradation activity of anaerobic sludge prepared by preincubation at 55 °C for new anaerobic biodegradation test. Polym. Degrad. Stab. 2010, 95, 1349–1355. [Google Scholar]
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Dỗ, N.L.; Nguyễn, T.H.; Lê, T.M.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L.; et al. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int. Biodeterior. Biodegrad. 2013, 83, 77–84. [Google Scholar] [CrossRef]
- Wu, C.S. Preparation and Characterization of Polyhydroxyalkanoate Bioplastic-Based Green Renewable Composites from Rice Husk. J. Polym. Environ. 2014, 22, 384–392. [Google Scholar] [CrossRef]
- Haig, S.; Morrish, L.; Morton, R.; Wilkinson, S. Film Reprocessing Technologies and Collection Schemes; WRAP (Waste & Resources Action Programme): Banbury, UK, 2018. [Google Scholar]
- Degli-Innocenti, F. Biodegradation of plastics and ecotoxicity testing: When should it be done. Front. Microbiol. 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: A review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef] [Green Version]
- Woolnough, C.A.; Charlton, T.; Yee, L.H.; Sarris, M.; Foster, L.J.R. Surface changes in polyhydroxyalkanoate films during biodegradation and biofouling. Polym. Int. 2008, 57, 1042–1051. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Villanova, J.; Cesar, G.; Gavara, R.; Hernandez-Munoz, P. Compostable properties of antimicrobial bioplastics based on cinnamaldehyde cross-linked gliadins. Chem. Eng. J. 2015, 262, 447–455. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Theisen, H.; Vigil, S.A. Integrated Solid Waste Management: Engineering Principles And Management Issues; McGraw-Hill Education: New York, NY, USA, 1993; ISBN 0070632375. [Google Scholar]
- Bhatt, R.; Shah, D.; Patel, K.C.; Trivedi, U. PHA-rubber blends: Synthesis, characterization and biodegradation. Bioresour. Technol. 2008, 99, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sudo, M.; Ohta, K.; Sugimura, T.; Yamada, H.; Aoki, T. Biodegradation of nylon4 and its blend with nylon6. J. Appl. Polym. Sci. 2002, 86, 2307–2311. [Google Scholar] [CrossRef]
- Volova, T.G.; Boyandin, A.N.; Vasiliev, A.D.; Karpov, V.A.; Prudnikova, S.V.; Mishukova, O.V.; Boyarskikh, U.A.; Filipenko, M.L.; Rudnev, V.P.; Bá Xuân, B.; et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as Promising Candidate for Polylactic Acid Type Bioplastic Degradation. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of polymers: A review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Scott, G. ‘Green’ polymers. Polym. Degrad. Stab. 2000, 68, 1–7. [Google Scholar] [CrossRef]
- Fábio Rivas, L.; Aline Casarin, S.; Cavalcante Nepomuceno, N.; Isabele Alencar, M.; Augusto Marcondes Agnelli, J.; Souto de Medeiros, E.; de Oliveira Wanderley Neto, A.; Pinheiro de Oliveira, M.; Marcos de Medeiros, A.; Severino Ferreira Santos, A. Reprocessability of PHB in extrusion: ATR-FTIR, tensile tests and thermal studies. Polímeros 2017, 27, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Ariffin, H.; Nishida, H.; Hassan, M.A.; Shirai, Y. Chemical recycling of polyhydroxyalkanoates as a method towards sustainable development. Biotechnol. J. 2010, 5, 484–492. [Google Scholar] [CrossRef]
- Voinova, O.N.; Kozhevnikova, N.A.; Gladyshev, M.I.; Volova, T.G. Bioplastic degradation in natural reservoirs differing in ecological parameters. Dokl. Biol. Sci. 2007, 417, 426–428. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Bioplastics: Missing link in the era of Microplastics. Sci. Total Environ. 2019, 697, 1–14. [Google Scholar] [CrossRef]
- Wierckx, N.; Narancic, T.; Eberlein, C.; Wei, R.; Drzyzga, O.; Magnin, A.; Ballerstedt, H.; Kenny, S.T.; Pollet, E.; Avérous, L.; et al. Plastic Biodegradation: Challenges and Opportunities. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Biodegradation and Bioremediation; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers (Basel) 2019, 11, 1066. [Google Scholar] [CrossRef] [Green Version]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Müller, R. Biodegradation behaviour of polymers in liquid environments. In Handbook of Biodegradable Polymers; Walter de Gruyter GmbH: Berlin, Gemany, 2006. [Google Scholar]
- Hablot, E.; Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Blazy, C.; Narayan, R.; Hablot, E.; Blazy, Á.C.; Narayan, Á.R.; Dharmalingam, S.; et al. Effect of Simulated Weathering on Physicochemical Properties and Inherent Biodegradation of PLA/PHA Nonwoven Mulches. J. Polym. Environ. 2014, 22, 417–429. [Google Scholar] [CrossRef]
- Mutmainna, I.; Tahir, D.; Lobo Gareso, P.; Ilyas, S. Synthesis composite starch-chitosan as biodegradable plastic for food packaging. J. Phys. Conf. Ser. 2019, 1317, 012053. [Google Scholar] [CrossRef]
- Nissa, R.C.; Fikriyyah, A.K.; Abdullah, A.H.D.; Pudjiraharti, S. Preliminary study of biodegradability of starch-based bioplastics using ASTM G21-70, dip-hanging, and Soil Burial Test methods. IOP Conf. Ser. Earth Environ. Sci. 2019, 277, 012007. [Google Scholar] [CrossRef]
- Martins-Franchetti, S.M.; De Campos, A.; Marconato, J.C. The Influence of Soil and Landfill Leachate Microorganisms in the Degradation of PVC/PCL Films Cast from DMF. Polímeros 2012, 22, 220–227. [Google Scholar]
- Wu, C.S. A comparison of the structure, thermal properties, and biodegradability of polycaprolactone/chitosan and acrylic acid grafted polycaprolactone/chitosan. Polymer (Guildf) 2005, 46, 147–155. [Google Scholar] [CrossRef]
- Di Franco, C.R.; Cyras, V.P.; Busalmen, J.P.; Ruseckaite, R.A.; Vázquez, A. Degradation of polycaprolactone/starch blends and composites with sisal fibre. Polym. Degrad. Stab. 2004, 86, 95–103. [Google Scholar] [CrossRef]
- Innocenti, F.D.; Razza, F.; Fieschi, M.; Bastioli, C.; Fieschi, S.; Donizetti, V.G. Life Cycle Management in bioplastics production. In Proceedings of the 3rd International Conference on Life cycle management, Zurich, Switzerland, 27–29 August 2007. [Google Scholar]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Ruggero, F.; Chiumenti, A. Fate of bioplastics in composting. In Proceedings of the 16th International Waste Management and Landfill Symposium Santa Margherita di Pula, Sardinia, Italy, 2–6 October 2017. [Google Scholar]
- Weng, Y.X.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Ahn, H.K.; Huda, M.S.; Smith, M.C.; Mulbry, W.; Schmidt, W.F.; Reeves, J.B. Biodegradability of injection molded bioplastic pots containing polylactic acid and poultry feather fiber. Bioresour. Technol. 2011, 102, 4930–4933. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Dell, E.; Lewis, C.; Trabold, T.A.; Diaz, C.A. Anaerobic biodegradation of bioplastic packaging materials. In Proceedings of the 21st IAPRI World Conference on Packaging 2018-Packaging: Driving a Sustainable Future, Zhuhai, China, 19–22 June 2018; DEStech Publications Inc.: Lancaster, PA, USA, 2019; pp. 730–737. [Google Scholar]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Mesophilic anaerobic biodegradation test and analysis of eubacteria and archaea involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2014, 110, 278–283. [Google Scholar] [CrossRef]
- Zhang, W.; Heaven, S.; Banks, C.J. Degradation of some EN13432 compliant plastics in simulated mesophilic anaerobic digestion of food waste. Polym. Degrad. Stab. 2018, 147, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic anaerobic biodegradation test and analysis of eubacteria involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2013, 98, 1182–1187. [Google Scholar] [CrossRef]
- Benn, N.; Zitomer, D. Pretreatment and Anaerobic Co-digestion of Selected PHB and PLA Bioplastics. Front. Environ. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Yu, J. Production of Biodegradable Thermoplastic Materials from Organic Wastes 1–6. U.S. Patent No 7,141,400, 28 November 2006. [Google Scholar]
- Abou-Zeid, D.M.; Müller, R.J.; Deckwer, W.D. Degradation of natural and synthetic polyesters under anaerobic conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic biodegradation tests of poly(lactic acid) under mesophilic and thermophilic conditions using a new evaluation system for methane fermentation in anaerobic sludge. Int. J. Mol. Sci. 2009, 10, 3824–3835. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- California State University; Greene, J. Mechatronic Engineering and Manifacturing Technology. In PLA and PHA Biodegradation in the Marine Environment; CalRecycle: Sacramento, CA, USA, 2012. [Google Scholar]
- Sridewi, N.; Bhubalan, K.; Sudesh, K. Degradation of commercially important polyhydroxyalkanoates in tropical mangrove ecosystem. Polym. Degrad. Stab. 2006, 91, 2931–2940. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Bioplastics Guide Bioplastics Standards & Certifications—ASTM, ISO, CEN, DIN Certco, Vincotte-Bioplastics Guide|Bioplastics Guide. Available online: http://www.bioplastics.guide/ref/bioplastics/standards-and-certifications/ (accessed on 2 February 2020).
- OECD Test No. 208 Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006; pp. 1–21. ISBN 9789264070066.

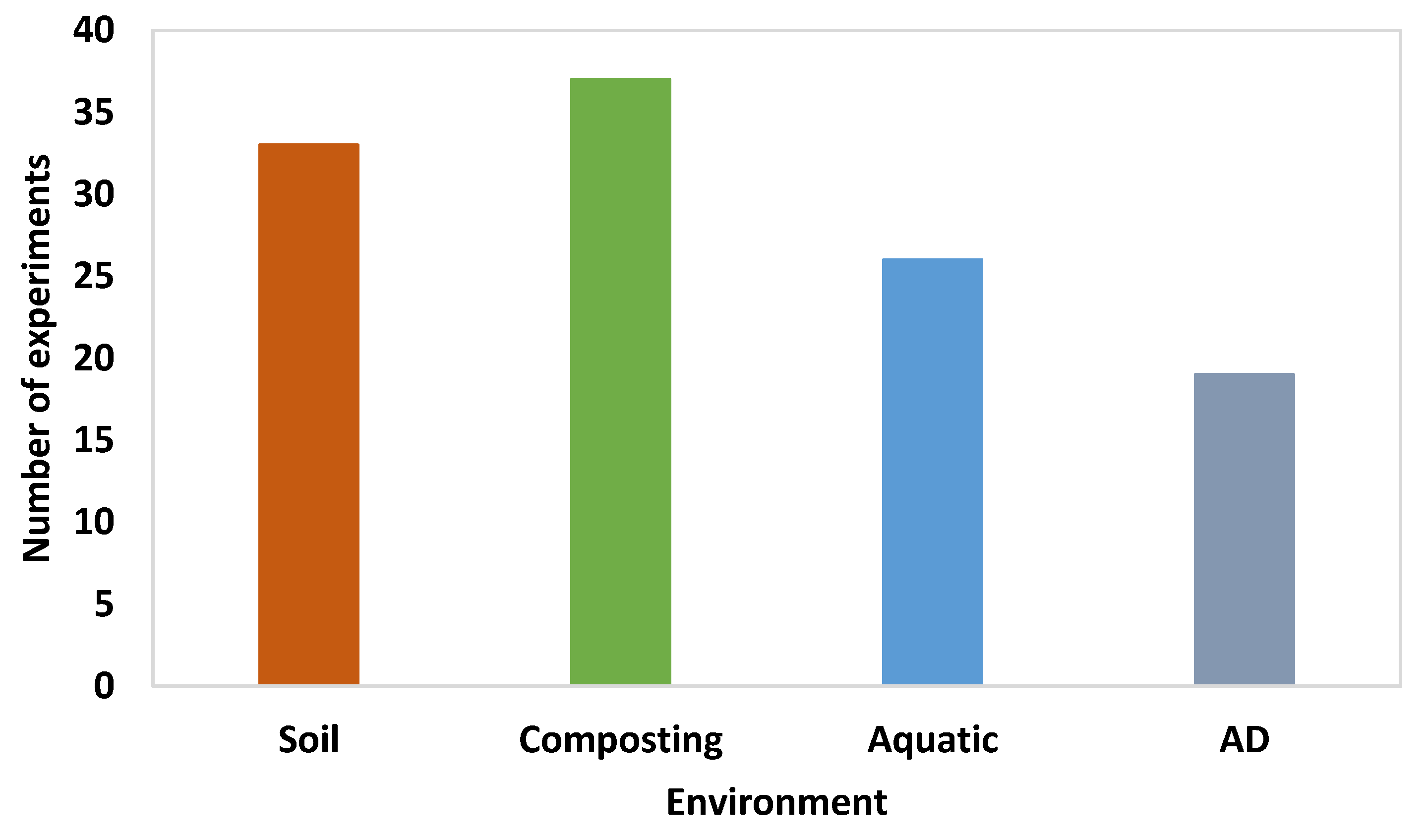
| Type | Biodegradability | Source | Example | References |
|---|---|---|---|---|
| Conventional (petrochemical) plastics | Non-Biodegradable | Fossil Fuel Resources (petroleum, natural gas) | PE, PS, PVC, PP, PC, PU | [2,10,28] |
| Bioplastics | Bio-based & Non-Biodegradable | Renewable Resources (corn, sugar cane, soybean) | Bio-PE, Bio-PET, Bio-PVC, Bio-PU | [28,55] |
| Biodegradable | Fossil Resources (petroleum, natural gas) | PBS, PCL, PBAT | [28,43] | |
| Bio-based & Biodegradable | Renewable Resources (corn, sugar cane) | PHA, PHB, PLA | [54,56] |
| Source of Bioplastic | Use | Ref. |
|---|---|---|
| Cellulose-based | Packaging | [5] |
| Sponge cloths | [73] | |
| Electronics applications | [5] | |
| Starch-based | Films | [74] |
| Rigid materials (such as plates and cutlery and foams) | [74,75] | |
| Packaging | [30,75] | |
| Medical products | [30,75] | |
| Agriculture | [30,39,76] | |
| Carrier bags | [16,77] | |
| PLA-based | Packaging | [30,38,74,78] |
| Disposable or durable goods | [74] | |
| Electronics applications | [38] | |
| Fibres | [38] | |
| Medical products | [30] | |
| Building and construction | [78] | |
| Agricultural field | [30,38] | |
| Textiles and films | [64] | |
| PHA-based | Medical products | [30,74,79,80,81,82] |
| Agriculture | [30,74] | |
| Packaging | [30,82] | |
| Petroleum-based | Medical products | [30] |
| Packaging | [30] | |
| Agriculture | [39] |
| Microorganisms | Anaerobic Bacteria and Archaea | Aerobic Bacteria, Archaea and Fungi | ||
|---|---|---|---|---|
| Process | Thermophilic Digestion | Mesophilic Digestion | Industrial Composting | Home Composting |
| Temperature Conditions | 50–60 °C | ≤35 °C | 50–60 °C | ≤35 °C |
| Bioplastics | ||||
| Chemical pulp | √ | √ | √ | √ |
| Mechanical pulp | X | X | √ | √ |
| Starch | √ | √ | √ | √ |
| Starch/PLA | √ | X | X | X |
| Starch/PCL | X | √ | √ | √ |
| PLA | √ | X | √ | X |
| PHA | √ | √ | √ | √ |
| PBAT | X | X | √ | √ |
| Source of Bioplastic | Name of Bioplastic | Type of Environment | Conditions | Scale | Biodegradation Indicator | Biodegradability (%) | Period of Biodegradability (Days) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Bio-based | PLA-based | PLA | Soil | 30% moisture | Buried at a depth of 12–15 cm in boxes of alluvial-type soil | Weight loss | 10 | 98 | [32] |
| PLA (powdered) | Soil | 25 °C, 60% humidity | 50 g-soil/pot | Weight loss | 13.8 | 28 | [39] | ||
| PLA/NPK fertilizer (63.5/37.5%) | Soil | 30 °C, 80% humidity | Buried in the topsoil located outside natural environment | Weight loss | 37.4 | 56 | [12] | ||
| PLA/NPK fertilizer/EFB (25/37.5/37.5%) | Soil | 30 °C, 80% humidity | Buried in the topsoil; likely in the lab | Weight loss | 43 | 56 | [12] | ||
| PLA/sisal fiber (SF) (60/40%) | Soil | 30% moisture | Buried at a depth of 12–15 cm in boxes of alluvial-type soil | Weight loss | >60 | 98 | [32] | ||
| PHA-based | PHB | Soil | – | Lab-scale container | Weight loss | 64.3 | 180 | [91] | |
| PHB | Microbial culture from soil | Aerobic | 3 L sealable, sterile container | Weight loss | ~18 | 18 | [128] | ||
| PHA | Soil | 35% moisture | Buried at a depth of 12–15 cm in boxes of alluvial-type soil | Weight loss | 35 | 60 | [124] | ||
| PHA | Soil/compost (90/10%) | 25 °C, 65% humidity | Lab-scale desiccator | Produced CO2 | 40–50 | 15 | [117] | ||
| PHA | Soil | 20 °C, 60% moisture | 2 L wide mouth jar | Produced CO2 | 48.5 | 280 | [54] | ||
| PHBV | Microbial culture from soil | – | 3 L sealable, sterile container | Weight loss | ~41 | 18 | [128] | ||
| PHB films | Soil (Hoa Lac, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 98 | ~365 | [123] | ||
| PHBV films | Soil (Hoa Lac, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 61 | ~365 | [123] | ||
| PHB pellets | Soil (Hoa Lac, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 55 | ~365 | [123] | ||
| PHBV pellets | Soil (Hoa Lac, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 35 | ~365 | [123] | ||
| PHB films | Soil (Dam Bai, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 47 | ~365 | [123] | ||
| PHBV films | Soil (Dam Bai, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 14 | ~365 | [123] | ||
| PHB pellets | Soil (Dam Bai, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 28 | ~365 | [123] | ||
| PHBV pellets | Soil (Dam Bai, Vietman) | Natural conditions—15 cm depth | Close-meshed gauze jackets | Weight loss | 8 | ~365 | [123] | ||
| PHA/Rice Husk (RH) (60/40%) | Soil | 35% moisture | Buried at a depth of 12–15 cm in boxes of alluvial-type soil | Weight loss | >90 | 60 | [124] | ||
| Starch-based | Starch-based | Soil | 20 °C, 60% moisture | 2 L wide mouth jar | Produced CO2 | 14.2 | 110 | [54] | |
| Mater-Bi plastic carrier bags | Soil | 25 °C | Lab-scale cylinder (30–20 cm) | Weight loss | 37 | 90 | [22] | ||
| Mater-Bi plastic carrier bags | Soil | Uncontrolled | Real filed—5 cm depth | Weight loss | 3.4 | 90 | [22] | ||
| Starch/chitosan (35/65) | Soil | Stockpiled sample on the ground | n.a. | Weight loss | 80 | 14 | [149] | ||
| Starch/chitosan (35/65) | Soil | Soil burial test method | Lab-scale | Weight loss | 96 | 28 | [102] | ||
| Cassava starch/glycerol (3/1) | Compost soil | Soil Burial Test., room temperature | Lab-scale | Weight loss | 30 | 10 | [150] | ||
| Cellulose-based | Rice straw bioplastics | Soil | Undefined | Lab-scale | Weight loss | ≈100 | 103 | [5] | |
| Sponge cloth (cellulose-based) | Synthetic soil containing compost | Aerobic, 58 °C | Laboratory-scale controlled composting conditions | Weight loss | >80 | 154 | [73] | ||
| Nylon4 (Polyamides, Bio-based) | Composted soil | 25 °C, pH = 7.5–7.6, 80% humidity | Buried in containers made of PP | Weight loss | 100 | 120 | [133] | ||
| Petroleum-based | PBS-based | PBS (films) | Soil | 25 °C, 60% humidity | 100 g-soil/pot | Weight loss | 1.2 | 28 | [39] |
| PBS (powdered) | Soil | 25 °C, 60% humidity | 50 g-soil/pot | Weight loss | 16.8 | 28 | [39] | ||
| PBS/starch (films) (50/50%) | Soil | 25 °C, 60% humidity | 100 g-soil/pot | Weight loss | 7.2 | 28 | [39] | ||
| PBS/starch (powdered) (50/50%) | Soil | 25 °C, 60% humidity | 50 g-soil/pot | Weight loss | 24.4 | 28 | [39] | ||
| PCL-based | PCL | Soil | 28 °C, 60% humidity | Lab-scale | Weight loss | 90 | 60 | [151] | |
| PCL | Soil and leachate | 28 °C, 60% humidity | Lab-scale | Weight loss | 22 | 60 | [151] | ||
| PCL | Alluvial-type soil | Samples buried at 15 cm depth, 60% humidity | Lab-scale | Weight loss | <5 | 48 | [152] | ||
| PCL/chitosan (80/20) | Alluvial-type soil | Samples buried at 15 cm depth, 60% humidity | Lab-scale | Weight loss | 20 | 48 | [152] | ||
| PCL/starch (75/25) | Soil | 20 °C, 40% humidity | Plastic bottles | Weight loss | 37 | 270 | [153] | ||
| Source of Bioplastic | Name of Bioplastic | Type of Environment | Conditions | Scale | Biodegradation Indicator | Biodegradability (%) | Period of Biodegradability (Days) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Bio-based | PLA-based | PLA | Compost | 58 °C | lab-scale compost reactor (bottle of 1 L volume) | Produced CO2 | 13 | 60 | [159] |
| PLA | Compost | 65 °C, pH = 8.5, 63% humidity | Wooden box (0.6 mx 0.3 mx 0.1 m) 1.2 m above ground and 1 m inside the compost pile | Produced CO2 | 84 | 58 | [155] | ||
| PLA | Compost | 55 °C, 70% moisture | Laboratory scale composting setup | Produced CO2 | ~70 | 28 | [158] | ||
| PLA | Compost | Aerobic, 58 °C, 60% humidity | Lab-scale | Weight loss | 60 | 30 | [41] | ||
| PLA | Synthetic material containing compost | Aerobic, 58 °C | Polypropylene reactor (L = 330 mm, W = 180 mm, H = 130 mm) | Weight loss | 63.6 | 90 | [15] | ||
| PLA | Synthetic material containing compost | 58 °C | Laboratory-scale plastic reactor | Weight loss | 100 | 28 | [56] | ||
| PLA/PFF/starch (80/5/15%)a | Compost | 58 °C | Lab-scale compost reactor (bottle of 1 L volume) | Produced CO2 | 53 | 60 | [159] | ||
| PLA/Soft wood (70/30%) | Compost | Aerobic, 58 °C, 60% humidity | - | Weight loss | 40 | 30 | [41] | ||
| PLA/corn (90/10%) | Synthetic material containing compost | Aerobic, 58 °C | Polypropylene reactor (L = 330 mm, W = 180 mm, H = 130 mm) | Weight loss | 79.7 | 90 | [15] | ||
| PLA+Nano-SiO2 film | Composting | Aerobic, 58 °C, 55% water | Reactor size of 37 cm × 17 cm × 25.5 cm | Disintegration with respect to PLA | 9.4 | 130 | [129] | ||
| PLA+Clay film | Composting | Aerobic, 58 °C, 55% water | Reactor size of 37 cm × 17 cm × 25.5 cm. | Disintegration with respect to PLA | 34 | 130 | [129] | ||
| PLA+Nano-CaCO3 film | Composting | Aerobic, 58 °C, 55% water | Reactor size of 37 cm × 17 cm × 25.5 cm. | Disintegration with respect to PLA | 48 | 130 | [129] | ||
| PLA/PHB (75/25%) | Synthetic material containing compost | 58 °C | Laboratory-scale plastic reactor | Weight loss | 100 | 35 | [56] | ||
| PLA/corn (90/10%) | Synthetic material containing compost | Aerobic, 58 °C | Polupropylene reactor (L = 330 mm, W = 180 mm, H = 130 mm) | Weight loss | 79.7 | 90 | [15] | ||
| PHA-based | PHA | Soil/compost (90/10%) | 25 °C, 65% humidity | Lab-scale desiccator | Produced CO2 | 40–50 | 15 | [117] | |
| PHB | Compost | 58 °C | Static composting vessel | Produced CO2 | 79.9 | 110 | [157] | ||
| PHB | Compost | 55 °C, 70% moisture | Laboratory scale composting setup | Produced CO2 | ~80 | 28 | [158] | ||
| Starch-based | Bioplastic (made from potato almidon) | Compost | Aerobic, 58 °C | Laboratory-scale test with PP reactors | Weight loss | ~85 | 90 | [4] | |
| Mater-Bi bioplastic (60% starch + 40% resin) | Compost | Aerobic, 23 °C, 55% moisture | A PVC rotary drum, L = 95 cm & Di = 55 cm with 200 L capacity | Weight loss | 26.9 | 72 | [26] | ||
| Plastarch | Compost | Aerobic, 55 °C, 60% moisture | 4 L vessel made of PVC pipe | Produced CO2 | 50 | 85 | [54] | ||
| Mater-Bi plastic carrier bags | Compost | 25 °C | Lab-scale cylinder (30–20 cm) | Weight loss | 43 | 90 | [22] | ||
| Cassava starch/glycerol (3/1) | Compost soil | Soil Burial Test., room temperature | Lab-scale | Weight loss | 30 | 10 | [150] | ||
| Cellulose-based | CA (produced from fiber flax) | Municipal solid waste mixture (compost) | - | Bench-scale simulated composting, batch reaction vessel | Weight loss | 44 | 14 | [63] | |
| CA (produced from cotton linters) | Municipal solid waste mixture (compost) | - | Bench-scale simulated composting, batch reaction vessel | Weight loss | 35 | 14 | [63] | ||
| Sponge cloth from renewable resources, organic cotton mesh | Compost | 1 m depth—15.7 °C average outside temperature | Industrial scale | Weight loss | 20 | 84 | [51] | ||
| Sponge cloth (Cellulose, Cotton mesh, Water, Salts, Pigments) | Compost | 1 m depth—15.7 °C average outside temperature | Industrial scale | Weight loss | 80 | 84 | [51] | ||
| Sponge cloth (70% Cellulose, 30% Cotton) | Compost | 1 m depth—15.7 °C average outside temperature | Industrial scale | Weight loss | ≈ 100 | 84 | [51] | ||
| Sponge cloth (75% Cellulose, 25% Cotton) | Compost | 1 m depth—15.7 °C average outside temperature | Industrial scale | Weight loss | ≈ 100 | 84 | [51] | ||
| Sponge cloth (cellulose-based) | Synthetic material containing compost | Aerobic, 58 °C | Laboratory-scale controlled composting conditions | Weight loss | >80 | 154 | [73] | ||
| Nylon4 (Polyamides, Bio-based) | Composted soil | 25 °C, pH = 7.5–7.6, 80% humidity | Buried in containers made of PP | Weight loss | 100 | 120 | [133] | ||
| Petroleum-based | PBS-based | PBS | Compost | Aerobic, 58–65 °C, pH = 7–8, 50–55% moisture | Glass hermetic flask with a capacity of 1000 mL | Produced CO2 | 90 | 160 | [35] |
| PBS/soy meal (75/25%) | Compost | Aerobic, 58–60 °C, pH = 7–8, 50–55% moisture | Glass hermetic flask with a capacity of 1000 mL | Produced CO2 | 90 | 100 | [35] | ||
| PBS/canola meal (75/25%) | Compost | Aerobic, 58–60 °C, pH = 7–8, 50–55% moisture | Glass hermetic flask with a capacity of 1000 mL | Produced CO2 | 90 | 100 | [35] | ||
| PBS/corn gluten meal (75/25%) | Compost | Aerobic, 58–60 °C, pH = 7–8, 50–55% moisture | Glass hermetic flask with a capacity of 1000 mL | Produced CO2 | 90 | 100 | [35] | ||
| PBS/switch grass (75/25%) | Compost | Aerobic, 58–60 °C, pH = 7–8, 50–55% moisture | Glass hermetic flask with a capacity of 1000 mL | Produced CO2 | 90 | 170 | [35] | ||
| PCL-based | PCL/starch | Compost | Aerobic, 25 °C | 500 mL glass bottle | Produced CO2 | 88 | 44 | [1] | |
| PCL | Compost | Aerobic, 50 °C, pH = 7–8.5 | Experimental system, mini-reactor with a cylinder made of pyrex glass and rubber stoppers with glass pipes for aeration | Produced CO2 | 38 | 6 | [112] | ||
| Source of Bioplastic | Name of Bioplastic | Type of Environment | Conditions | Scale | Biodegradation Indicator | Biodegradability (%) | Period of Biodegradability (Days) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Bio-based | PLA-based | PLA | Sludge | Anaerobic, 37 °C | Lab-scale, 10 L stainless steel bottle | Produced CO2 | 29–49 | 277 | [161] |
| PLA | Sludge | Anaerobic, 55 °C | Lab-scale, 10 L stainless steel bottle | Produced CO2 | 80 | 30–50 | [111] | ||
| PLA | AD | Anaerobic, 52 °C | Lab-scale | Comparison with respect to theoretical BMP | 90 | 36 | [160] | ||
| PLA powder | Sludge | Anaerobic, 55 °C | Lab-scale | Biogas production | 90 | 60 | [167] | ||
| PLA | Sludge | Anaerobic, 55 °C | Lab-scale | n.a. | 75 | 75 | [163] | ||
| PLA | Sludge | Anaerobic, 55 °C | Lab-scale, 10-L bottle | Produced biogas | 85 | 60 | [122] | ||
| PHA-based | PHBs | AD | Anaerobic digestion—untreated PHB—35 °C | Lab-scale | Conversion to biogas | 67 | 175 | [164] | |
| PHBs | AD | Anaerobic digestion—pretreated PHB—35 °C | Lab-scale | Conversion to biogas | 91 | 175 | [164] | ||
| PHB | Sludge | Anaerobic, 55 °C | Lab-scale | Produced biogas | 90 | 14 | [163] | ||
| PHB | Sludge | Anaerobic, 37 °C | Lab-scale, 10 L stainless steel bottle | Produced CO2 | 90 | 9 | [161] | ||
| PHB | AD | Anaerobic | Lab-scale | Weight loss—Biogas production | 90 | 9 | [166] | ||
| Starch-based | Plastarch | AD | Anaerobic, 37 °C | 2 L laboratory scale batch reactor | Produced CO2 | 26.4 | 50 | [54] | |
| Mater-Bi plastic carrier bags | AD | Untreated bioplastic—35 °C | Lab-scale 1-L bottle | Weight loss | 23–30 | 15–30 | [77] | ||
| Mater-Bi plastic carrier bags | AD | NaOH pretreated bioplastic—35 °C | Lab-scale 1-L bottle | Weight loss | 73 | 15 | [77] | ||
| Mater-Bi plastic carrier bags | AD | Untreated bioplastic—55 °C | Lab-scale 1-L bottle | Weight loss | 28–41 | 15–30 | [77] | ||
| Petroleum-based | PBS-based | PBS | Landfill | Anaerobic, 25 °C | 500 mL glass bottle | Produced CO2 | 2 | 100 | [1] |
| PCL-based | PCL/starch | Landfill | Anaerobic, 25 °C | 500 mL glass bottle | Produced CO2 | 83 | 139 | [1] | |
| PCL | Sludge | Anaerobic, 37 °C | Lab-scale, 10 L stainless steel bottle | Produced CO2 | 3–22 | 277 | [161] | ||
| PCL | Sludge | Anaerobic, 55 °C | Lab-scale, 10 L stainless steel bottle | Produced CO2 | 75 | 40–75 | [161] | ||
| Source of Bioplastic | Name of Bioplastic | Type of Environment | Conditions | Scale | Biodegradation Indicator | Biodegradability (%) | Period of Biodegradability (Days) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Bio-based | PLA-based | PLA | Freshwater | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | <2 | 365 | [168] |
| PLA | Sea water | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | <2 | 365 | [168] | ||
| PLA | Marine | 30 °C | Lab-scale | CO2 production | 3.1–5.7 | 180–365 | [169] | ||
| PLA | Marine | 30 °C | Lab-scale | CO2 production | 4.5–8.4 | 180–365 | [169] | ||
| PLGA | Freshwater | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | 100 | 270 | [168] | ||
| PLGA | Sea water | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | 100 | 270 | [168] | ||
| PHA-based | PHB | Freshwater | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | 8.5 | 365 | [168] | |
| PHB | Sea water | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | 8.5 | 365 | [168] | ||
| PHB | Sea water | 25 °C | Lab-scale | BOD bio-degradability | 80 | 14 | [11] | ||
| PHB | Sea water | Static incubation, 21 °C | Laboratory-scale 121 mL flask | Weight loss | 99 | 49 | [171] | ||
| PHB | Sea water | Dynamic incubation, 12–22 °C, pH = 7.9–8.1 | Aquarium tank with continuously flowing seawater | Weight loss | 30 | 90 | [171] | ||
| PHBV | Sea water | Static incubation, 21 °C | Laboratory-scale 121 mL flask | Weight loss | 99 | 49 | [171] | ||
| PHBV | Sea water | Dynamic incubation, 12–22 °C, pH = 7.9–8.1 | Aquarium tank with continuously flowing seawater | Weight loss | 30 | 90 | [171] | ||
| PHB | River water | Real condition ~20 °C | Eutrophic recreation reservoir (surface = 0.32 km2 & Dmax = 8 m) | Weight loss | 43.5 | 42 | [121] | ||
| PHB | Brackish water sediment | 32 °C, pH = 7.06 | Nylon mesh jachet in a PVC column at the depth of 20 cm from the sediment surface | Weight loss | 100 | 56 | [170] | ||
| PHB | Marine water | 28.75 °C, pH = 7–7.5, average salinity 34‰ | Specimens on stainless-steel frames in a floating platform in seawater | Weight loss | 58 | 160 | [134] | ||
| Starch-based | Mater-Bi bioplastic | Marine water with sediment | Room temperature | Oxitop BOD Respirometer System, glass reactor of 82 mL | BOD biodegradability | 68.9 | 236 | [3] | |
| Mater-Bi plastic carrier bags | Freshwater marsh | 25 °C/uncontrolled | Lab-scale 5-L flask /In situ | Weight loss | 1.5 | 90 | [22] | ||
| Mater-Bi plastic carrier bags | Seawater | 25 °C/uncontrolled | Lab-scale 5-L flask/In situ | Weight loss | 1.5 | 90 | [22] | ||
| Cassava starch/glycerol (3/1) | Mixed culture | Aerobic, Aspergillus niger culture | Lab-scale | Weight loss | 20 | 10 | [150] | ||
| Cassava starch/glycerol (3/1) | Mixed culture | Dip-hanging method, Aerobic, 30 °C | Lab-scale | Weight loss | 11 | 10 | [150] | ||
| Petroleum-based | PCL-based | PCL | Inoculum from a municipal wastewater treatment plant | Aerobic, 30 °C, pH = 7 | Respirometer, 500 mL bottle | Weight loss | 7.6 | 28 | [111] |
| PCL/starch | Inoculum from a municipal wastewater treatment plant | Aerobic, 30 °C, pH = 7 | Respirometer, 500 mL bottle | Weight loss | 53 | 28 | [111] | ||
| PCL | Freshwater | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | <2 | 365 | [168] | ||
| PCL | Sea water | 25 °C—16 h light and 8 h dark | Lab-scale | Weight loss | <2 | 365 | [168] | ||
| PCL | Aquatic | Anaerobic, 55 °C | Lab-scale | Produced biogas | 80 | 50 | [163] | ||
| Environment or Process | Standard | Notes/Requirements |
|---|---|---|
| Composting | EN 13432 | Criteria that must be taken into account to label a packaging material as compostable: at least 90% of the organic material is converted into CO2 within 6 months; after 3 months’ composting and subsequent sieving through a 2 mm sieve, no more than 10% residue may remain, as compared to the original mass; no negative influence on the composting process or plant growth is permitted. Biodegradability is evaluated by O2 consumption and CO2 generation. |
| ISO 17088 | This specification is intended to establish the requirements for the labelling of plastic products and materials, including packaging made from plastics, as “compostable” or “compostable in municipal and industrial composting facilities” or “biodegradable during composting”. | |
| ASTM D6400 | The standard lasts from a minimum of 90 days to up to 180 days This standard includes elemental analysis, phytotoxicity tests, and mesh filtration of the resulting particles, necessary for labeling of plastics designed to be aerobically composted in municipal or industrial facilities. | |
| ASTM D6868 | Standard specification for labeling of end items that incorporate plastics and polymers as coatings or additives with paper and other substrates designed to be aerobically composted in municipal or industrial facilities. | |
| ASTM D5338 | This test method determines the degree and rate of aerobic biodegradation of plastic materials on exposure to a controlled-composting environment under laboratory conditions, at thermophilic temperatures. This test method is designed to yield a percentage of conversion of carbon in the sample to carbon dioxide. | |
| ISO 14855 | The standard determines ultimate aerobic biodegradability and disintegration of plastic materials under controlled composting conditions. Test requirements include standard testing for a minimum of 90 days and then the biodegradation results are determined after an analysis of evolved CO2. | |
| ISO 20200 | The standard specifies a method of determining the degree of disintegration of plastic materials when exposed to a laboratory-scale composting environment. The method is not applicable to the determination of the biodegradability of plastic materials under composting conditions. Further testing is necessary to be able to claim compostability. | |
| ISO 16929 | The standard is used to determine the degree of disintegration of plastic materials in a pilot-scale aerobic composting test under defined conditions. It cannot be used to determine the aerobic biodegradability of a test material. | |
| EN 14806 | This laboratory scale test method using synthetic waste aims at simulating the environmental conditions found in industrial composting plants to assess the disintegration process of packaging materials exposed to this environment. This test does not replace the acceptance disintegration test as specified in EN 14045, in accordance with EN 13432. | |
| EN 14045 | This standard is used to evaluate the disintegration of packaging materials in a pilot-scale aerobic composting test under defined conditions. The material is mixed with biowaste and spontaneously composted for 12 weeks. Disintegration is measured by the calculation of a mass balance. Additionally, this method can be used for visual perception and photographic documentation of the disintegration of packaging materials and to evaluate the effect of their addition on the composting process. Other methods should be used to measure the biodegradability of the packaging materials. | |
| AS 5810 | This Australian standard specifies requirements and procedures to determine whether a plastic material is biodegradable in home composting conditions and provides the basis to allow labelling of materials or products made from plastics as ‘home compostable’, for use in home composting systems. | |
| UNI 11183 | The norm defines the biodegradability requirements of plastic materials to be anaerobically treated at room temperature. | |
| NF T 51-800 | French norm specifying the requirements for plastics suitable for home composting. | |
| Composting and anaerobic digestion (AD) | ISO 18606 | As with EN 13432, packaging is considered recoverable by organic recycling only if all the individual components meet the requirements. For each of the packaging components the following four aspects are addressed: biodegradation; disintegration during biological waste treatment process; negative effects on the biological process; negative effects on the quality of the resulting compost, including the presence of high levels of regulated metals and other substances hazardous to the environment. |
| EN 14995 | Requirements and procedures for the evaluation of the compostability and anaerobic treatment of plastics. Disintegration of the material during the biological treatment and effect on the quality of the final product are considered indexes of biodegradability. | |
| Soil | EN 17033 | This document specifies the requirements for biodegradable films, manufactured from thermoplastic materials, to be used for mulch applications in agriculture and horticulture. |
| NF U 52-001 | French standard used for the classification of biodegradable materials for agriculture and horticulture purposes (mulching products) | |
| ISO 17556 | This document specifies a method for determining the ultimate aerobic biodegradability of plastic materials in soil by measuring the oxygen demand in a closed respirometer or the amount of carbon dioxide evolved. If a non-adapted soil is used as an inoculum, the test simulates the biodegradation processes which take place in a natural environment; if a pre-exposed soil is used, the method can be used to investigate the potential biodegradability of a test material. | |
| ASTM D5988-18 | This test method determines the degree of aerobic biodegradation by measuring evolved carbon dioxide as a function of time for a plastic exposed to soil. | |
| AD—Landfilling | ASTM D5511-02 | This test method covers the determination of the degree and rate of anaerobic biodegradation of plastic materials in high-solids environments (more than 30% total solids) under anaerobic conditions and static non-mixed conditions. The methanogenic inoculum is derived from anaerobic digesters operating only on pretreated household waste. This test method may also resemble some conditions in biologically active landfills where the gas generated is recovered and biogas production is even actively promoted. |
| Landfilling | ASTM D5526 | The standard evaluates the percentage of conversion of carbon in the test sample to carbon in the gaseous form (CH4 and CO2) under conditions that resemble landfill conditions. |
| AD | ISO 15985 | The standard specifies a method for the evaluation of the ultimate anaerobic biodegradability of plastics based on organic compounds under high-solids anaerobic-digestion conditions by measurement of evolved biogas and the degree of disintegration at the end of the test. |
| ISO 11734 | The standard gives a method for the evaluation of the ultimate biodegradability of organic compounds in digested sludge at a given concentration by anaerobic microorganisms. | |
| ASTM D5210-92 | This test method determines the degree and rate of anaerobic biodegradation of synthetic plastic materials on exposure to anaerobic-digester municipal sewage sludge from a wastewater treatment plant, under laboratory conditions. | |
| Anaerobic—Aqueous system | ISO 14853 | The method determines the ultimate anaerobic biodegradation of plastic materials in an aqueous system. Biodegradation is measured by biogas production. |
| Marine Environment | ASTM D6691 | This test method, conducted under controlled laboratory conditions, is used to determine the degree and rate of aerobic biodegradation of plastic materials exposed to a pre-grown population of at least ten aerobic marine microorganisms of known genera or the indigenous population existing in natural seawater. |
| ASTM D6692 | This test method is used to determine the degree of aerobic biodegradation of polymeric compounds utilized in plastic materials by determining the level of respiration of such radiolabeled carbon compounds to radiolabeled carbon dioxide. The test is designed to utilize the naturally occurring microbes in seawater as the inoculum for the enrichment and subsequent mineralization (biodegradation) of the test polymer using it as a carbon and energy source resulting in a carbon dioxide as an end product. | |
| ASTM D7473-12 | This test method is used to determine the weight loss as a function of time of non-floating plastic materials, when incubated under changing, open, marine aquarium conditions, which is representative of aquatic environments near the coasts and near the bottom of a body of water in the absence of sunlight, particularly UV and visible portions of the spectrum. | |
| OECD 306 | The test gives a first impression of biodegradability in seawater. If the result is positive (>70% DOC removal; >60% ThOD), it may be concluded that there is a potential for biodegradation in the marine environment. | |
| ISO 16221 | This standard specifies five methods to determine the ultimate aerobic biodegradability of organic compounds in the marine environment by aerobic microorganisms in static aqueous test systems. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. https://doi.org/10.3390/su12156030
Folino A, Karageorgiou A, Calabrò PS, Komilis D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability. 2020; 12(15):6030. https://doi.org/10.3390/su12156030
Chicago/Turabian StyleFolino, Adele, Aimilia Karageorgiou, Paolo S. Calabrò, and Dimitrios Komilis. 2020. "Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review" Sustainability 12, no. 15: 6030. https://doi.org/10.3390/su12156030
APA StyleFolino, A., Karageorgiou, A., Calabrò, P. S., & Komilis, D. (2020). Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability, 12(15), 6030. https://doi.org/10.3390/su12156030







