L-Arginine-Incorporated Cement Mortar as Sustainable Artificial Reefs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Test Methods
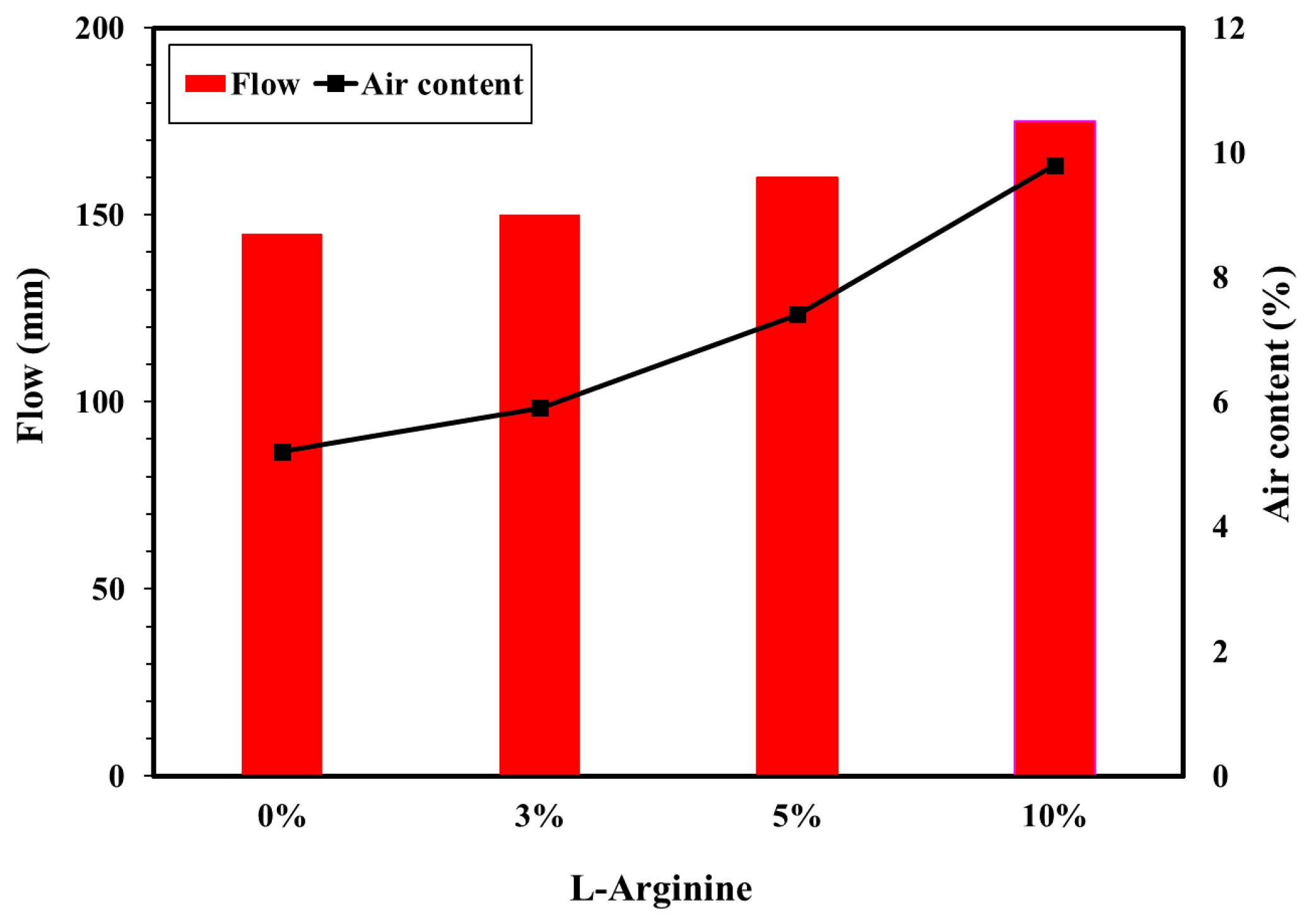
2.3.1. Flow/Slump Value and Air Content
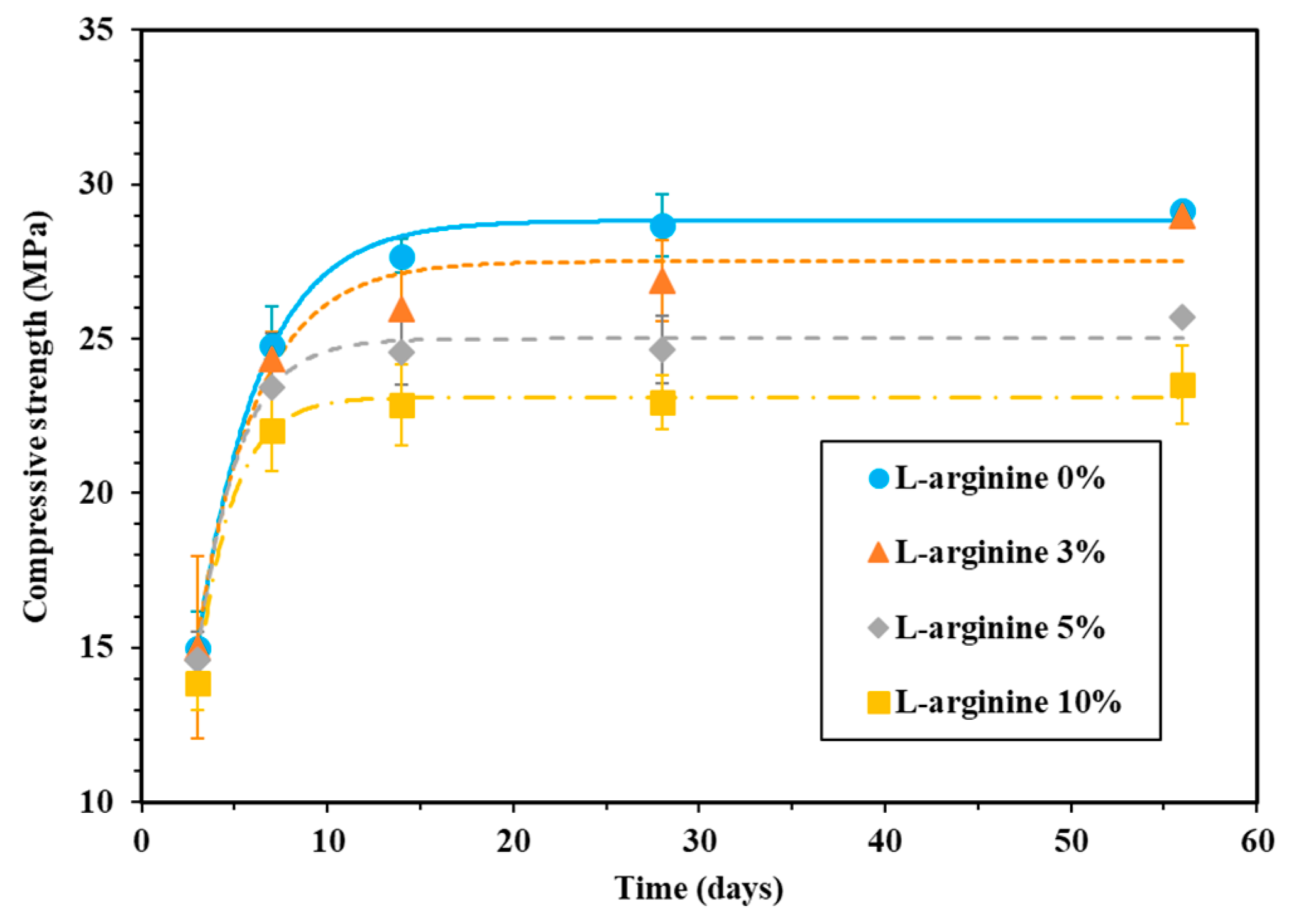
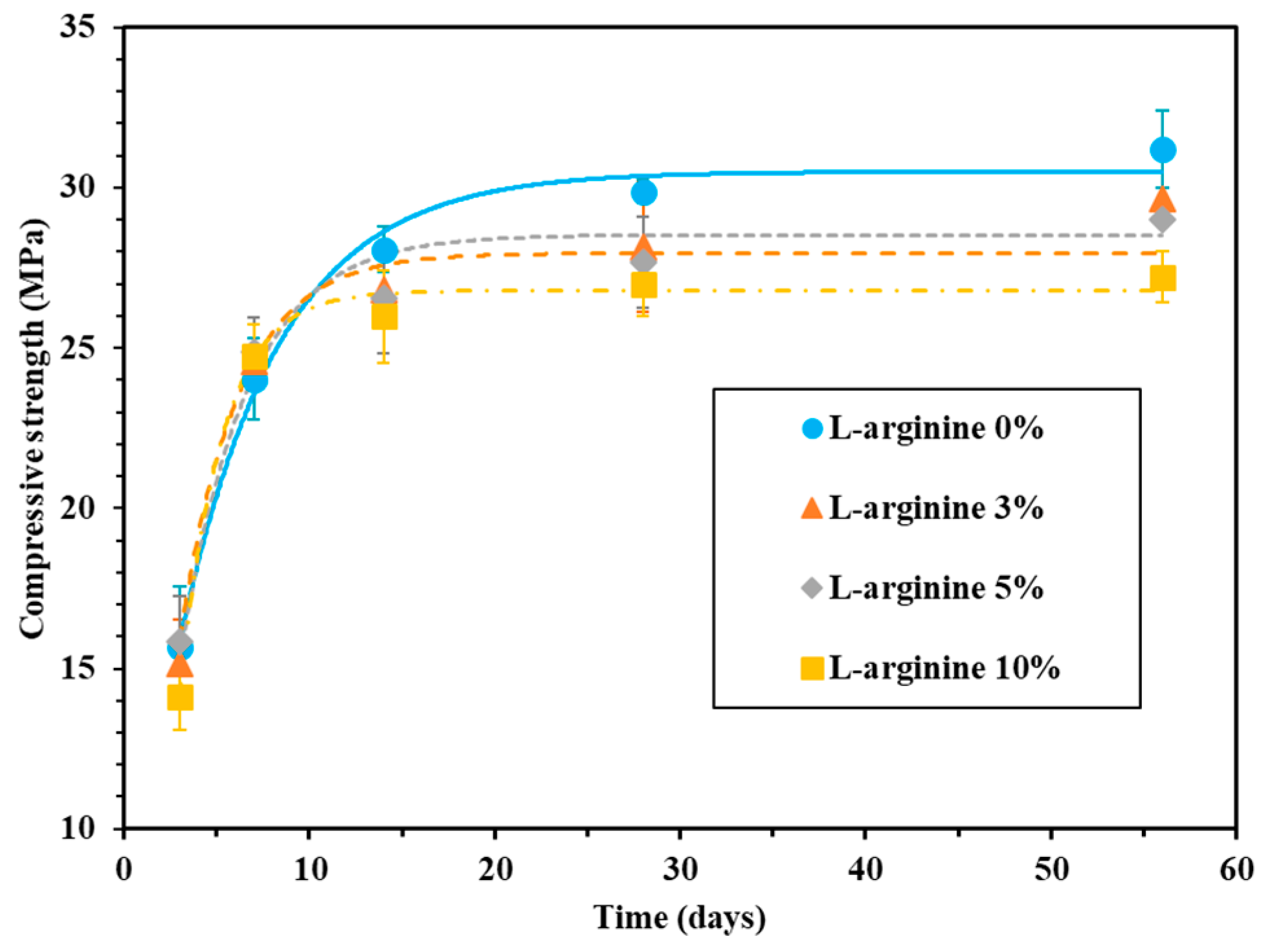
2.3.2. Compressive Strength
2.3.3. Leaching Test for L-Arginine
2.3.4. pH Determination
2.3.5. Dissolved Nitrogen
2.3.6. Evaluation of Chlorophyll-A Amount
3. Results and Discussion
3.1. Properties of Cement Mortar with L-Arginine
3.1.1. Flow/Slump and Air Content of Fresh Cement Mortar
3.1.2. Compressive Strength
3.2. L-Arginine Extraction from Hardened Cement Mortar
3.2.1. Extraction of L-Arginine in Distilled Water
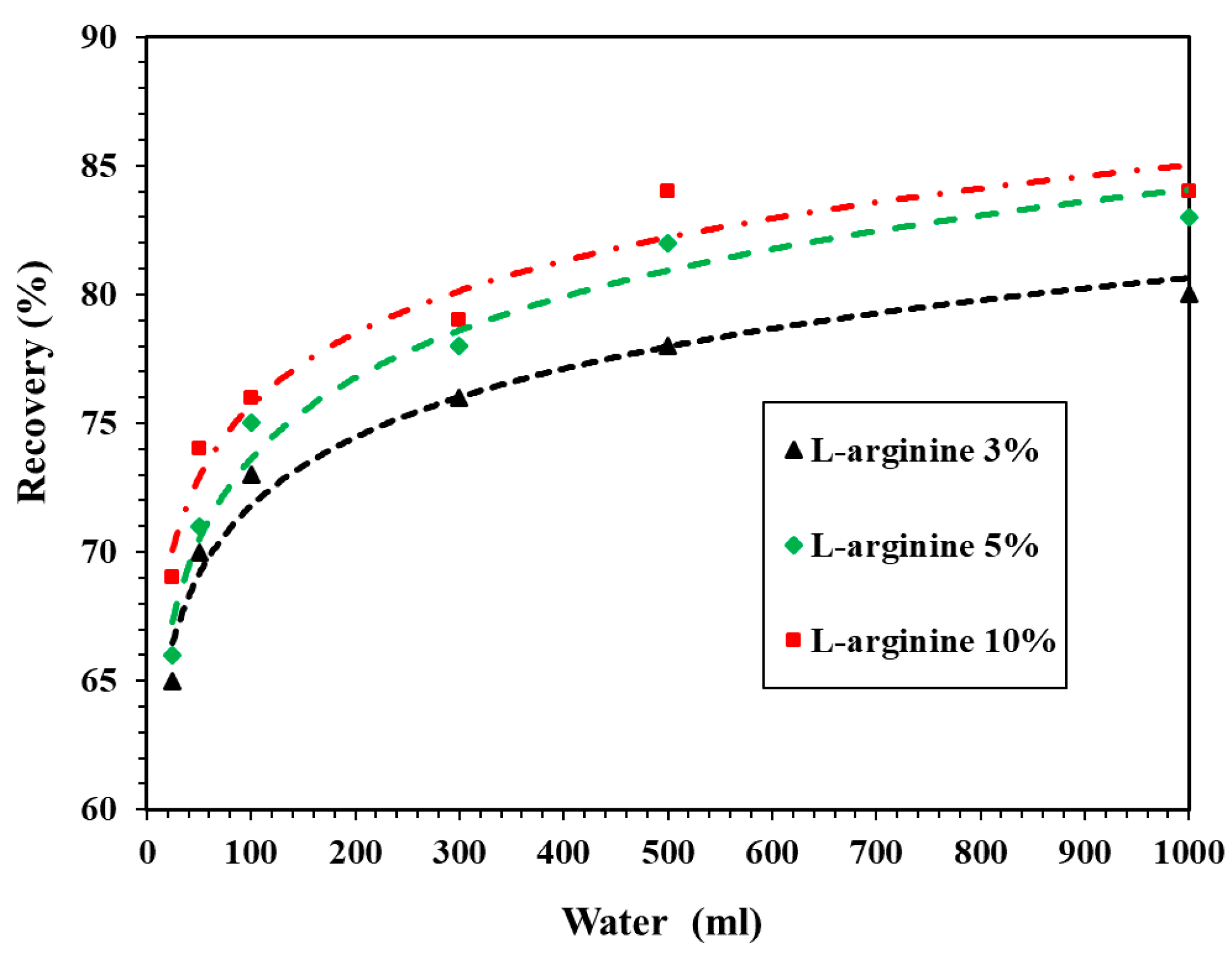
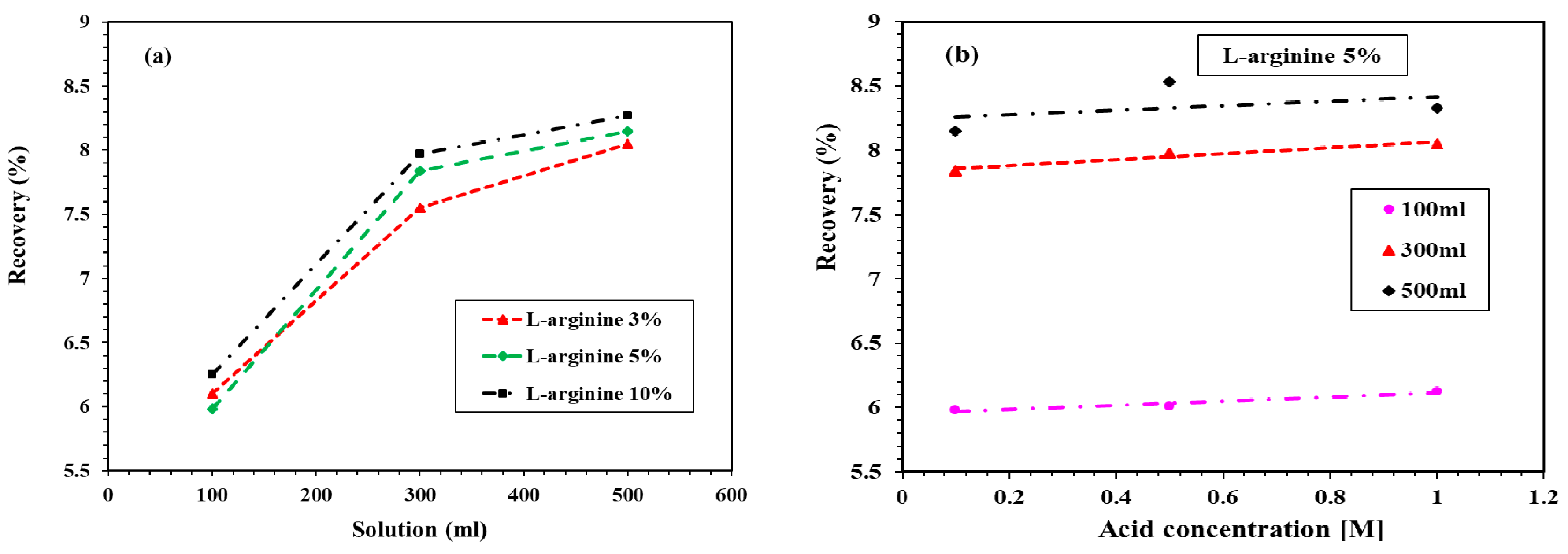
3.2.2. Recovery of Undissolved L-Arginine in HCl
3.3. Evaluation of the Impact of the Environmental Activity of L-Arginine-Containing Mortar
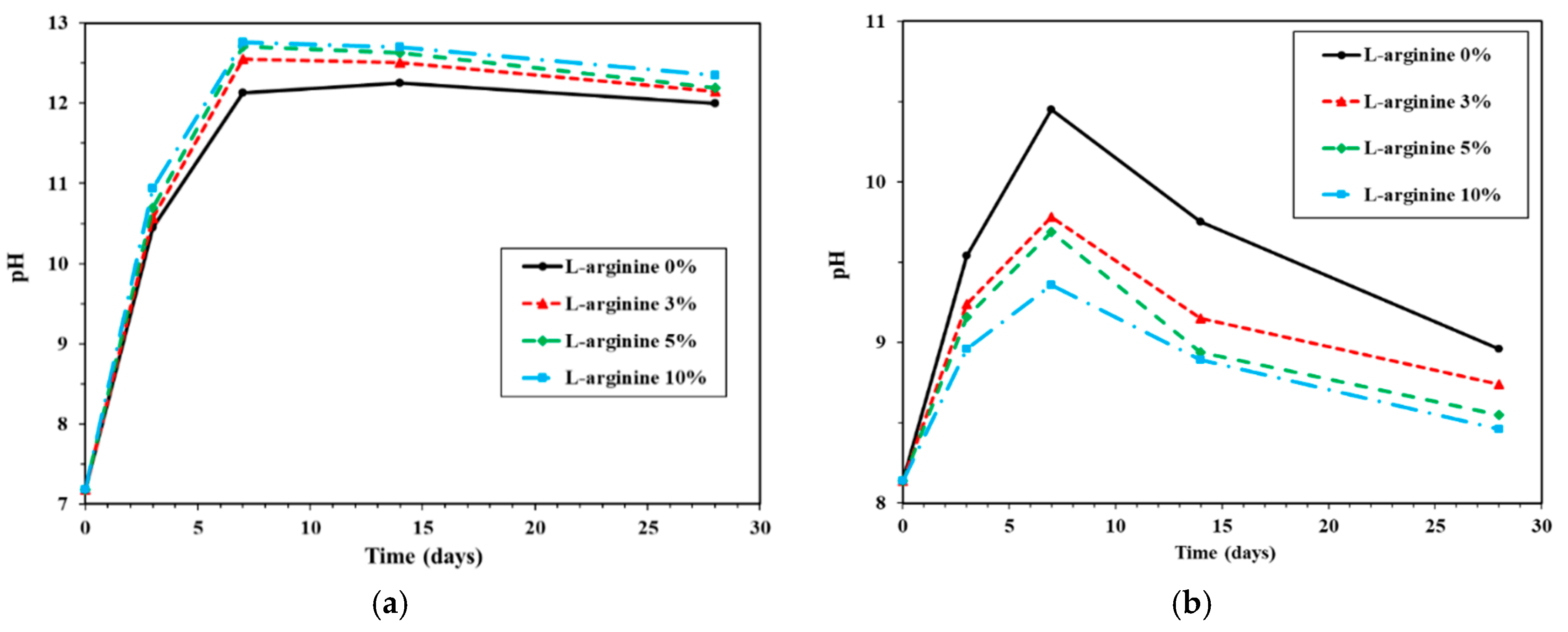
3.3.1. Change of pH Value According to the Type of Water Used to Immerse Cement Mortar with L-Arginine
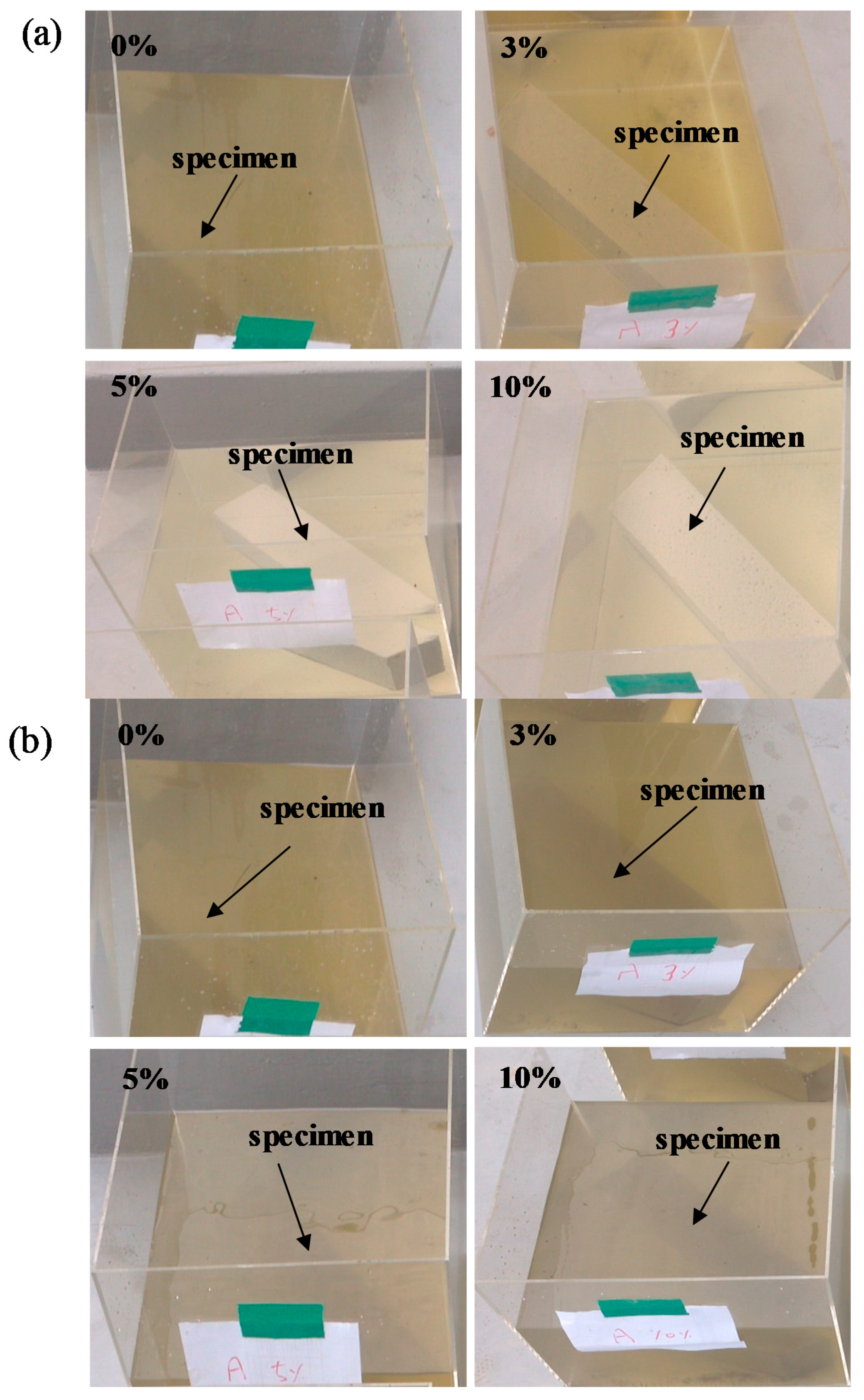
3.3.2. Time Required to Change the Color of River Water after Immersion of L-Arginine-Containing Cement Mortar
3.3.3. Total Dissolved Nitrogen in Distilled Water
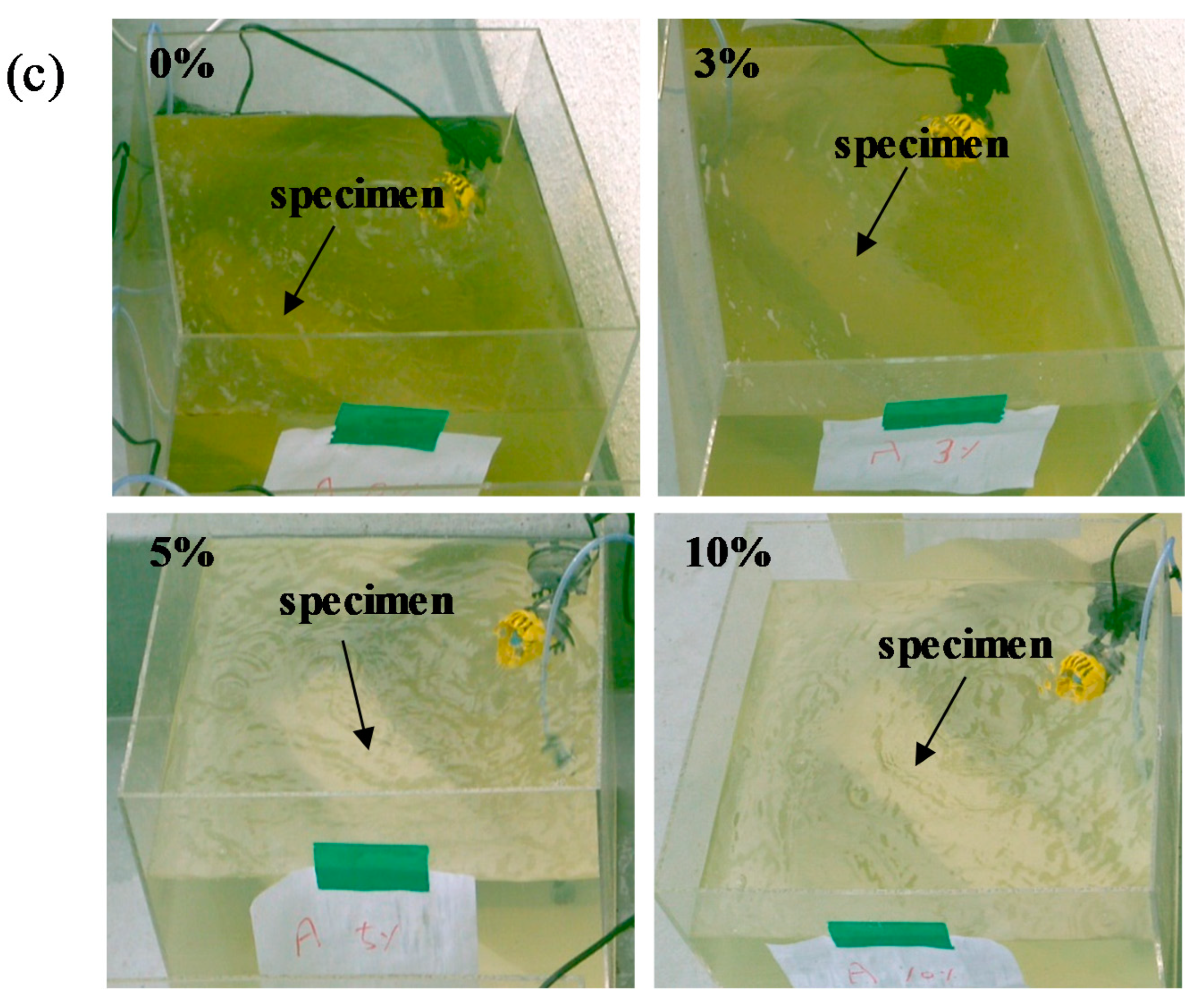
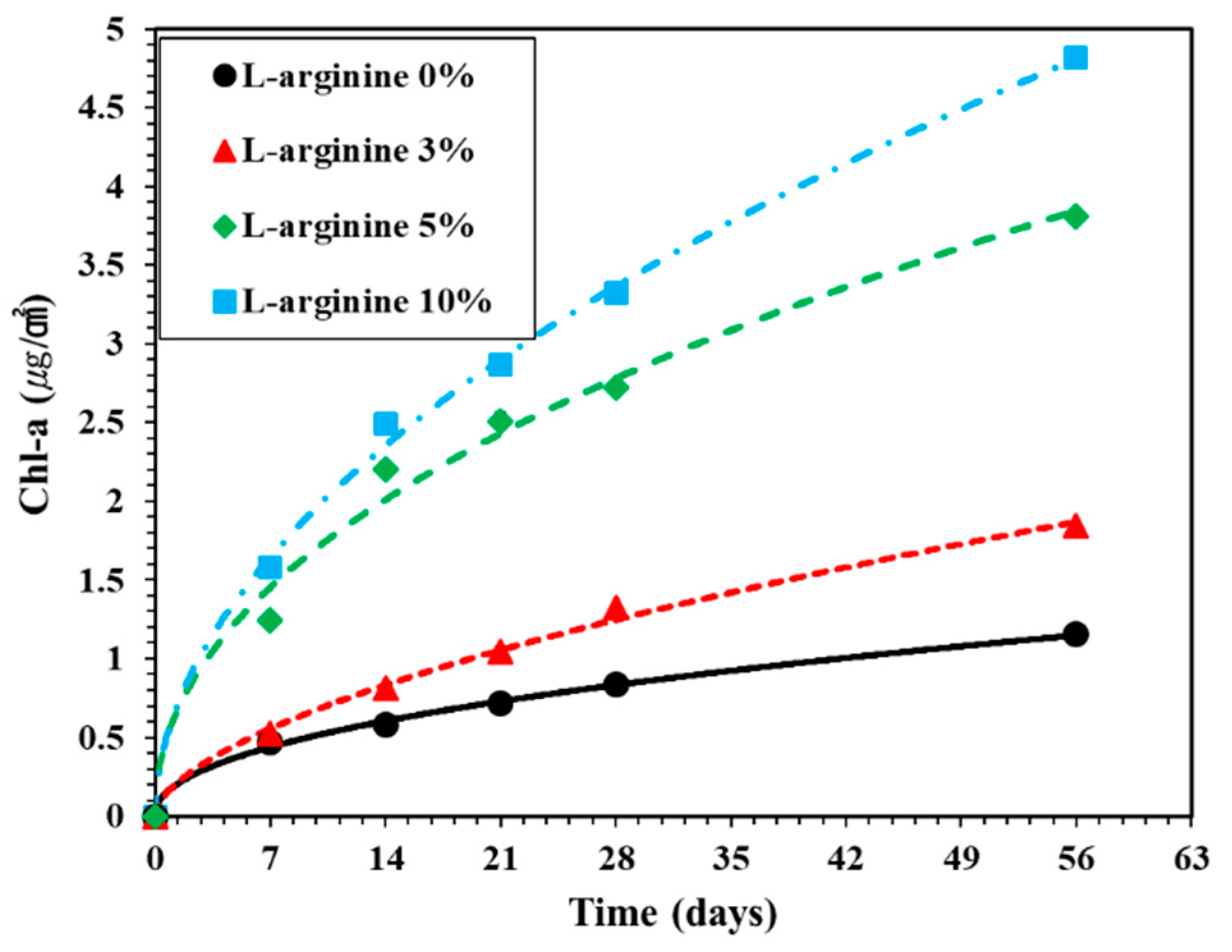
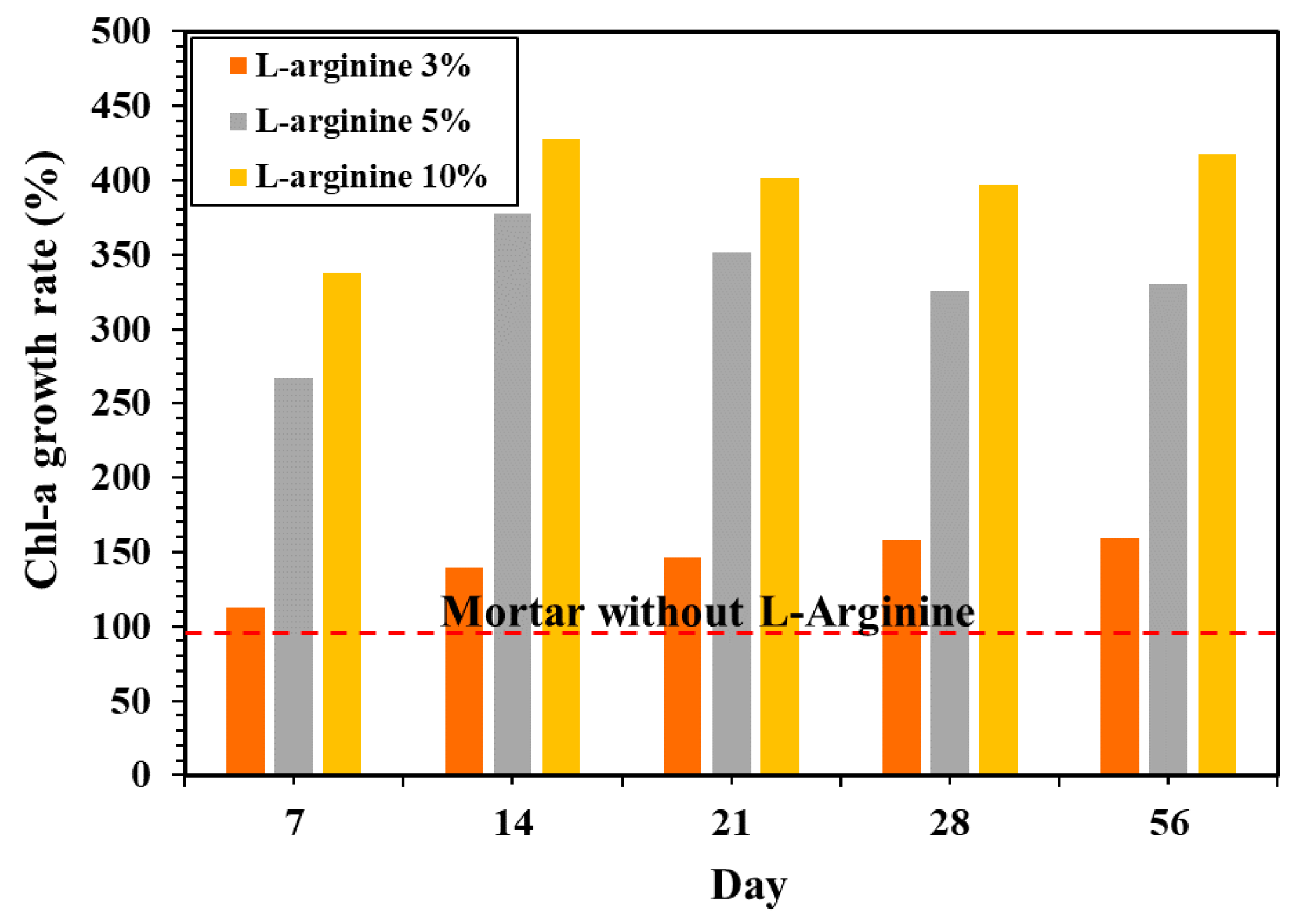
3.3.4. Growth of Chlorophyll-A on Mortar Surface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firth, L.B.; Knights, A.M.; Thompson, R.C.; Mieszkowska, N.; Bridger, D.; Evans, A.J.; Moore, P.J.; O’Connor, N.E.; Sheehan, E.V.; Hawkins, S.J. Ocean sprawl: Challenges and opportunities for biodiversity management in a changing world. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 193–269. [Google Scholar]
- Aguilera, M.A.; Broitman, B.R.; Thiel, M. Spatial variability in community composition on a granite breakwater versus natural rocky shores: Lack of microhabitats suppresses intertidal biodiversity. Mar. Pollut. Bull. 2014, 87, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.G.; Bulleri, F. Intertidal seawalls–new features of landscape in intertidal environments. Landsc. Urban Plann. 2003, 62, 159–172. [Google Scholar] [CrossRef]
- Chapman, M.G. Paucity of mobile species on constructed seawalls: Effects of urbanization on biodiversity. Mar. Ecol. Prog. Ser. 2003, 264, 21–29. [Google Scholar] [CrossRef]
- Firth, L.B.; Thompson, R.C.; White, F.J.; Schofield, M.; Skov, M.W.; Hoggart, S.P.G.; Jackson, J.; Knights, A.M.; Hawkins, S.J. The importance of water-retaining features for biodiversity on artificial intertidal coastal defence structures. Divers. Distrib. 2013, 19, 1275–1283. [Google Scholar] [CrossRef]
- Moschella, P.S.; Abbiati, M.; Åberg, P.; Airoldi, L.; Anderson, J.M.; Bacchiocchi, F.; Bulleri, F.; Dinesen, G.E.; Frost, M.; Gacia, E.; et al. Low-crested coastaldefence structures as artificial habitats for marine life: Using ecological criteria in design. Coast. Eng. 2005, 52, 1053–1071. [Google Scholar] [CrossRef]
- McManus, R.S.; Archibald, N.; Comber, S.; Knights, A.M.; Thompson, R.C.; Firth, L.B. Partial replacement of cement for waste aggregates in concrete coastal and marine infrastructure: A foundation for ecological enhancement? Ecol. Eng. 2018, 120, 655–667. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Leaching behavior of major trace elements from concrete: Effect of fly ash and GGBS. Cem. Concr. Compos. 2015, 58, 129–139. [Google Scholar] [CrossRef]
- Warati, G.K.; Darwish, M.M.; Feyessa, F.F.; Ghebrab, T. Suitability of scoria as fine aggregate and its effect on the properties of concrete. Sustainability 2019, 11, 4647. [Google Scholar] [CrossRef]
- Amin, M.N.; Murtaza, T.; Shahzada, K.; Khan, K.; Adil, M. Pozzolanic potential and mechanical performance of wheat straw ash incorporated sustainable concrete. Sustainability 2019, 11, 519. [Google Scholar] [CrossRef]
- Ekström, T. Leaching of concrete: Experiments and modeling. In Division Building Materials; University of Lund: Lund, Sweden, 2001. [Google Scholar]
- Gaitero, J.J.; Campillo, I.; Guerrero, A. Reduction of the calcium leaching rate of cement paste by addition of silica nanoparticles. Cem. Concr. Res. 2008, 38, 1112–1118. [Google Scholar] [CrossRef]
- Ertelt, M.J.; Raith, M.; Eisinger, J.; Grosse, C.U.; Lieleg, O. Bacterial additives improve the water resistance of mortar. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Nosouhian, F.; Mostofinejad, D.; Hasheminejad, H. Concrete durability improvement in a sulfate environment using bacteria. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]
- Al-Salloum, Y.; Abbas, H.; Sheikh, Q.I.; Hadi, S.; Alsayed, S.; Almusallam, T. Effect of some biotic factors on microbially-induced calcite precipitation in cement mortar. Saudi J. Biol. Sci. 2017, 24, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X.; Özyurt, N. Improved strength and durability of fly ash-amended concrete by microbial calcite precipitation. Ecol. Eng. 2011, 37, 554–559. [Google Scholar] [CrossRef]
- Rose, W.C. The nutritional significance of the amino acids. Physiol. Rev. 1938, 18, 109–136. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudli ´nska-Chylak, A.; Czeczotko, M.; Rejman, K. Food products as sources of protein and amino acids—the case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef]
- Chen, Q.-F.; Huang, X.-Y.; Li, H.-Y.; Yang, L.-J.; Cui, Y.-S. Recent progress in perennial buckwheat development. Sustainability 2018, 10, 536. [Google Scholar] [CrossRef]
- Schier, H.E.; Eliot, K.A.; Herron, S.A.; Landfried, L.K.; Migicovsky, Z.; Rubin, M.J.; Miller, A.J. Comparative analysis of perennial and annual phaseolus seed nutrient concentrations. Sustainability 2019, 11, 2787. [Google Scholar] [CrossRef]
- Khaled, K.F.; Al-Mhyawi, S.R. Electrochemical and density function theory investigations of l-arginine as corrosion inhibitor for steel in 3.5% NaCl. Int. J. Electrochem. Sci. 2013, 8, 4055–4072. [Google Scholar]
- Gowri, S.; Sathiyabama, J.; Rajendran, S. Corrosion inhibition effect of carbon steel in sea water by l-arginine-Zn2+ system. Int. J. Chem. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Ootsuki, N.; Hirayama, S.; Miyazato, S.; Yokozeki, Y. Fundamental study on estimating of Ca leaching from mortar and the deterioration of mortar. J. Constr. Manag. Eng. JSCE 1999, 634, 293–302. [Google Scholar]
- Haga, K.; Toyohara, M.; Sutou, S.; Kaneko, M.; Kobayahi, Y.; Kozawa, T. Alteration of cement hydrate by dissolution, (I)alteration test of hydrate cement paste by water-permeation using centrifugal force. Trans. At. Energy Soc. Jpn. 2002, 1, 20–29. [Google Scholar] [CrossRef]
- Hartwich, P.; Vollpracht, A. Influence of leachate composition on the leaching behaviour of concrete. Cem. Concr. Res. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A.; Sloot, H.A. pH dependent leaching characterization of major and trace elements from fly ash and metakaolin geopolymers. Cem. Concr. Res. 2019, 125, 1–14. [Google Scholar] [CrossRef]
- ASTM C150. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM C33. Standard Specification for Concrete Aggregates; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C1437. Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM C230. Standard Specification for Flow Table for Use in Tests of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM C185. Standard Test Method for Air Content of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM C109. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Rowell, D.L.; Vo-Dinh, T.; Gauglitz, G. Chemical and Biological Sensing Technologies Ii, Soil Science: Methods and Applications, Soil Acidity and Alkalinity; Longman Scientific and Technical: Harlow, UK, 1996; Volume 3, pp. 153–174. [Google Scholar]
- ASTM D4972-13. Standard Test Method for pH of Soils; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Behnood, A.; Tittelboom, K.V.; Belie, N.D. Methods for measuring pH in concrete: A review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- ASTM D8083. Standard Test Method for Total Nitrogen, and Total Kjeldahl Nitrogen (TKN) by Calculation in Water by High Temperature Catalytic Combustion and Chemiluminescence Detection; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM D3731. Standard Practices for Measurement of Chlorophyll Content of Algae in Surface Waters; ASTM International: West Conshohocken, PA, USA, 1987. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Behbahani, A.E.; Soltanzadeh, F.; Jomeh, M.E.; Zadeh, Z.S. Sustainable approaches for developing concrete and mortar using waste seashell. Eur. J. Environ. Civ. Eng. 2019. [Google Scholar] [CrossRef]
- Hellier, M.D.; Thirumalai, C.; Holdsworth, C.D. The effect of amino acids and dipeptides on sodium and water absorption in man. Gut 1973, 14, 41–45. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Wingertzahn, M.A.; Teichberg, S. L-arginine in low concentration improves rat intestinal water and sodium absorption from oral rehydration solutions. Gut 1997, 40, 602–607. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants–do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Negim, E.S.; Kozhamzharova, L.; Khatib, J.; Bekbayeva, L.; Williams, C. Effects of surfactants on the properties of mortar containing styrene/methacrylate superplasticizer. Sci. World J. 2014, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sylvie, C.-A.; Isabelle, C. Foams: Structure and Dynamics; Cantat, I., Cohen-Addad, S., Elias, F., Graner, F., Hhler, R., Pitois, O., Rouyer, F., Saint-Jalmes, A., Cox, S., Eds.; Oxford University Press: Walton Street, UK, 2013. [Google Scholar]
- Du, L.; Folliard, K. Mechanisms of air-entrainment in concrete. Cem. Concr. Res. 2005, 351, 463–1471. [Google Scholar] [CrossRef]
- Shah, S.P.; Choi, S.; Janse, D.C. Strain softening of concrete in compression. Proc. Fract. Mech. Concr. Struct. 1996, 3, 1827–1841. [Google Scholar]
- Zheng, X.; Ji, T.; Easa, S.M.; Ye, Y. Evaluating feasibility of using sea water curing for green artificial reef Concrete. Constr. Build. Mater. 2018, 187, 545–552. [Google Scholar] [CrossRef]
- Chen, C.; Ji, T.; Zhuang, Y.; Lin, X. Workability, mechanical properties and affinity of artificial reef concrete. Constr. Build. Mater. 2015, 98, 227–236. [Google Scholar] [CrossRef]
- Jones, M.R.; Macphee, D.E.; Chudek, J.A.; Hunter, G.; Lannergrand, R. Studies using 27 Al MAS NMR of AFm and AFt phases and the formation of Friedel’s salt. Cem. Concr. Res. 2003, 33, 177–182. [Google Scholar] [CrossRef]
- Suryavanshi, A.J.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, T.; Lin, X.; Chen, C.; Yang, Z. Biogenic sulfuric acid corrosion resistance of new artificial reef concrete. Constr. Build. Mater. 2018, 158, 33–41. [Google Scholar] [CrossRef]
- Ramesh, K.G.B.; Kesavan, V. A review analysis of cement concrete strength using sea water. Mater. Today Proc. 2020, 22, 983–986. [Google Scholar] [CrossRef]
- Sagi, A.A.; Moreno, E.I.; Andrade, C. Evolution of pH during in-situ leaching in small concrete cavities. Cem. Concr. Res. 1997, 27, 1747–1759. [Google Scholar]



| Division | Properties |
|---|---|
| Formula | C6H14N4O2 |
| Molecular weight | 174.20 g/mol |
| pH | 12.0 at 25 °C |
| Melting point | 222 °C |
| Water solubility | 87.1 g/L at 20 °C - completely soluble |
| Chemical Composition | Cement (wt.%) |
|---|---|
| SiO2 | 23.20 |
| Al2O3 | 5.03 |
| Fe2O3 | 2.92 |
| CaO | 62.40 |
| MgO | 2.07 |
| SO3 | 2.34 |
| K2O | 0.59 |
| Na2O | 0.26 |
| Loss of ignition | 1.19 |
| W/B | L-Arginine (%) | Mix Composition (kg/m3) | |||
|---|---|---|---|---|---|
| Water | Cement | Sand | L-Arginine | ||
| 0.5 | 0 | 255 | 510 | 1530 | 0 |
| 3 | 494.7 | 15.3 | |||
| 5 | 484.5 | 25.5 | |||
| 10 | 459 | 51 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-M.; Myung, N.V.; Lee, H.-S.; Singh, J.K. L-Arginine-Incorporated Cement Mortar as Sustainable Artificial Reefs. Sustainability 2020, 12, 6346. https://doi.org/10.3390/su12166346
Yang H-M, Myung NV, Lee H-S, Singh JK. L-Arginine-Incorporated Cement Mortar as Sustainable Artificial Reefs. Sustainability. 2020; 12(16):6346. https://doi.org/10.3390/su12166346
Chicago/Turabian StyleYang, Hyun-Min, Nosang V. Myung, Han-Seung Lee, and Jitendra Kumar Singh. 2020. "L-Arginine-Incorporated Cement Mortar as Sustainable Artificial Reefs" Sustainability 12, no. 16: 6346. https://doi.org/10.3390/su12166346
APA StyleYang, H.-M., Myung, N. V., Lee, H.-S., & Singh, J. K. (2020). L-Arginine-Incorporated Cement Mortar as Sustainable Artificial Reefs. Sustainability, 12(16), 6346. https://doi.org/10.3390/su12166346








