Abstract
Sludge from carwash wastewater treatment plants has been evaluated as substitute for lime paste, as well as its behavior in cement mortars. Dry sludge waste was used with (CSlud) and without (USlud) pretreatment and have been characterized. The pastes were prepared with weight replacement of 5, 10, 15, and 20% of sludge. The formation of calcium silicate hydrate was determined by TGA, both in lime and cement pastes. The compressive strength properties were evaluated in mortars. It was found the mixtures which present the best results were those of 5 and 10% for USlud, and 10 and 20% for CSlud.
1. Introduction
The carwash industry requires high amount of fresh water to effectively remove pollutants. It is estimated that an average of 150–600 l of wastewater are generated for each washing process, depending on the car washing facilities and the size of the car [1,2]. Among the pollutants most found in wastewater of this type are free oil-grease, oil/water emulsion, sand-dust, salts, surfactants, and hydraulic fluid being discharged to water channels or to domestic wastewater treatment plants in developing countries like Turkey, Colombia and Brazil, among others [1]. Different treatments have been applied to this type of water, such as the membrane process, flocculation, adsorption, flotation, chemical coagulation, membrane bioreactor, electrocoagulation, and chemical oxidation [1,2,3,4,5,6,7]. The studies found in the literature have focused on the treatment of wastewater, while in the case of sludge, no valorization and/or recovery has been reported.
The most common treatment for this type of waste is incineration, which is used due to the high content of pollutant present in the wastewater sludge. Energy has been obtained from this process; the sludge has been dried (humidity less than 20%) and afterwards it is taken to a gasifier through the combustion of synthesis gas in a cogeneration engine [8]. The most widely used treatments is co-combustion, which is normally done with coal, and emissions have been found to be well below environmental limits [9,10]. The main product of the combustion process is fly ash, which is estimated to be 80% of the total ash produced. The properties of fly ash can vary and differ greatly on combustion method, raw material origin, type of emission control systems, particle size, and storage methods, among others [11,12,13,14]. Likewise, its fine texture and its high chemical reactivity favors reactions with other compounds that may be present in the medium and some authors have shown that these can contaminate the soils and groundwater by leachates produced by their inadequate disposal [15,16,17].
According to some authors, fly ash is characterized by a combination of crystalline and amorphous phases, which have low density, high electrical conductivity, high specific surface area, low cation exchange capacity, and high water retention capacity [12,13,15]. For this reason, the development of technologies that contribute to remove or reduce the risk of fly ash has been recommended [18]. Within these technologies are low-temperature thermal degradation [19], microwave degradation [20], extraction-UV degradation [21], and ball-milling mechanochemical degradation [22]. However, many of these fly ashes contain compounds such as silicon and aluminum, which have been found to be highly reactive in both lime and cement pastes, and then widely used as a replacement and/or additive in concrete [23,24,25,26]. Therefore, the use of these as a replacement for cement has been increasing in recent decades, with the growth of waste combustion systems in the world.
Concrete is the most produced and used construction material in the world, because of its cost-effectiveness and resistance properties. Despite this, its production generates high environmental impacts, contributing 5% of global CO2 emissions [27]. This is due to the incineration of limestone, where extreme temperatures (up to 1400 °C) are required, generating high levels of energy dispersion. The excessive use of energy in this production and the amount of CO2 released has presented the requirement of finding ecological substitutes for cement in recent decades, to reduce the greenhouse effect emissions currently generated [28,29]. Furthermore, using additions in the cement industry also satisfies different technical as well as environmental reasons. The mixes are based on the easiness on chemically reacting the released lime with the cement during the hydration process, forming stable cement products over time which are permanently insoluble in water and resistant to different aggressive exposures [30,31,32].
According to Vashistha et al. [33], in recent years, different attempts have been made to increase the incorporation of alternative materials, but there are still deficiencies in the type of ash to be used for taking advantage on its greater potential. This expansion is due to the technical and environmental advantages that these mixtures have produced compared to conventional Portland cement, and it is expected that more materials will be tested and will continue to rise over time [34,35]. Despite this, the global availability of fly ash is approximately 800 million tons [36], considering that in developing countries there are few alternatives for waste incineration treatment. Hence, this amount could increase in the coming years according to the conversion carried out in those countries.
This paper presents a study of the use of carwash sludge as a replacement in lime pastes, through reactivity at different curing ages. The mixtures which present the major results from the previous analysis were used for the manufacture of mortars. Reactivity and compressive strength tests were performed to determine the impact on the addition of these fly ash from carwash in the reactivity and mechanical properties.
2. Materials and Methods
The sludge used in this project was obtained from FERRAUTOS carwash company located in the city of Bogotá. NaOH was acquired from MATERQUIM S.A.S (Bogotá, Colombia) and the cement from ARGOS (Medellín, Colombia). The sludge was dried at 105 °C in a forced convection oven for 24 h. Subsequently, it were crushed and sieved with a 60 μm mesh (Figure 1). Part of this material was calcined at 650 °C in a muffle for 4 h. In this way, two material samples were obtained, calcined sludge (CSlud) and uncalcined sludge (USlud).

Figure 1.
Untreated and treated sludge from MATERQUIM S.A.S. (a) Untreated sludge and (b) calcined sludge.
2.1. Chemical Composition of the Raw Material
The chemical composition of the sludge samples was analyzed by X-ray fluorescence (XRF) in a ZSX Primus Rigaku® (Tokyo, Japan) spectrophotometer (Table 1). Both sludge samples were classified as F-type fly ash or silico-aluminous ash according to the ASTM-C618 standard, because the total content of SiO2, Al2O3, and Fe2O3 added up to more than 70% in both cases, 85.26% for dry sludge (CSlud) and 86.49% for calcined sludge (USlud). The content of CaO and SO3 was less than 9% for CSlud and 10% for USlud. This type of ash, having a low lime content and a combination of silica, aluminum, and iron, may have binding properties in the presence of pozzolanic activators (lime or cement). In addition, the ashes are also hydrophilic and have pozzolanic properties, which allows them to have excellent durability, low permeability to chloride ions, and does not present adverse expansion when highly reactive aggregates are incorporated into the concrete [37]. Prevention of chloride seizure is mainly due to the presence of aluminum. Alumina and chlorine combine chemically to form the well-known Friedel salt. This prevents chlorine from penetrating into the structure; although chloride ions do not present a hazard to the concrete, they can corrode the reinforcing steel [31,38,39].

Table 1.
Chemical composition of the sludge according to XRF results.
Metals and elemental composition tests were also carried out in both sludges. Metal analysis was carried out using inductively coupled plasma optical emission spectrometry (ICP-OES) in an ICP-OES Thermo Scientific™ ICAP6500 DUO kit (Thermo Scientific, Waltham, MA, USA) equipment (Table 2). The elemental analysis for the quantification of N, C, S, H, and O was carried out following the ASTM-D5373-16 standard on the Elementar Vario Macro CHNS® (Elementar, Langenselbold, Germany). According to the test, the samples would be considered hazardous for disposal in sanitary landfills due to high aluminum and calcium content. It was observed that with the calcination process the sample CSlud had little carbon content, which indicates that it was an inert sample. Likewise, it was observed that high temperatures produce reactions in the sludge and support the increase in the concentration of Al and Fe, while elements such as Pb and Ni decrease their concentration as a result of high temperature.

Table 2.
Analysis of metals and elemental composition of the treated sludge.
2.2. Lime Pastes Preparation
The pastes were prepared according to the ASTM-C305 standard, using a water/lime mass ratio of 0.5, making lime weight substitutions of 5, 10, 15, and 20% with each of the sludge samples. The pastes were subjected to a curing process at 25 °C for 56 days, conversing the humidity of the sample. Samples of 10 g were taken from the pastes on days 1, 3, 7, 14, 28, and 56. Hydration processes were stopped with acetone, and the samples were dried at 60 °C for 1 h to guarantee water evaporation. Finally, a thermogravimetric analysis (TGA) was carried out with the TGA-5500 Discovery equipment (TA Instruments, New Castle, DE, USA). A temperature ramp between 30 and 600 °C with 10 °C min-1 heating rate and N2 as an inert atmosphere were used as working conditions.
2.3. Cement Mortars Preparation
From the major results of the reactivity tests with lime and in accordance with the TGA analysis carried out on the lime pastes at 28 days, the substitutions of each sample of sludge were selected. These presented high reactivity conditions for the preparation of the mortars. The mortars have been prepared according to the ASTM-C305 standard, using a mass water/cement ratio of 0.5 and molds one inch in diameter by two inches long. The samples underwent a curing process at 25 °C for 56 days. During this process, three mortars of each substitution were subjected to compressive strength tests in accordance with the regulations, on days 1, 7, 14, 28 and 56. These were carried out on a universal test press to determine the compressive strength. After the tests, 10 g of the remaining sample of the specimen were taken, and its hydration processes were stopped with acetone. Additionally, it was dried at 60 °C for 1 h to guarantee the evaporation of the water. This final sample was stored in a desiccator. Finally, a TGA analysis was accomplished on the TGA-5500 Discovery equipment (TA Instruments, New Castle, DE, USA), with the same conditions as previously mentioned (Section 2.2). This was done to check the formation of important gels in the process of curing and hydrating concrete.
3. Results and Discussion
3.1. Evaluation of the Reactivity of the Sludge/Lime Mixtures
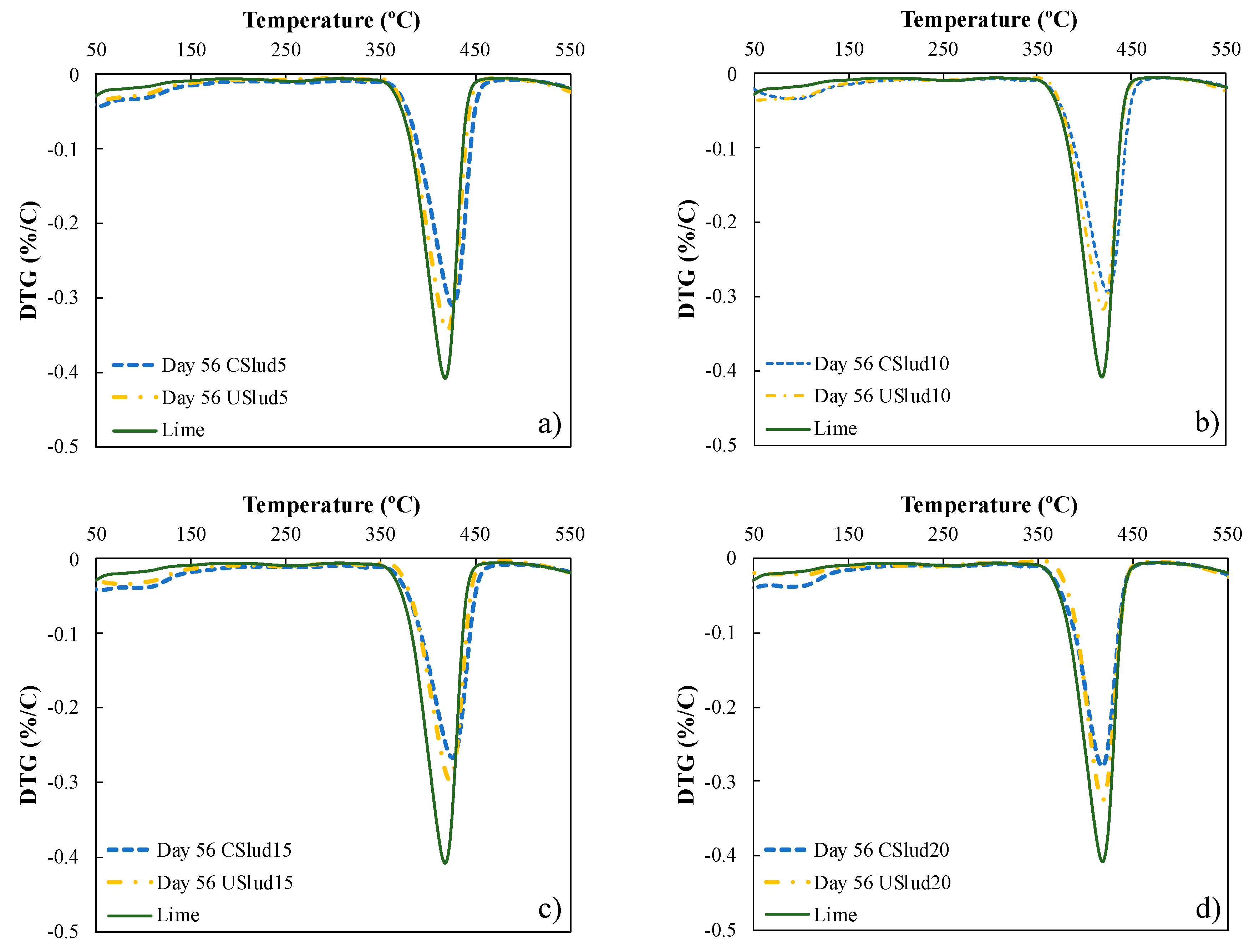
Figure 2 shows the evolution of the hydration process of lime pastes from both CSlud and USlud at 56 day of hydration compared to lime as a control. The transformation of portlandite (peak between 350 and 450 °C) to one of the phases of C-S-H gels (peak between 50 to 100 °C) is evident, for the two treatments carried out. However, the 5 and 10% substitutions for USlud and the 10 and 20% substitutions for CSlud presented higher peaks of decomposition of C-S-H gels. Likewise, when comparing each substitution on day 56 of hydration with respect to lime, it is observed that lime does not show peaks in the range between 50 and 100 °C. These peaks are clearer as the curing age passes and are due to the consumption of portlandite.
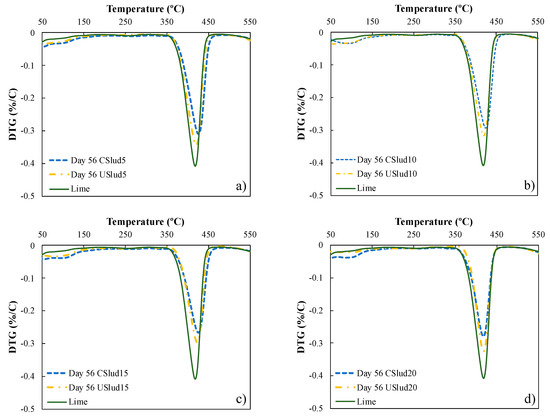
Figure 2.
Thermogravimetric analysis for samples at day 56 of hydration, (a) 5% substitution, (b) 10% substitution, (c) 15% substitution; and (d) 20% substitution.
The formation of C-S-H gels and not only portlandite is the ideal behavior in the hydration process, since in this way the compressive strength of any material associated with cement is presented [39].
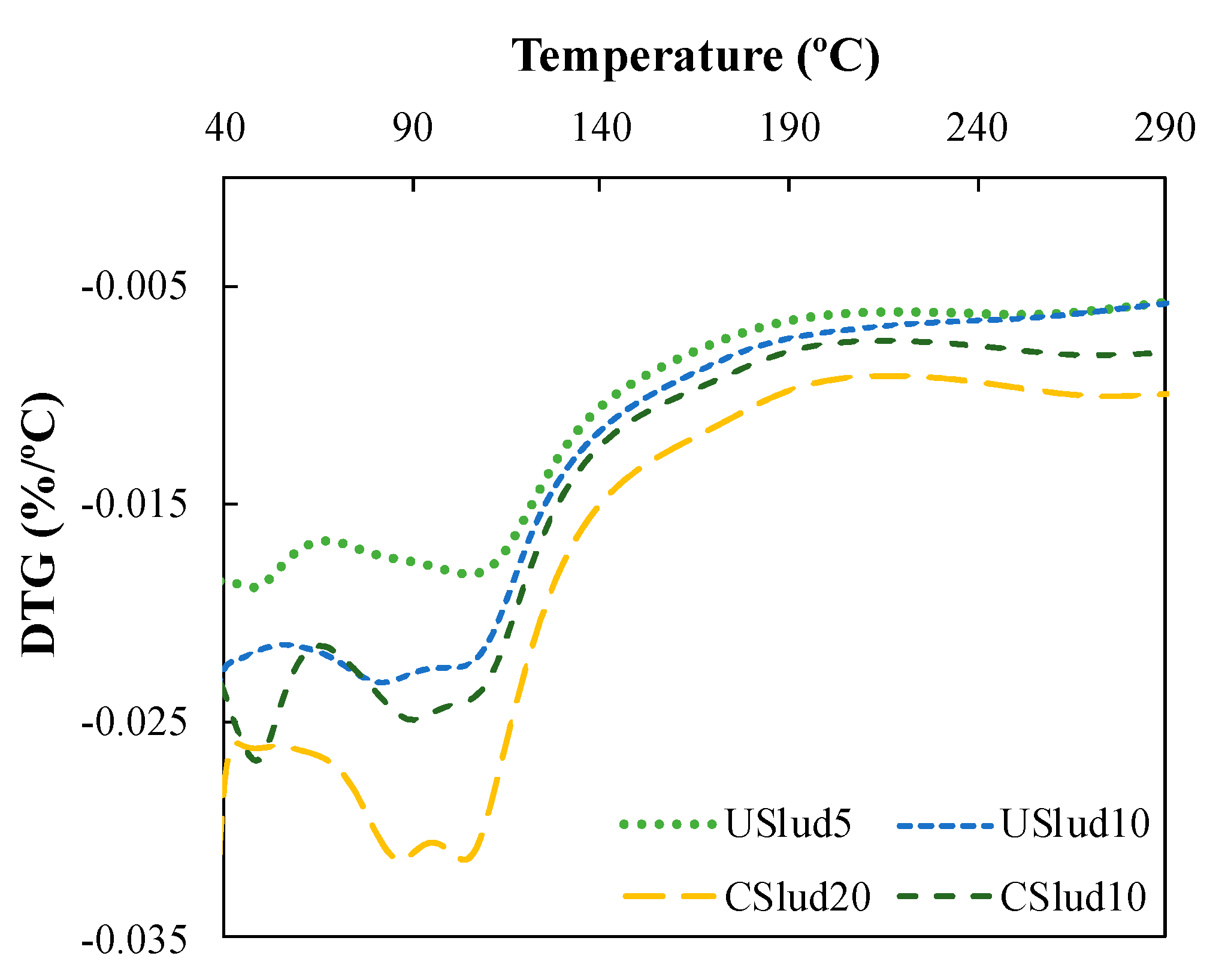
A hi-res TGA analysis was performed on the samples that showed the major reactivity according to the TGA (Figure 1); these were the 5 and 10% substitutions for USlud and the 10 and 20% substitutions for CSlud. The lowest replacements with both USlud and CSlud presented lower peaks between 40 to 50 °C which can be related to a constant amount of water within the sample and could be associated with the water adsorbed by the sample (Figure 3). On the other hand, there is a second peak between 75 to 110 °C, which could be related to the amount of water that increases with the curing time and in this temperature range. These hydrated phases could presumably be C-S-H gel [38,40]. Consequently, the mixtures are promising as a substitute for low proportion cement uses in low-performance manufacture inputs in construction. Similarly, this behavior is appropriate according to the characterization carried out on the sludge, where there is an F type fly ash, which is suitable for this type of replacement.
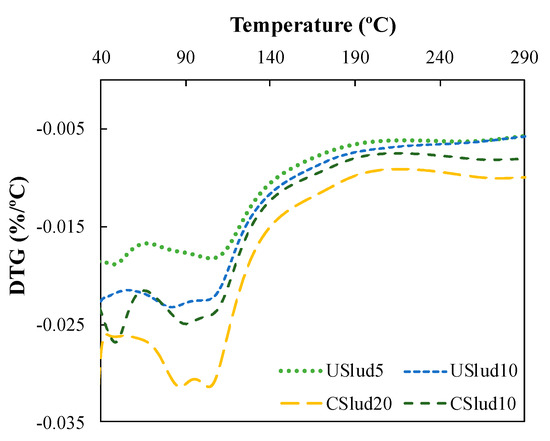
Figure 3.
Hi-res TGA analysis for the best replacements on 28 day.
Accordingly, the following mixtures have been taken to be used in the manufacture of the cement mortars. In the case of USlud, replacements of 5 and 10% have been used, while for CSlud, 10 and 20% of the cement have been replaced. Figure 2 shows that for USlud treatments the behavior was very similar to those reported in the literature and that as the ash/lime ratio increased, the reactivity was reduced and the formation of these C-S-H gels was not encouraged [41,42,43]. For the tests carried out with CSlud, different behavior occurred. It was found that the best mixtures were produced with the 10 and 20% substitutions, whereas for the 5 and 15% substitutions unfavorable results were presented.
Although all the evaluated samples were characterized by presenting adequate reactivity in the lime pastes, it was observed that the replacements with calcined sludge presented a clearer formation of the C-S-H gels, with the 20:80 ash/lime ratio being the one with the best conditions. Therefore, these four relations with cement mixes have been evaluated.
3.2. Sludge Reactivity in Cement from TGA Analysis
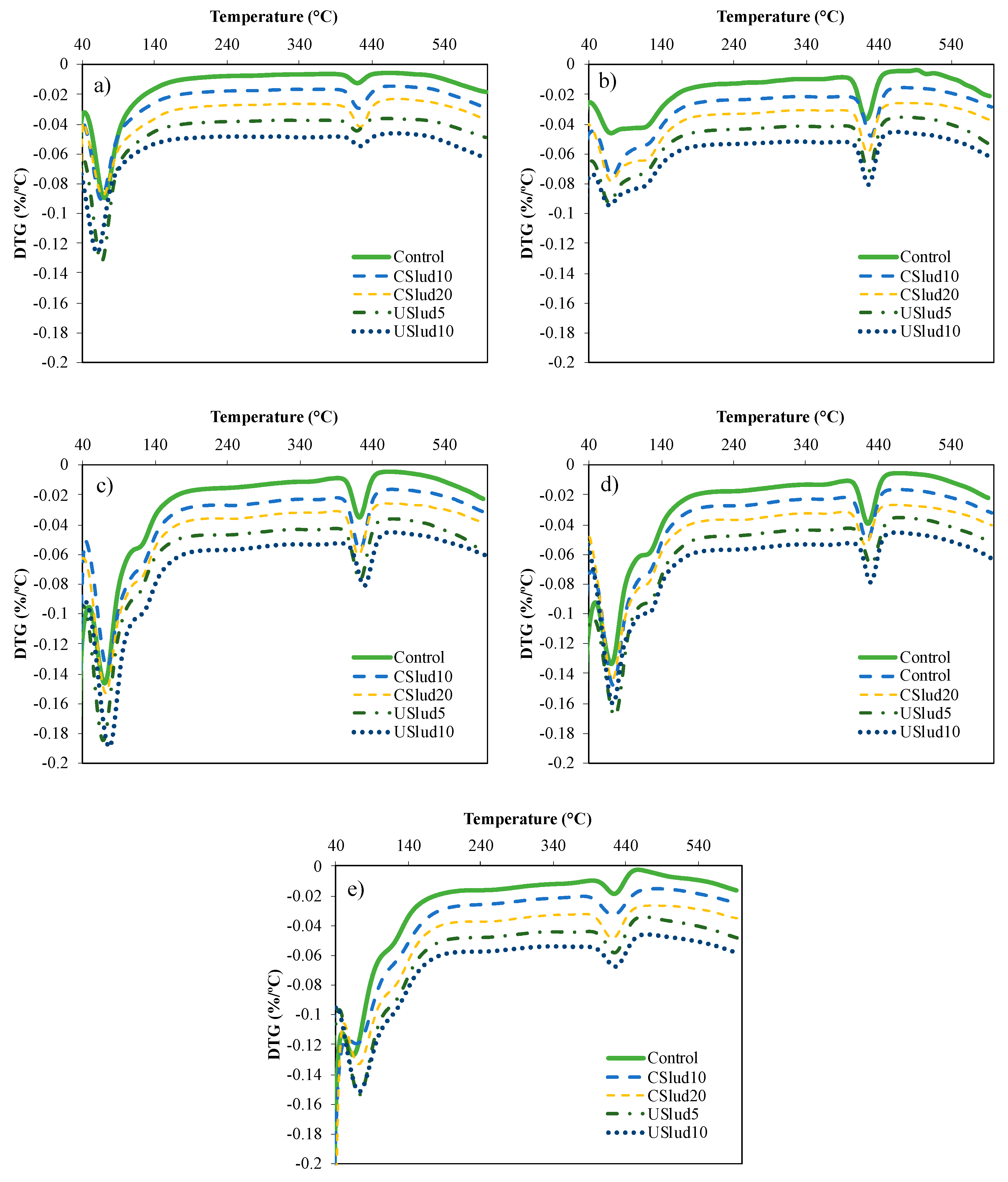
From the results with the lime pastes, the cement mortars were prepared with substitution of 5 and 10% for USlud and 10 and 20% for CSlud, in addition to the preparation of a control with only cement. As can be seen in Figure 4, in the cement control mixture, there was formation of portlandite and its respective transformation in some phase of C-S-H gels from 1 day. After 7 days it is possible to appreciate the formation of C-A-S-H gels (peak between 50 and 150 °C). This behavior is similar to all the tests carried out, observing the formation of very pronounced peaks in this temperature range.
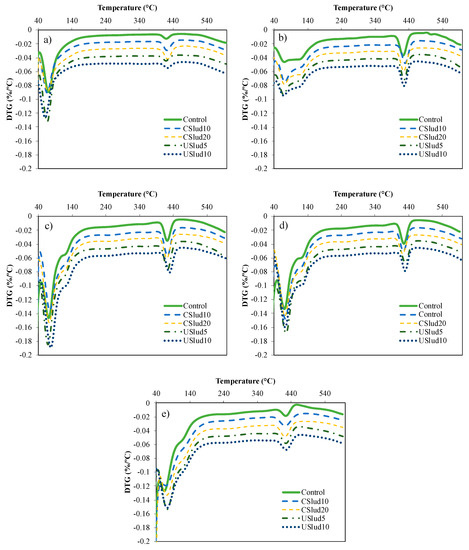
Figure 4.
Reactivity assessment by TGA analysis for the selected ratios. (a) 1st day, (b) 7th day, (c) 14th day, (d) 28th day, and (e) 56th day.
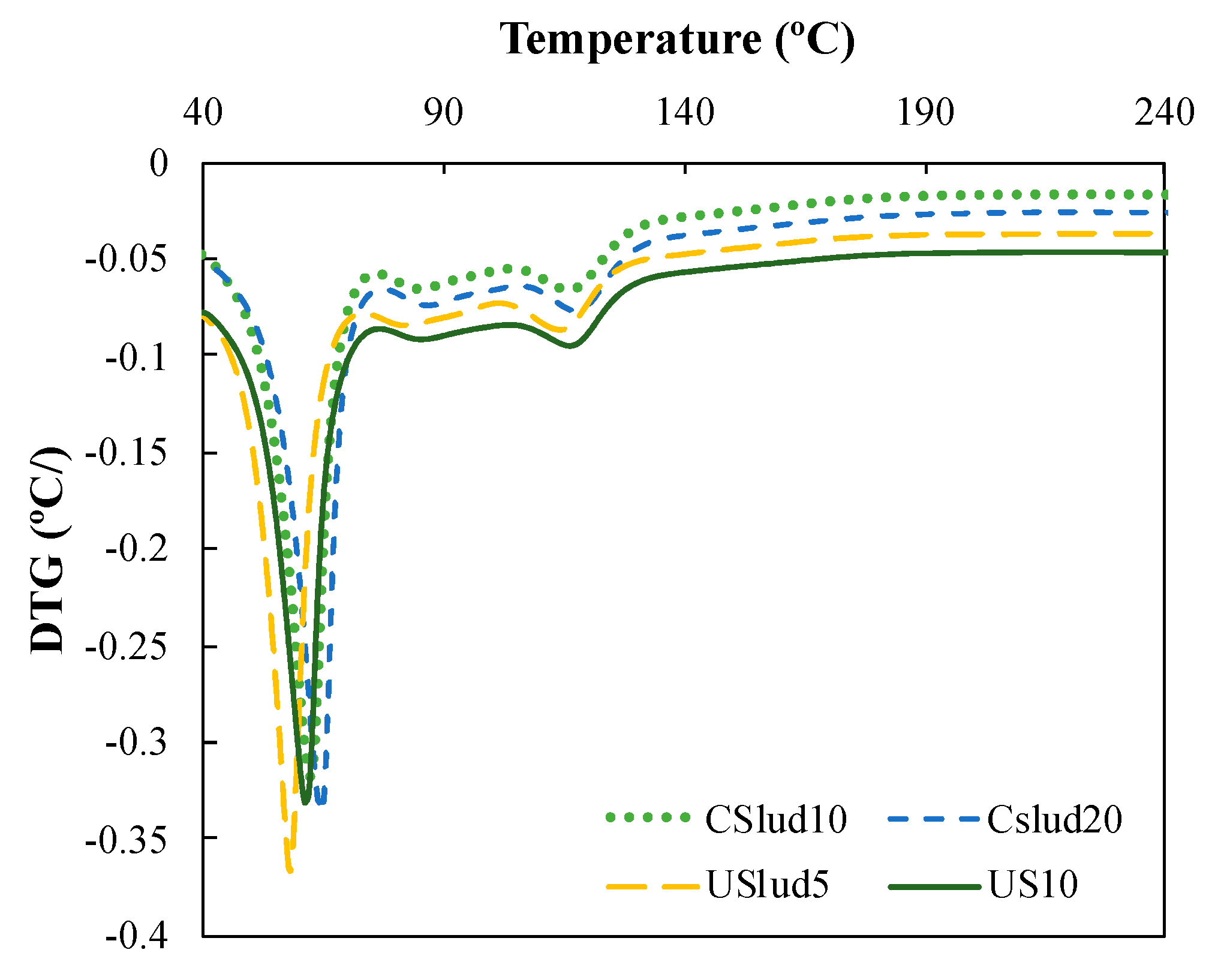
Figure 5 shows a hi-res TGA analysis, and the formation of three peaks can be observed: the first one is notoriously pronounced close to 70 °C, the second peak very low around 90 °C and the third peak near to 130 °C. These are associated with the C-S-H gels evidenced in the lime paste mixtures. The first loss of weight that is evident in both Figure 4 and Figure 5 is the result of dehydration reactions of various hydrates such as C-S-H gels, carboluminates, ettringite, etc. However, this loss is mainly due to the dehydration of C-S-H and C-A-S-H [44,45,46,47].
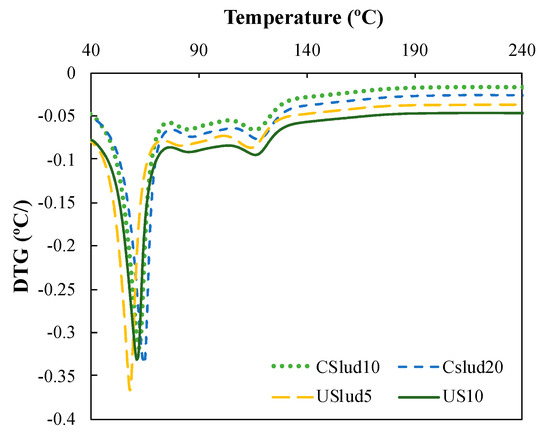
Figure 5.
Hi-res TGA analysis of CSlud10, CSlud20, USlud5, USlud10 substitutions on day 28.
The second peak of weight loss is observed between 350 and 500 °C, which corresponds to the dehydration of portlandite, another hydration product [44,45,47,48]. It can be seen in the four replacements made that the beginning and end of the decomposition of Ca(OH)2 varies, and according to some authors this is due the reaction enthalpy, which has been associated [46]. This enthalpy has been associated by some authors with the hydration time (curing age), type and amount of cement, and mineral addition used in the paste, as well as the water/cement ratio of the mixture [45,46,49,50,51].
Considering the water/lime mass ratio would vary between 0.3 and 0.7 generally, it is relevant to emphasize that if this ratio is sufficient, the hydration process progresses until all the cement is consumed. However, if the ratio is low, the reaction would be incomplete, leaving many of the cement particle unreacted [39]. This is due to the fact that it has been found that the workability of concrete is widely determined by the properties of fresh pastes, especially the rheology of the pastes, and the morphological and geometrical properties of the agglomerated structure [52,53,54,55]. Therefore, it would be appropriate to study this factor in new works to determine if the ratio used (0.5) in both lime pastes and cement pastes is appropriate.
3.3. Compressive Strength Tests
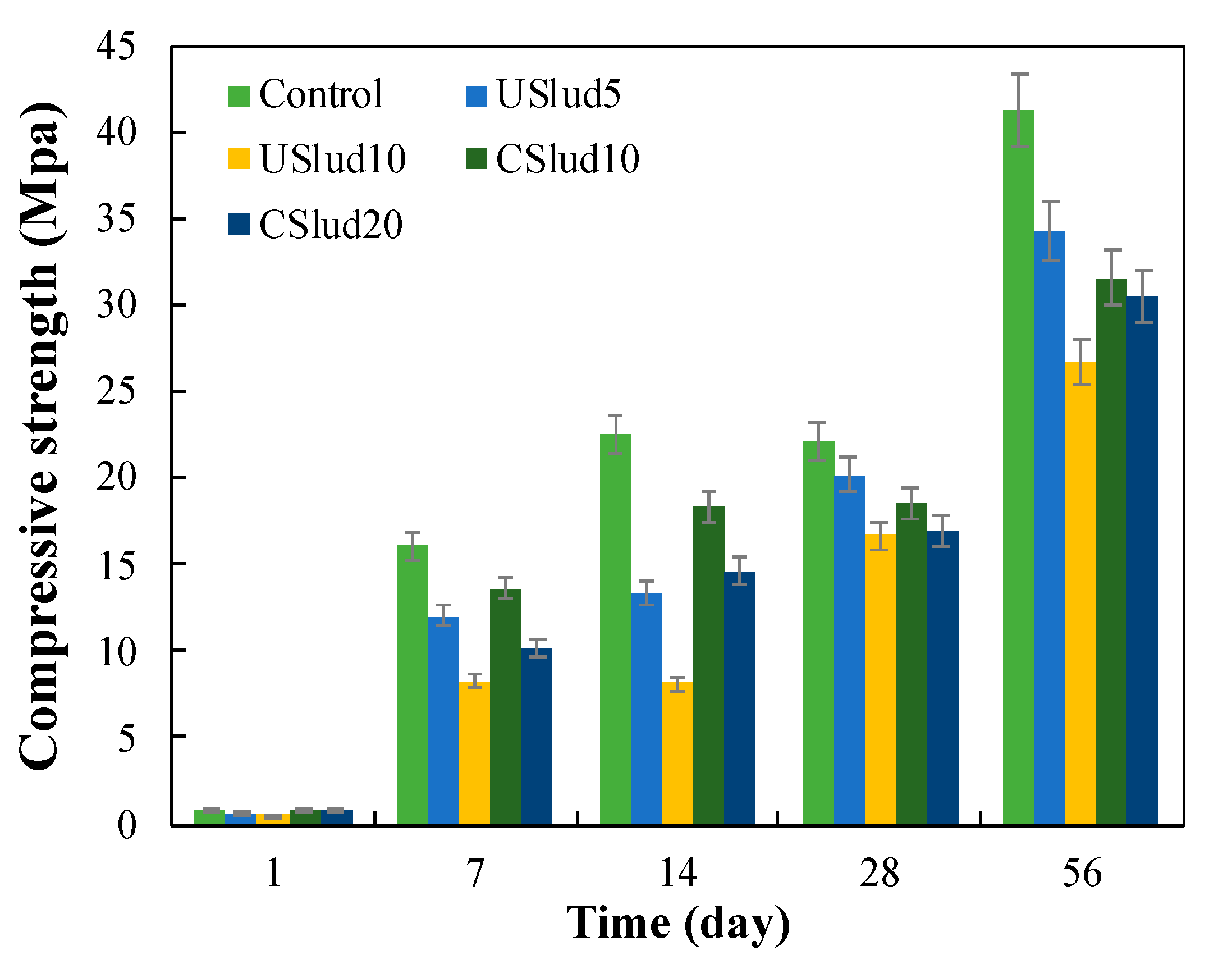
It was evidenced in all the tests carried out on compressive strength that this property increased over time (Figure 6), which is consistent with the results of TGA analysis and the formation of C-S-H gels from the first and seventh day of hydration (Figure 4). It was observed, as on the 56th day of hydration, all the treatments showed an increase in compressive strength according to the standard of the 28th day (ASTM-C105), but this indicates that these mixtures cannot be used for structural concrete, they must be used as low-performance inputs in construction or rendering mortars [30,31].
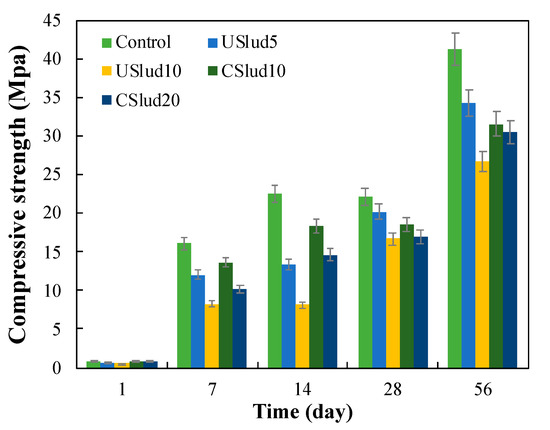
Figure 6.
Compressive strength of the ash-cement mixtures based on age.
Moreover, it is possible to say that the control treatments, USlud5 and CSlud10, have fulfilled with the compressive strength standards established for Type 4 cement for the 28th day of hydration according to the ASTM-C150 standard. These mixtures reached a compressive strength of 22.24, 20.22 and 18.60 MPa respectively, and the minimum compressive strength allowed for this class is 17.5 MPa. This type of cement is characterized by having a low heat of hydration and its chemical composition is mainly given by C3S and C2S [31,39,40]. On the other hand, only the cement control met the minimum compressive strength required for Type 5 cement (21 MPa). None of the treatments carried out fulfilled the required standard for type 1 cement. Type 5 cement is characterized by high resistance to sulfates. Its chemical composition has a contribution of C3A and C4AF even less than Type 4 cement [31,40]. The mixtures made with USlud10 and CSlud20 substitutions do not meet any type of standard, since they presented an average compressive strength of 16.72 and 16.98 MPa respectively, on the 28th day of testing.
After 56 days of curing, it was observed that all the evaluated treatments had a compressive strength higher than 25 MPa. Therefore, these replacements could be used as a substitute for cement, for the manufacture of low-performance inputs, because the acquisition of compressive strength is slower, and more days of cure are needed (above 28 days) due to C-S-H gel formation being lower compared to cement control.
3.4. Techno-Economic Analysis
Due to the composition of this type of sludge, it must be treated as a hazardous waste. Disposal and/or treatment is then expensive. Currently the cost per disposal of 1m3 of this type of waste is approximately $80, making it expensive, since there is a large amount of this type of waste. The production of this type of waste in Colombia is high, with an approximate fleet of seven million (motorcycles, cars, trucks, pick-ups, among others). Therefore, the generation of this type of sludge at present and in the coming years will be on the increase, with which its disposal will be highly expensive, if other forms of valorization and/or recovery are not found. The above will increase environmental impacts in water and soil. Likewise, the cost of 1 kg of cement in Colombia is $0.15, with all the environmental problems that its production generates, such as air pollution and its large CO2 contributions. These sludges after drying and without being incinerated can be used as a replacement in proportions of 5%. The use of this in the construction industry for the use of low-performance inputs implies an important advance in the recovery of this type of waste, which causes great problems in its disposal, involving high costs.
In studies on the production of mortars for the manufacture of low-performance inputs, other authors [56] have calculated that the cost of 1 tn of mortar with ashes from incineration plants can be around 29.46 Euro tn−1, while the price of only mortar in the market is 44 Euros tn−1. This shows that the decrease in price is more than 30%. Likewise, it is estimated that the consumption of cement excluding China worldwide for the year 2019 was 1.81 bnt [57]. With the addition of this type of sludge, it does not need prior treatment, such as incineration, which implies high costs both in its implementation and operation. The reduction in cement consumption and its environmental impacts would be very significant. However, it is necessary to continue with more detailed studies of this type and using mixtures of many types of fly ash, because in the market for waste and fly ash, there are many types of these that can be exploited.
With the results of this work, a contribution has been made to the circular economy and sustainable development. The recovery of this type of sludge that is generally disposed of in sanitary landfills would be valued (treated and untreated) for use as substitutes for cement in the manufacture of low-performance inputs and/or rendering mortars. With this, the entry of virgin raw materials such as limestone for the manufacture of cement and the production of waste is reduced, closing the loops or economic flows. With the analysis carried out, the costs of its implementation are quite interesting to be applied on a real scale. This type of sludge, according to the principles of the circular economy, is converted into nutrients, which are designed to be reintroduced into the production chain with high quality and are not disposed of again in the biosphere.
4. Conclusions
The reactivity of the sludge from a wastewater treatment plant from the carwash industry has been characterized and evaluated. Its properties and viability have been identified as cement substitutes in at least 5% with not calcined sludge and at least 10% with calcined sludge, giving them a specific use as an additive in the manufacture of low-performance inputs in construction and complying with the technical standards required for it. Therefore, the use of this material helps to reduce current environmental impacts caused by its inadequate disposal, both in open dumps and in sanitary landfills. Likewise, it was found that both calcined and not calcined sludge are highly reactive in the lowest substitutions. This is interesting since it is an industry which is growing in developing countries, and sanitary landfills are not suitable to receive this type of materials.
In the same way, with this work it is verified that the substitution of cement with this type of materials is appropriate because important parameters such as compressive strength are not compromised with replacements of up to 10%. According to the hydration reactions that occur, the formation of the C-S-H gels, important for increasing compressive strength, was verified by means of TGA. Finally, it is recommended to study different water/lime and/or water/cement ratios in order to determine their incidence on the hydration process.
Author Contributions
Conceptualization, J.F.S.; methodology, J.F.S.; validation, J.F.S., M.C.R.-F. and J.D.A.; formal analysis, M.C.R.-F. and J.F.S.; investigation, M.C.R.-F. and J.D.A.; resources, J.F.S.; data curation, M.C.R.-F., J.D.A. and J.F.S.; writing—original draft preparation, M.C.R.-F., J.F.S. and C.M.; writing—review and editing, J.F.S., M.C.R.-F. and C.M.; supervision, J.F.S.; project administration, J.F.S.; funding acquisition, J.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was carried out with financial support from the Department of Civil and Environmental Engineering at Universidad de los Andes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Firdaus, S. Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep. Purif. Technol. 2013, 104, 26–31. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Mahmood, Q.; Raja, I.A.; Malik, A.H.; Khan, M.S.; Wu, D. Chemical oxidation of carwash industry wastewater as an effort to decrease water pollution. Phys. Chem. Earth Parts ABC 2011, 36, 465–469. [Google Scholar] [CrossRef]
- Boussu, K.; Kindts, C.; Vandecasteele, C.; Van der Bruggen, B. Applicability of nanofiltration in the carwash industry. Sep. Purif. Technol. 2007, 54, 139–146. [Google Scholar] [CrossRef]
- Hamada, T.; Miyazaki, Y. Reuse of carwash water with a cellulose acetate ultrafiltration membrane aided by flocculation and activated carbon treatments. Desalination 2004, 169, 257–267. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Applicability of electrochemical methods to carwash wastewaters for reuse. Part 2: Electrocoagulation and anodic oxidation integrated process. J. Electroanal. Chem. 2010, 638, 236–240. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. More environmentally friendly vehicle washes: Water reclamation. J. Clean. Prod. 2012, 37, 115–124. [Google Scholar] [CrossRef]
- Gikas, P. Towards energy positive wastewater treatment plants. J. Environ. Manag. 2017, 203, 621–629. [Google Scholar] [CrossRef]
- Barbosa, R.; Lapa, N.; Boavida, D.; Lopes, H.; Gulyurtlu, I.; Mendes, B. Co-combustion of coal and sewage sludge: Chemical and ecotoxicological properties of ashes. J. Hazard. Mater. 2009, 170, 902–909. [Google Scholar] [CrossRef]
- Stasta, P.; Boran, J.; Bebar, L.; Stehlik, P.; Oral, J. Thermal processing of sewage sludge. Appl. Therm. Eng. 2006, 26, 1420–1426. [Google Scholar] [CrossRef]
- Jala, S.; Goyal, D. Fly ash as a soil ameliorant for improving crop production—A review. Bioresour. Technol. 2006, 97, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Singh, D.N.; Kolay, P.K. Simulation of ash–water interaction and its influence on ash characteristics. Prog. Energy Combust. Sci. 2002, 28, 267–299. [Google Scholar] [CrossRef]
- Pandey, V.C. Chapter 1—Fly ash properties, multiple uses, threats, and management: An introduction. In Phytomanagement of Fly Ash; Pandey, V.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–34. ISBN 978-0-12-818544-5. [Google Scholar]
- Tang, J.; Su, M.; Wu, Q.; Wei, L.; Wang, N.; Xiao, E.; Zhang, H.; Wei, Y.; Liu, Y.; Ekberg, C.; et al. Highly efficient recovery and clean-up of four heavy metals from MSWI fly ash by integrating leaching, selective extraction and adsorption. J. Clean. Prod. 2019, 234, 139–149. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, L.; Wang, L.; Wang, X.; Gong, J.; Yang, L.; Bai, J. Enhanced geopolymeric co-disposal efficiency of heavy metals from MSWI fly ash and electrolytic manganese residue using complex alkaline and calcining pre-treatment. Waste Manag. 2019, 98, 135–143. [Google Scholar] [CrossRef]
- Xiao, H.; Cheng, Q.; Liu, M.; Li, L.; Ru, Y.; Yan, D. Industrial disposal processes for treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans in municipal solid waste incineration fly ash. Chemosphere 2020, 243, 125351. [Google Scholar] [CrossRef]
- Sato, T.; Todoroki, T.; Shimoda, K.; Terada, A.; Hosomi, M. Behavior of PCDDs/PCDFs in remediation of PCBs-contaminated sediments by thermal desorption. Chemosphere 2010, 80, 184–189. [Google Scholar] [CrossRef]
- Qiu, Q.; Jiang, X.; Lü, G.; Chen, Z.; Lu, S.; Ni, M.; Yan, J.; Deng, X. Degradation of PCDD/Fs in MSWI fly ash using a microwave-assisted hydrothermal process. Chin. J. Chem. Eng. 2019, 27, 1708–1715. [Google Scholar] [CrossRef]
- Weidemann, E.; Lundin, L.; Boily, J.-F. Thermal decomposition of municipal solid waste fly ash and desorption of polychlorinated dibenzo-p-dioxins and furans from fly ash surfaces. Environ. Sci. Pollut. Res. 2016, 23, 22843–22851. [Google Scholar] [CrossRef]
- Yan, J.H.; Peng, Z.; Lu, S.Y.; Li, X.D.; Ni, M.J.; Cen, K.F.; Dai, H.F. Degradation of PCDD/Fs by mechanochemical treatment of fly ash from medical waste incineration. J. Hazard. Mater. 2007, 147, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; He, Y.; Lu, L.; Hu, S. Evolution of aluminate hydrate phases in fly ash-cement system under the sulfate conditions. Constr. Build. Mater. 2020, 252, 119045. [Google Scholar] [CrossRef]
- Hou, D.; Li, T.; Wang, P. Molecular Dynamics Study on the Structure and Dynamics of NaCl Solution Transport in the Nanometer Channel of CASH Gel. ACS Sustain. Chem. Eng. 2018, 6, 9498–9509. [Google Scholar] [CrossRef]
- Hou, D.; Li, T. Influence of aluminates on the structure and dynamics of water and ions in the nanometer channel of calcium silicate hydrate (C–S–H) gel. Phys. Chem. Chem. Phys. 2018, 20, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- da Silva, S.R.; de Oliveira Andrade, J.J. Investigation of mechanical properties and carbonation of concretes with construction and demolition waste and fly ash. Constr. Build. Mater. 2017, 153, 704–715. [Google Scholar] [CrossRef]
- Paris, J.M.; Roessler, J.G.; Ferraro, C.C.; DeFord, H.D.; Townsend, T.G. A review of waste products utilized as supplements to Portland cement in concrete. J. Clean. Prod. 2016, 121, 1–18. [Google Scholar] [CrossRef]
- Kosmatka, S.H.; Kerkhoff, B.; Panarese, W.C. Design and Control of Concrete Mixtures, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2002; ISBN 978-0-89312-217-1. [Google Scholar]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003; ISBN 0-13-064632-6. [Google Scholar]
- Siddique, R. Effect of fine aggregate replacement with Class F fly ash on the mechanical properties of concrete. Cem. Concr. Res. 2003, 33, 539–547. [Google Scholar] [CrossRef]
- Vashistha, P.; Kumar, V.; Singh, S.K.; Dutt, D.; Tomar, G.; Yadav, P. Valorization of paper mill lime sludge via application in building construction materials: A review. Constr. Build. Mater. 2019, 211, 371–382. [Google Scholar] [CrossRef]
- Malhotra, V.M. High-Performance High-Volume Fly Ash Concrete. Concr. Int. 2002, 24, 30–34. [Google Scholar]
- Mehta, P.K.; Monmohan, D. Sustainable High-Performance Concrete Structures. Concr. Int. Des. Constr. 2006, 7, 37–43. [Google Scholar]
- Mehta, P.K.; Claisse, P.; Naik, T.R. Sustainable Cements and Concrete for the Climate Change Era—A Review; Coventry University and The University of Wisconsin Milwaukee Centre for By-products Utilization: Ancona, Italy, 2010. [Google Scholar]
- Cáceres, S.H.; Quispe, G.B. Utilización de la ceniza volante en la dosificación del concreto como sustituto del cemento. Rev. Investig. Altoandinas J. High Andean Res. 2018, 20, 225–234. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7944-0. [Google Scholar]
- Peter, C.H. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0-7506-6256-7. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; ISBN 978-0-7277-2592-9. [Google Scholar]
- Gene, J.M.; Gaviria, X.; Saldarriaga, J.F. Evaluation of fly ash reactivity from incineration of hazardous waste in lime pastes. Chem. Eng. Trans. 2019, 75, 619–624. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Geng, B.; Ni, W.; Wang, J.; Qiu, X.; Cui, X.; Ren, C.; Zhang, S.; Xing, Y. Pozzolanic Reactivity and Hydration Products of Hedenbergite. Chem. Eng. Trans. 2017, 6270, 943–948. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Camões, A.; Branco, F.G.; Aguiar, J.B.; Fangueiro, R. Recycling of biomass and coal fly ash as cement replacement material and its effect on hydration and carbonation of concrete. Waste Manag. 2019, 94, 39–48. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Anjos, M.A.S.; Reis, R.; Camões, A.; Duarte, F.; Jesus, C. Evaluation of hydration of cement pastes containing high volume of mineral additions. Eur. J. Environ. Civ. Eng. 2019, 23, 987–1002. [Google Scholar] [CrossRef]
- Baert, G.; Hoste, S.; De Schutter, G.; De Belie, N. Reactivity of fly ash in cement paste studied by means of thermogravimetry and isothermal calorimetry. J. Therm. Anal. Calorim. 2008, 94, 485–492. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Handbook of Thermal Analysis of Construction Materials, 1st ed.; William Andrew: Norwich, CT, USA, 2002; ISBN 978-0-8155-1487-9. [Google Scholar]
- El-Shimy, E.; Abo-El-Enein, S.A.; El-Didamony, H.; Osman, T.A. Physico-chemical and Thermal Characteristics of Lime-silica Fume Pastes. J. Therm. Anal. Calorim. 2000, 60, 549–556. [Google Scholar] [CrossRef]
- Dweck, J.; Buchler, P.M.; Coelho, A.C.V.; Cartledge, F.K. Hydration of a Portland cement blended with calcium carbonate. Thermochim. Acta 2000, 346, 105–113. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Zhao, K.-F.; Xie, F.; Li, H.; Wang, D. Rheology and agglomerate structure of fresh geopolymer pastes with different Ms ratio of waterglass. Constr. Build. Mater. 2020, 250, 118881. [Google Scholar] [CrossRef]
- Romagnoli, M.; Leonelli, C.; Kamse, E.; Lassinantti Gualtieri, M. Rheology of geopolymer by DOE approach. Constr. Build. Mater. 2012, 36, 251–258. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1989; ISBN 0-444-87469-0. [Google Scholar]
- Laskar, A.I.; Bhattacharjee, R. Effect of Plasticizer and Superplasticizer on Rheology of Fly-Ash-Based Geopolymer Concrete. Mater. J. 2013, 110, 513–518. [Google Scholar] [CrossRef]
- Sikalidis, C.A.; Zabaniotou, A.A.; Famellos, S.P. Utilisation of municipal solid wastes for mortar production. Resour. Conserv. Recycl. 2002, 36, 155–167. [Google Scholar] [CrossRef]
- Armstrong, T. World Cement Consumption Rises by 2.8% in 2019. Available online: https://www.prnewswire.com/news-releases/world-cement-consumption-rises-by-2-8-in-2019--300996142.html (accessed on 23 June 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).