Identification of New Biocontrol Agent against Charcoal Rot Disease Caused by Macrophomina phaseolina in Soybean (Glycine max L.)
Abstract
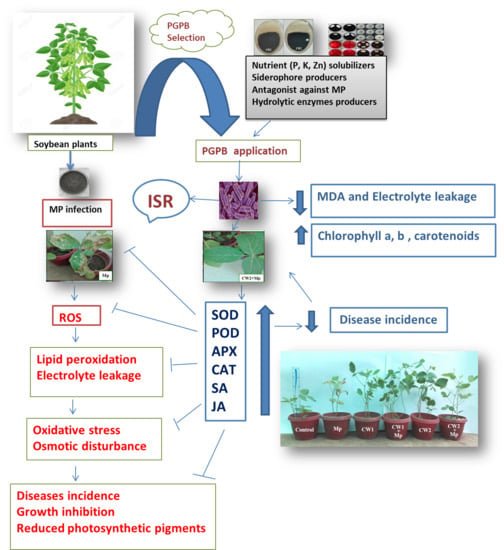
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Isolation of Bacteria
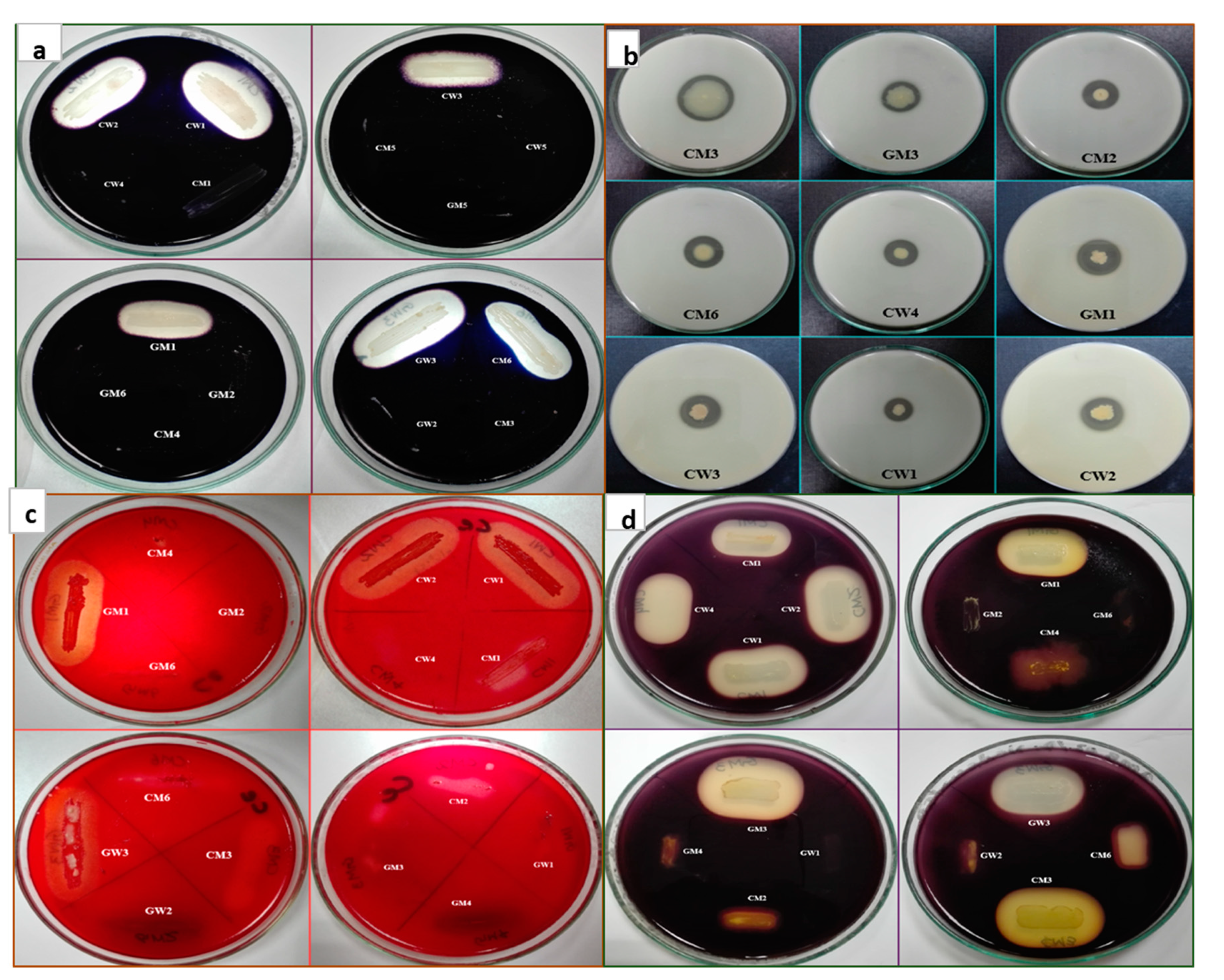
2.2. Screening of Bacteria for Production of Hydrolytic Enzymes
2.3. Screening of Bacterial Isolates for Antagonism Against the Pathogen
2.4. In Vitro Experiment
2.5. Sequencing of 16S rRNA Gene of Potent Biocontrol Strains for Molecular Identification
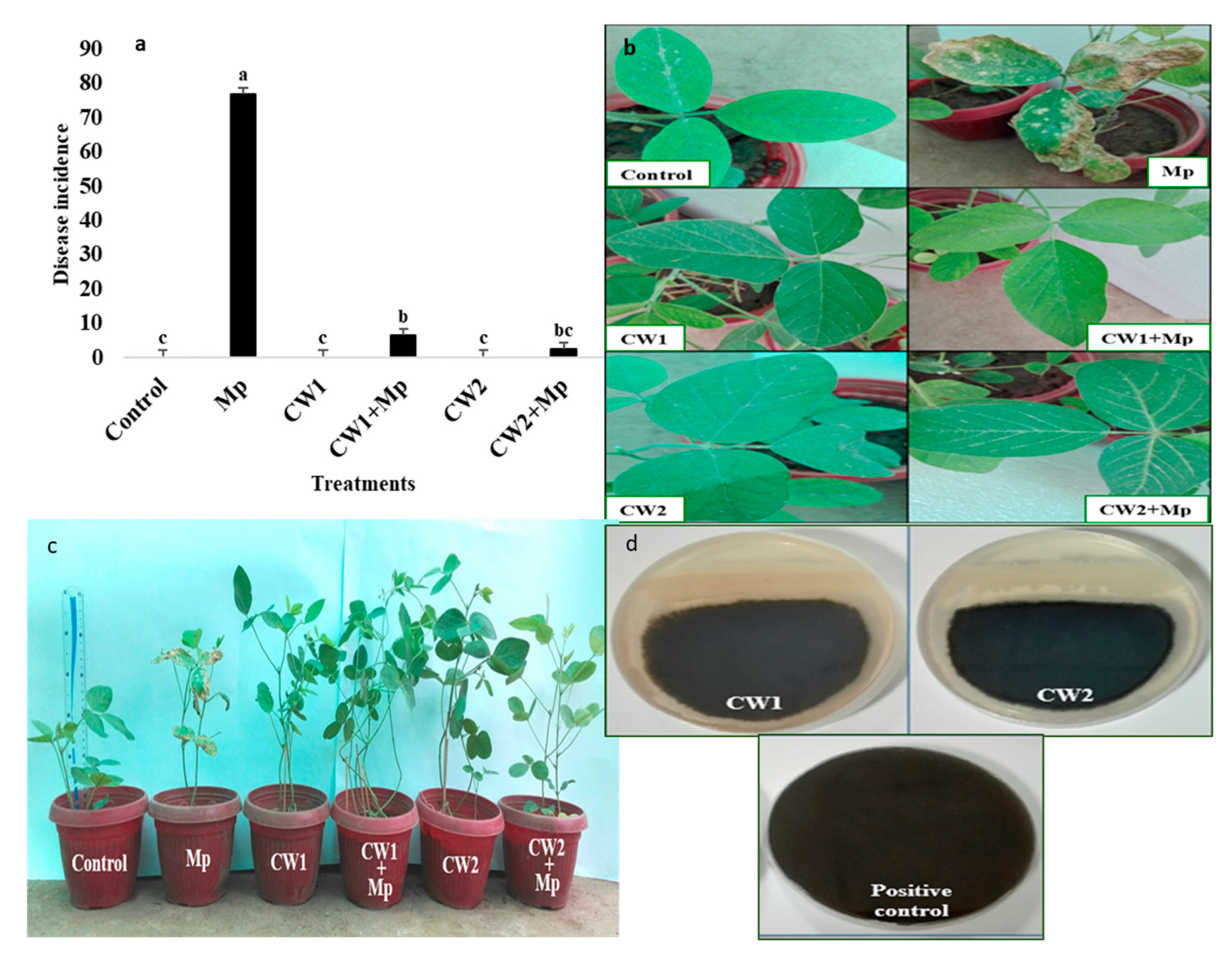
2.6. Pot Experiment
2.6.1. Relative Water Content (RWC)
2.6.2. Analysis of Photosynthetic Pigments
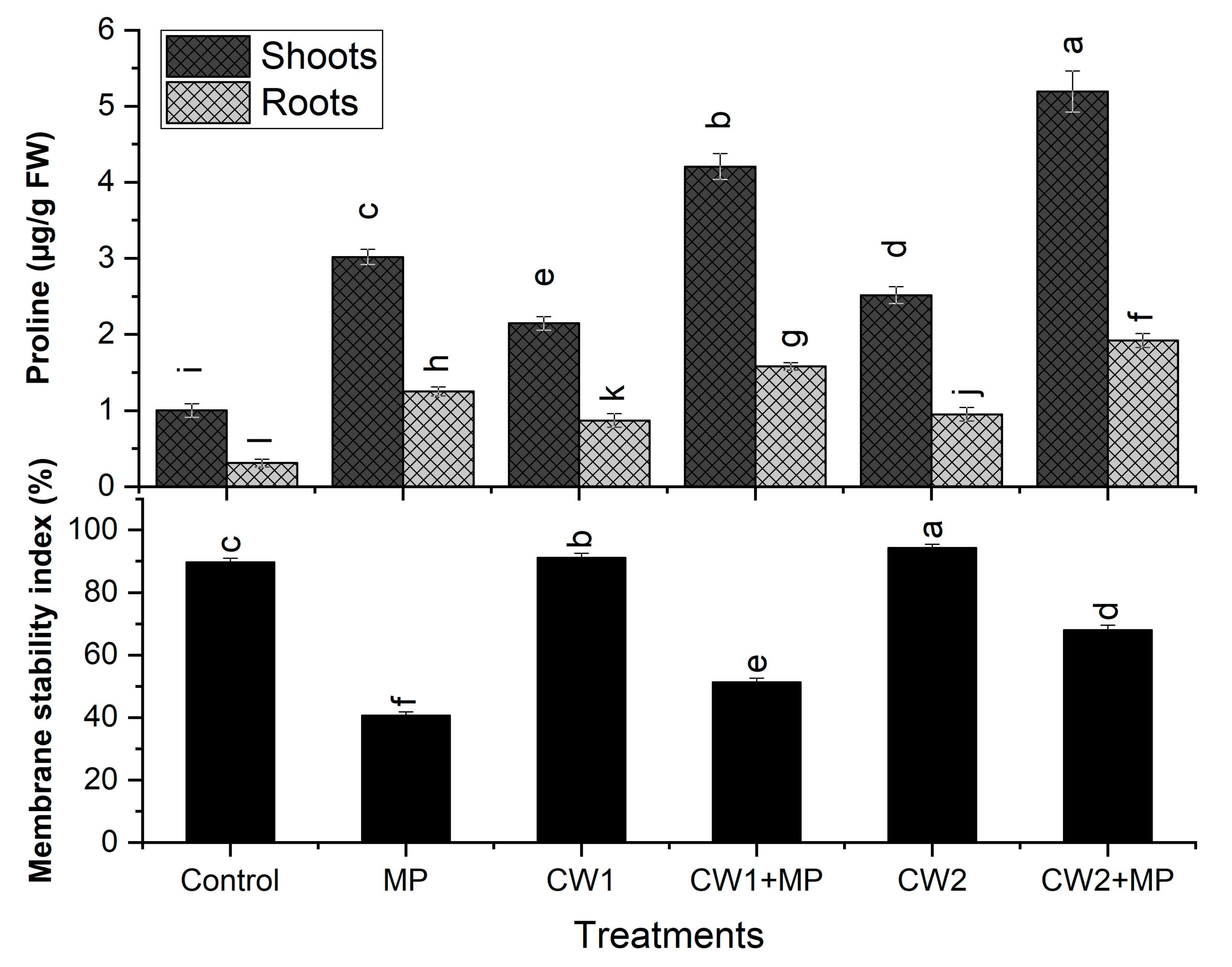
2.6.3. Estimation of Proline Content
2.6.4. Determination of Membrane Electrolytic Leakage
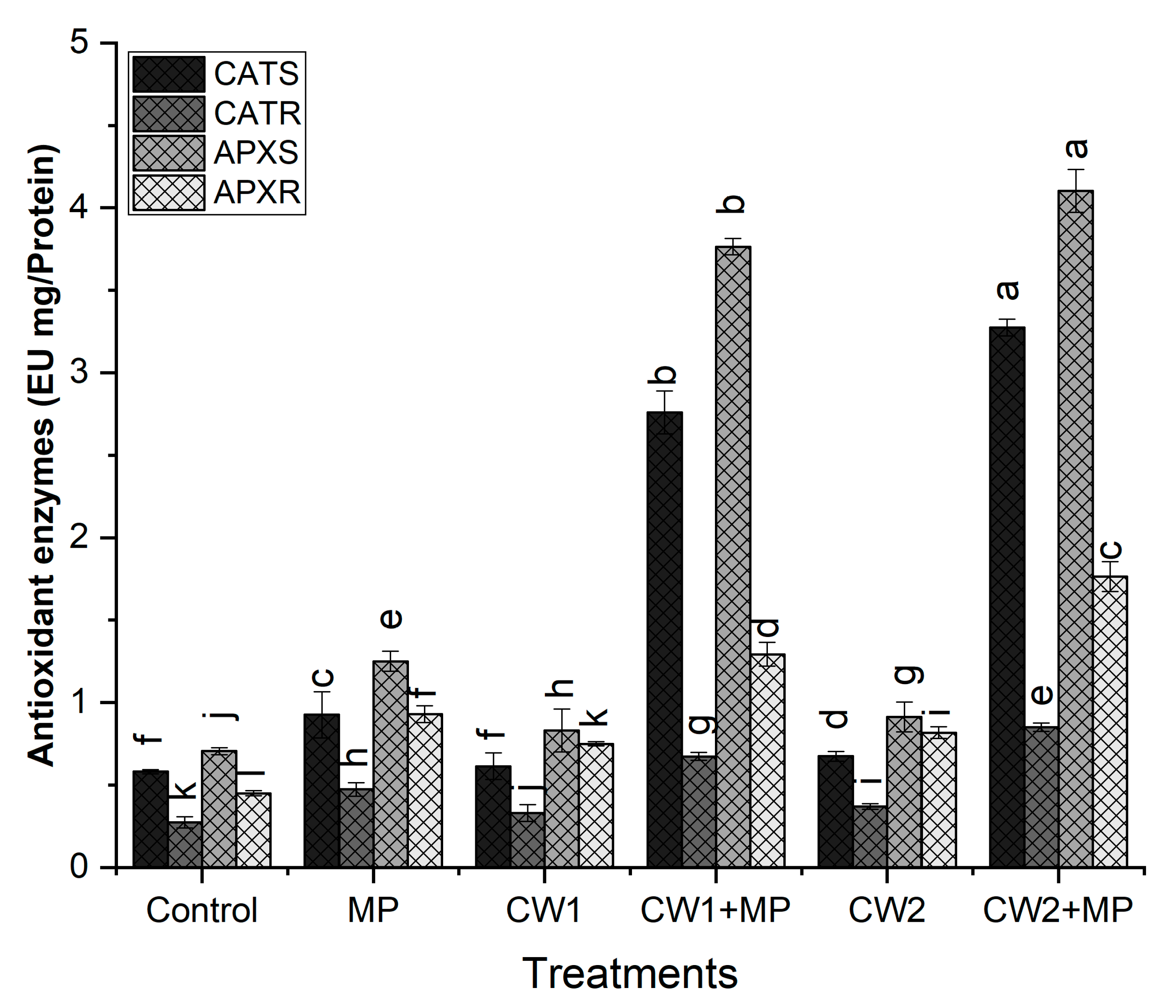
2.6.5. Analysis of Enzymatic Antioxidants
Preparation of Enzyme Extracts
Superoxide Dismutase (SOD)
Peroxidase (POD)
Catalase (CAT)
Ascorbate Peroxidase (APX)
2.7. Quantification of Phytohormones
2.8. Data Analysis
3. Results
3.1. Plant Growth-Promoting (PGP) Traits of Bacteria Isolated from Hot Springs
3.2. Hydrolytic Enzymes Production and Antagonistic Activity Against M. phaseolina
3.3. Post-Harvest Assays for In Vitro Experiment
3.4. Identification of Potent Biocontrol Agents
- Pot Experiment
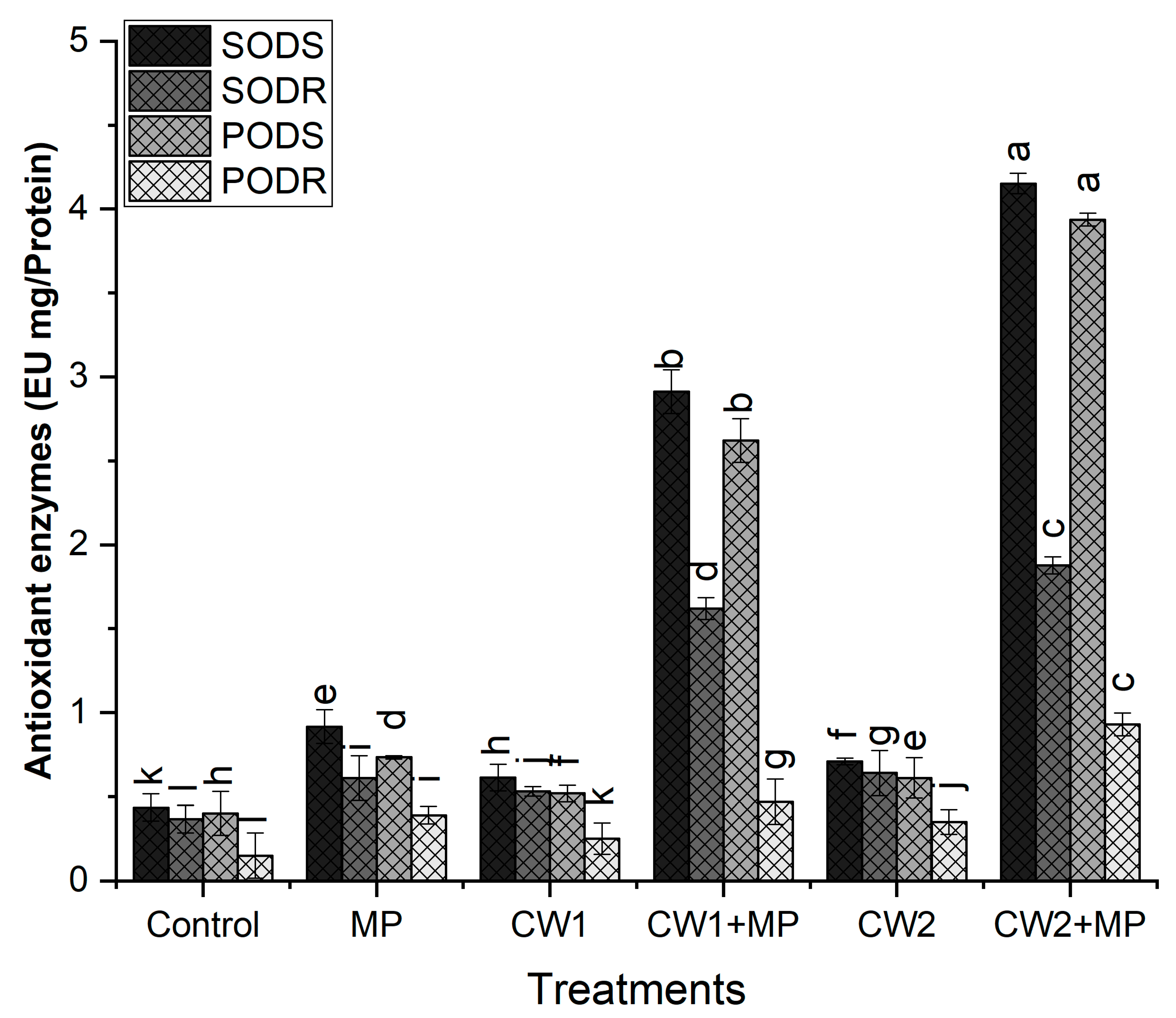
- Superoxide Dismutase (SOD)
- Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hungria, M.; Mendes, I.C. Nitrogen Fixation with Soybean: The Perfect Symbiosis? In Biological Nitrogen Fixation; de Bruijin, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1009–1024. [Google Scholar]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.; Canteri, M.G.; Goncalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. PeerJ. 2020, 8, e7905. [Google Scholar] [CrossRef] [Green Version]
- Castaño-Miquel, L.; Mas, A.; Teixeira, I.; Segui, J.; Perearnau, A.; Thampi, B.N.; Schapire, A.L.; Rodrigo, N.; La Verde, G.; Manrique, S.; et al. SUMOylation Inhibition Mediated by Disruption of SUMO E1-E2 Interactions Confers Plant Susceptibility to Necrotrophic Fungal Pathogens. Mol. Plant. 2017, 10, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Coser, S.M.; Reddey, R.V.C.; Zhang, J.; Mueller, D.S.; Mengistu, A.; Wise, K.A.; Allen, T.W.; Singh, A.; Singh, A.K. Genetic Architecture of Charcoal Rot (Macrophomina phaseolina) Resistance in Soybean Revealed Using a Diverse Panel. Front. Plant. Sci. 2017, 8, 1626. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [Green Version]
- Mufti, R.; Bano, A. PGPR-induced defense responses in the soybean plant against charcoal rot disease. Eur. J. Plant. Pathol. 2019, 155, 983–1000. [Google Scholar] [CrossRef]
- Ramezani, M.; Shier, W.T.; Abbas, H.K.; Tonos, J.L.; Baird, R.E.; Sciumbato, G.L. Soybean Charcoal Rot Disease Fungus Macrophomina phaseolina in Mississippi Produces the Phytotoxin (−)-Botryodiplodin but No Detectable Phaseolinone. J. Nat. Prod. 2007, 70, 128–129. [Google Scholar] [CrossRef]
- Kannojia, P.; Choudhary, K.K.; Srivastava, A.K.; Singh, A.K. Chapter Four—PGPR Bioelicitors: Induced Systemic Resistance (ISR) and Proteomic Perspective on Biocontrol. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 67–84. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.a.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.N.; Sahar, N.; Shah, S.Z.; Afghan, S.; Hafeez, F.Y. Suppression of red rot disease by Bacillus sp. based biopesticide formulated in non-sterilized sugarcane filter cake. BioControl 2015, 60, 691–702. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abdallah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of Phosphorus in Soil Connection with the Vital Activity of Some Microbial Species. Microbiology 1948, 1, 362–370. [Google Scholar]
- Hu, X.; Chen, J.; Guo, J. Two Phosphate- and Potassium-solubilizing Bacteria Isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of Zinc Solubilizing Bacteria in Growth Promotion and Zinc Content of Wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef] [Green Version]
- Lorck, H. Production of Hydrocyanic Acid by Bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Pontecorvo, G.; Roper, J.A.; Chemmons, L.M.; Macdonald, K.D.; Bufton, A.W.J. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 5, 141–238. [Google Scholar] [PubMed]
- Bibi, F.; Strobel, G.A.; Naseer, M.I.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Microbial Flora Associated with the Halophyte–Salsola imbricate and Its Biotechnical Potential. Front. Microbiol. 2018, 9, 65. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, S.; Wray, V.; Nimtz, M.; Prakash, A.; Johri, B.N. Characterization of an antifungal compound produced by Bacillus sp. strain A5F that inhibits Sclerotinia sclerotiorum. J. Basic Microbiol. 2012, 52, 670–678. [Google Scholar] [CrossRef]
- Afzal, A.; Khokhar, S.N.; Jabeen, B.; Asad, S.A. Phosphate Solubilizing Bacteria Associated with Vegetables Roots in Different Ecologies. Pak. J. Bot. 2013, 45, 535–544. [Google Scholar]
- Zia, M.A.; Yasmin, H.; Shair, F.; Jabeen, Z.; Mumtaz, S.; Hayat, Z.; Shah, S.Z.H.; Afghan, S.; Hafeez, F.Y.; Hassan, M.N. Glucanolytic Rhizobacteria Produce Antifungal Metabolites and Elicit ROS Scavenging System in Sugarcane. Sugar Tech. 2019, 21, 244–255. [Google Scholar] [CrossRef]
- Rais, A.; Shakeel, M.; Malik, K.; Hafeez, F.Y.; Yasmin, H.; Mumtaz, S.; Hassan, M.N. Antagonistic Bacillus spp. reduce blast incidence on rice and increase grain yield under field conditions. Microbiol. Res. 2018, 208, 54–62. [Google Scholar] [CrossRef]
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE 2020, 15, e0231348. [Google Scholar] [CrossRef]
- Sambrook, J.F.; Russell, D. Molecular Cloning: A Laboratory Manual (3-Volume Set), 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Doubledee, M.D.; Rupe, J.C.; Rothrock, C.S.; Bajwa, S.G. Effect of root infection by Macrophomina phaseolina on stomatal conductance, canopy temperature and yield of soybean. Can. J. Plant. Pathol. 2018, 40, 272–283. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the Water Relations of the Cotton Plant. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Shoaf, W.T.; Lium, B.W. Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Engineering drought tolerant tomato plants over-expressing BcZAT12 gene encoding a C2H2 zinc finger transcription factor. Phytochemistry 2013, 85, 44–50. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Vetter, J.L.; Steinberg, M.P.; Nelson, A.I. Quantitative Determination of Peroxidase in Sweet Corn. J. Agric. Food Chem. 1958, 6, 39–41. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Reports 2018, 19, e00273. [Google Scholar] [CrossRef]
- Thangarajan, S.; Gopalakrishnan, V.K. Enzymatic and non-enzymatic antioxidant properties of Tylophora pauciflora wight and arn.—An in vitro study. Asian J. Pharm. Clin. Res. 2013, 6, 68–71. [Google Scholar]
- Engelberth, J.; Schmelz, E.A.; Alborn, H.T.; Cardoza, Y.J.; Huang, J.; Tumlinson, J.H. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal. Biochem. 2003, 312, 242–250. [Google Scholar] [CrossRef]
- Heinisch, O. Steel, RGD, and JH Torrie: Principles and Procedures of Statistics. (With special Reference to the Biological Sciences.) McGraw-Hill Book Company, New York, Toronto, London 1960, 481 S., 15 Abb.; 81 s 6 d. Biom. Z. 1962, 4, 207–208. [Google Scholar] [CrossRef]
- Patel, K.S.; Naik, J.H.; Chaudhari, S.; Amaresan, N. Characterization of culturable bacteria isolated from hot springs for plant growth promoting traits and effect on tomato (Lycopersicon esculentum) seedling. C. R. Biol. 2017, 340, 244–249. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Krishna, R.; Prakash, S.; Yadav, J.; Singh, V. Characterization and Screening of Thermophilic Bacillus Strains for Developing Plant Growth Promoting Consortium from Hot Spring of Leh and Ladakh Region of India. Front. Microbiol. 2018, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. 2017, 7, 381. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech. 2020, 10, 36. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Ruiz-Valdiviezo, V.M.; Velázquez, E.; Ruiz-Lau, N.; Rogel-Hernández, M.A.; Villalobos-Maldonado, J.J.; Rincón-Rosales, R. Plant growth-promoting potential of bacteria associated to pioneer plants from an active volcanic site of Chiapas (Mexico). Appl. Soil Ecol. 2020, 146, 103390. [Google Scholar] [CrossRef]
- Xia, Y.; Farooq, M.A.; Javed, M.T.; Kamran, M.A.; Mukhtar, T.; Ali, J.; Tabassum, T.; Rehman, S.; Munis, M.F.H.; Chaudhary, H.J.; et al. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant. Physiol. Biochem. 2020, 151, 640–649. [Google Scholar]
- Oumer, O.J.; Abate, D. Screening and Molecular Identification of Pectinase Producing Microbes from Coffee Pulp. Biomed. Res. Int. 2018, 2018, 2961767. [Google Scholar] [CrossRef]
- Balakireva, A.V.; Zamyatnin, A.A., Jr. Cutting Out the Gaps Between Proteases and Programmed Cell Death. Front. Plant. Sci. 2019, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Illakkiam, D.; Anuj, N.L.; Ponraj, P.; Shankar, M.; Rajendhran, J.; Gunasekaran, P. Proteolytic enzyme mediated antagonistic potential of Pseudomonas aeruginosa against Macrophomina phaseolina. Indian J. Exp. Biol. 2013, 51, 1024–1031. [Google Scholar]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.-J.; Alghamdi, S.S.; Siddique, K.H.M. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant. Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G.M.; Ramaraj, G.B.; Ponnusamy, P.; Sekar, J.; Ramalingam, P.V. Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol. Control. 2019, 129, 55–64. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Brandan, C.P.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Decrease in the incidence of charcoal root rot in common bean (Phaseolus vulgaris L.) by Bacillus amyloliquefaciens B14, a strain with PGPR properties. Biol. Control. 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K.; Miransari, M. Plant growth promoting rhizobacteria to alleviate soybean growth under abiotic and biotic stresses. In Abiotic and Biotic Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 131–155. [Google Scholar]
- Ullah, H.; Yasmin, H.; Mumtaz, S.; Jabeen, Z.; Naz, R.; Nosheen, A.; Hassan, M.N. Multi-trait Pseudomonas spp. isolated from monocropped wheat (Triticum aestivum L). suppress Fusarium root and crown rot. Phytopathology 2019, 110, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS ONE 2017, 12, e0181201. [Google Scholar] [CrossRef] [Green Version]
- Omara, A.E.-D.; Hauka, F.; Afify, A.; El-Din, M.N.; Kassem, M. The Role of Some PGPR Strains to Biocontrol Rhizoctonia Solani in Soybean and Enhancement the Growth Dynamics and Seed Yield. Environ. Biodivers. Soil Secur. 2017, 1, 47–59. [Google Scholar]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant. Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- De los Santos-Villalobos, S.; Guzmán-Ortiz, D.A.; Gómez-Lim, M.A.; Délano-Frier, J.P.; de Folter, S.; Sánchez-García, P.; Peña-Cabriales, J.J. Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biol. Control. 2013, 64, 37–44. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M. ‘Turan, M.; Sahin, F. Plant Growth-Promoting Rhizobacteria (Pgpr) Increase Yield, Growth and Nutrition of Strawberry Under High-Calcareous Soil Conditions. J. Plant. Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant. Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Front. Plant. Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.K.; Gupta, A.; Fatima, U.; and Senthil-Kumar, M. AtGBF3 confers tolerance to Arabidopsis thaliana against combined drought and Pseudomonas syringae stress. Environ. Exp. Bot. 2019, 168, 103881. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Koç, E.; İşlek, C.; Arici, Y.K. Spermine and its interaction with proline induce resistance to the root rot pathogen Phytophthora capsici in pepper (Capsicum annuum). Hortic. Environ. Biotechnol. 2017, 58, 254–267. [Google Scholar] [CrossRef]
- Agarwal, P.; Patel, K.; Agarwal, P.K. Ectopic Expression of JcWRKY Confers Enhanced Resistance in Transgenic Tobacco Against Macrophomina phaseolina. DNA Cell Biol. 2018, 37, 298–307. [Google Scholar] [CrossRef]
- Gupta, A.; Sinha, R.; Fernandes, J.L.; Abdelrahman, M.; Burritt, D.J.; Tran, L.S.P. Phytohormones regulate convergent and divergent responses between individual and combined drought and pathogen infection. Crit. Rev. Biotechnol. 2020, 40, 320–340. [Google Scholar] [CrossRef]




| Strain | Siderophore Production Index | Phosphate Solubilization Index | Potassium Solubilization Index | Zinc Solubilization Index | HCN Production Ability |
|---|---|---|---|---|---|
| GM1 | − | 2.32 ± 0.01 ef | − | − | + |
| GM2 | − | − | − | − | + |
| GM3 | 4.45 ± 0.01 g | − | − | 3.66 ± 0.33 g | + |
| GM4 | 4.25 ± 0.01 g | 2.70 ± 0.01 d | 7.91 ± 0.01 c | + | |
| GM5 | 5.67 ± 0.33 d | 4.11 ± 0.01 c | 6.70 ± 0.01 b | 3.12 ± 0.01 h | − |
| GM6 | 5.27 ± 1.41 ef | 2.25 ± 0.01 ef | 3.15 ± 0.01 d | 5.15 ± 0.01 e | − |
| GW1 | 6.46 ± 0.01 c | 2.46 ± 0.03 e | 2.33 ± 0.01 g | 4.07 ± 0.01 f | − |
| GW2 | 4.44 ± 0.01 g | 2.25 ± 0.01 f | 6.43 ± 0.01 c | 9.33 ± 0.33 b | + |
| GW3 | 8.29 ± 0.01 b | 2.32 ± 0.01 ef | − | 3.30 ± 0.0 gh | − |
| CM1 | 5.04 ± 0.01 ef | − | − | − | + |
| CM2 | 4.37 ± 0.03 g | − | − | 5.54 ± 0.01 d | + |
| CM3 | 5.40 ± 0.01 de | 2.36 ± 0.03 ef | − | 2.47 ± 0.01 i | + |
| CM4 | 8.63 ± 0.01 ab | − | − | 3.33 ± 0.01 gh | − |
| CM5 | 3.86 ± 0.01 h | − | 2.67 ± 0.01 f | 5.33 ± 0.33 de | + |
| CM6 | 4.47 ± 0.01 g | − | − | 5.17 ± 0.01 e | + |
| CW1 | − | − | − | 1.58 ± 0.09 j | + |
| CW2 | 8.67 ± 0.33 a | 4.44 ± 0.01 b | 7.65 ± 0.01 a | 13.46 ± 0.03 a | + |
| CW3 | − | 2.17 ± 0.01 f | − | − | − |
| CW4 | 4.94 ± 0.01 f | − | − | − | − |
| CW5 | 4.36 ± 0.03 g | 2.18 ± 0.01 f | − | 9.13 ± 0.03 b | + |
| Positive Control | R2b | GuSM5 | GuSM5 | GuSM4 | SLH4 |
| 5.66 ± 0.33 d | 4.89 ± 0.33 a | 2.96 ± 0.01 e | 5.13 ± 0.03 e | +++ |
| Strain | Amylase Production Index | Cellulase Production Index | Pectinase Production Index | Protease Production Index | Percentage Inhibition of M. phaseolina |
|---|---|---|---|---|---|
| GM1 | 2.56 ± 0.01 c | 2.77 ± 0.01 c | 3.32 ± 0.01 bc | 3.35 ± 0.01 b | 31.96 ± 1.14 b |
| GM2 | − | − | − | − | − |
| GM3 | − | 2.23 ± 0.01 f | 3.34 ± 0.01 b | 2.37 ± 0.01 e | 4.73 ± 0.14 h |
| GM4 | − | − | − | − | 3.57 ± 0.02 h |
| GM5 | − | − | 2.65 ± 0.01 ef | − | 4.72 ± 0.03 h |
| GM6 | − | − | − | − | − |
| GW1 | − | − | − | − | − |
| GW2 | − | − | − | − | 27.12 ± 0.72 e |
| GW3 | 2.57 ± 0.01 bc | 2.77 ± 0.01 c | 2.58 ±0.01 f | − | 28.80 ± 0.92 d |
| CM1 | − | 2.23 ± 0.01 f | 3.33 ± 0.33 bc | − | − |
| CM2 | − | 2.36 ± 0.06 e | 2.47 ± 0.01 f | 3.25 ± 0.01 bc | − |
| CM3 | − | 2.77 ± 0.01 c | 2.89 ± 0.01 de | 2.20 ± 0.01 e | − |
| CM4 | − | − | − | − | 8.22 ± 0.06 g |
| CM5 | − | − | − | − | − |
| CM6 | 2.27 ± 0.01 d | 2.66 ± 0.01 d | 2.41 ± 0.01 f | 3.33 ± 0.33 b | − |
| CW1 | 2.63 ± 0.08 b | 2.87 ± 0.01 b | 2.92 ± 0.01 de | 2.63 ± 0.03 d | 30.26 ± 1.13 c |
| CW2 | 2.92 ± 0.01 a | 3.03 ± 0.01 a | 3.68 ± 0.01 a | 3.57 ± 0.01 a | 41.7 ± 0.59 a |
| CW3 | 2.18 ± 0.01 e | 2.77 ± 0.02 c | 3.03 ± 0.01 cd | 3.23 ± 0.01 bc | 12.08 ± 0.68 f |
| CW4 | − | − | 3.33 ± 0.31 bc | 3.05 ± 0.01 c | − |
| CW5 | − | − | − | − | − |
| Treatments | Shoot Length (cm) | Root Length (cm) | Leaf Area (cm2) | Disease Incidence | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| Control | 12 ± 0.62 h | 11 ± 0.45 h | 1.5 ± 0.13 g | 0 | 0.74 ± 0.005 e | 0.63 ± 0.007 h |
| MP | 5 ± 0.26 j | 4.5 ± 0.9 i | 0.5 ± 0.14 i | 75 ± 0.92 a | 0.32 ± 0.007 j | 0.32 ± 0.01 k |
| GM1 | 15 ± 0.92 e | 12.5 ± 0.22 g | 1.8 ± 0.08 d | 0 | 0.8 ± 0.006 d | 0.69 ± 0.03 g |
| GM1+ MP | 11.7 ± 0.26 g | 11 ± 0.26 h | 0.5 ± 0.13 i | 10 ± 0.12 d | 0.4 ± 0.01 i | 0.44 ± 0.01 j |
| GW2 | 17 ± 0.41 c | 14 ± 0.71 d | 1.6 ± 0.03 f | 0 | 0.8 ± 0.01 d | 0.74 ± 0.02 e |
| GW2+ MP | 13 ± 0.57 i | 13 ± 0.9 f | 0.7 ± 0.05 h | 12 ± 0.13 c | 0.44 ± 0.01 h | 0.43 ± 0.06 j |
| GW3 | 16 ± 0.26 d | 13.5 ± 0.26 e | 1.7 ± 0.13 e | 0 | 0.85 ± 0.01 c | 0.75 ± 0.02 d |
| GW3+ MP | 12 ± 0.85 h | 11 ± 0.49 h | 0.8 ± 0.01 h | 15 ± 0.11 b | 0.56 ± 0.01 g | 0.47 ± 0.1 i |
| CW1 | 18 ± 0.36 b | 15 ± 0.55 c | 2.4 ± 0.05 b | 0 | 0.9 ± 0.01 b | 0.87 ± 0.02 b |
| CW1+ MP | 14 ± 0.26 g | 14 ± 0.26 d | 2 ± 0.13 d | 5 ± 0.09 e | 0.7 ± 0.01 f | 0.7 ± 0.02 f |
| CW2 | 20 ± 0.55 a | 16 ± 0.72 a | 2.5 ± 0.12 a | 0 | 0.8 ± 0.01 d | 0.96 ± 0.01 a |
| CW2+ MP | 15 ± 0.42 f | 15.5 ± 0.81 b | 2.3 ± 0.03 c | 2 ± 0.07 f | 0.98 ± 0.01 a | 0.75 ± 0.11 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmin, H.; Naz, R.; Nosheen, A.; Hassan, M.N.; Ilyas, N.; Sajjad, M.; Anjum, S.; Gao, X.; Geng, Z. Identification of New Biocontrol Agent against Charcoal Rot Disease Caused by Macrophomina phaseolina in Soybean (Glycine max L.). Sustainability 2020, 12, 6856. https://doi.org/10.3390/su12176856
Yasmin H, Naz R, Nosheen A, Hassan MN, Ilyas N, Sajjad M, Anjum S, Gao X, Geng Z. Identification of New Biocontrol Agent against Charcoal Rot Disease Caused by Macrophomina phaseolina in Soybean (Glycine max L.). Sustainability. 2020; 12(17):6856. https://doi.org/10.3390/su12176856
Chicago/Turabian StyleYasmin, Humaira, Rabia Naz, Asia Nosheen, Muhammad Nadeem Hassan, Noshin Ilyas, Muhammad Sajjad, Seemab Anjum, Xiangkuo Gao, and Zhide Geng. 2020. "Identification of New Biocontrol Agent against Charcoal Rot Disease Caused by Macrophomina phaseolina in Soybean (Glycine max L.)" Sustainability 12, no. 17: 6856. https://doi.org/10.3390/su12176856
APA StyleYasmin, H., Naz, R., Nosheen, A., Hassan, M. N., Ilyas, N., Sajjad, M., Anjum, S., Gao, X., & Geng, Z. (2020). Identification of New Biocontrol Agent against Charcoal Rot Disease Caused by Macrophomina phaseolina in Soybean (Glycine max L.). Sustainability, 12(17), 6856. https://doi.org/10.3390/su12176856