Recovery of Lithium from Simulated Secondary Resources (LiCO3) through Solvent Extraction
Abstract
:1. Introduction
- Solvating Extractant: This is an organic carbon-based group consisting of alcohols, ketones, esters, and ethers. The extraction mechanism can be called an oxygen-donating solvation reaction when the metal is solvated by the organic oxygen extractant [18,19]. Research has been published regarding the result of using solvating extractant in experiments to recover trivalent lanthanides using tributylphosphate (TBP). The results showed that better extraction efficiency could be achieved with extractants with higher atomic numbers [20]. For our experiment, we selected n-butanol to study extraction efficiency compared with other groups.
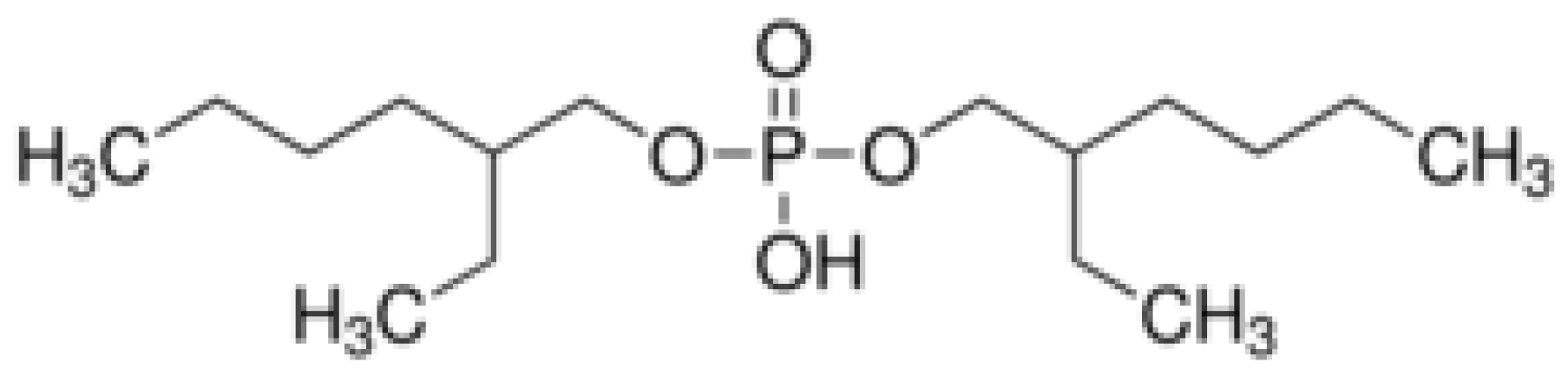
- Acidic (or Cation Exchange) Extractant: Two classes of acid are commonly used to represent acidic extractants—carboxylic acid and organo-phosphoric acids. Phosphorous acid extractants that are well known in the metal separation field are di-2-ethylhexylphosphoric acid (D2EHPA), 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (EHEHPA, HEHEHP, P507, PC88A), phosphinic acid, and di-2,4,4-trimethyl pentyl phosphinic acid (Cyanex 272) [20,21,22]. In terms of extraction efficiency of acidic extractants, it was reported that in rare earth separation from nitrate solution, D2EHPA could separate rare earth at low pH, and middle rare earth was recovered in the first stage at very high efficiency (above 95%), then light rare earth recovery happened at the second stage of stripping, with very promising results [20,21]. The acidic extractant in our experiment is bis(2-ethylhexyl) phosphate (DEHPA).
- Synergistic Extractant: The expectation was that a synergistic extractant would show better performance [18,19,23,24]. Synergistic systems can be found in mixtures of acid extractants and neutral extractants, such as in a metal ion extraction study using the synergistic system of trioctylphosphine oxide (TOPO) and trialkylphosphine oxide (TRPO) extractants [25], and the extraction of trivalent rare earth in a mixture of sec-octylphenoxy acetic acid (CA12) and Cyanex 301 in n-heptane [26].
2. Materials and Methods
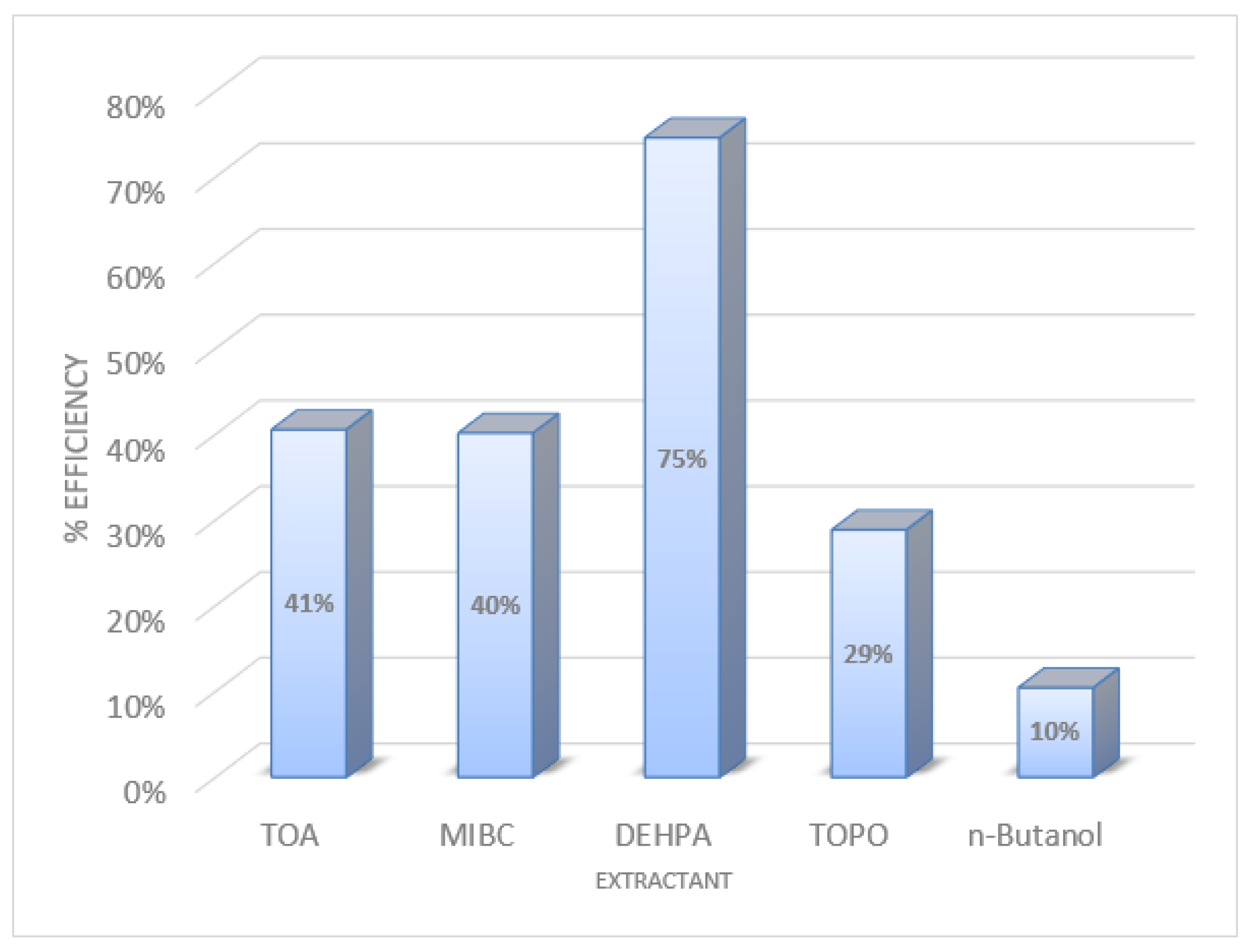
2.1. Extraction Efficiency of Various Extracts
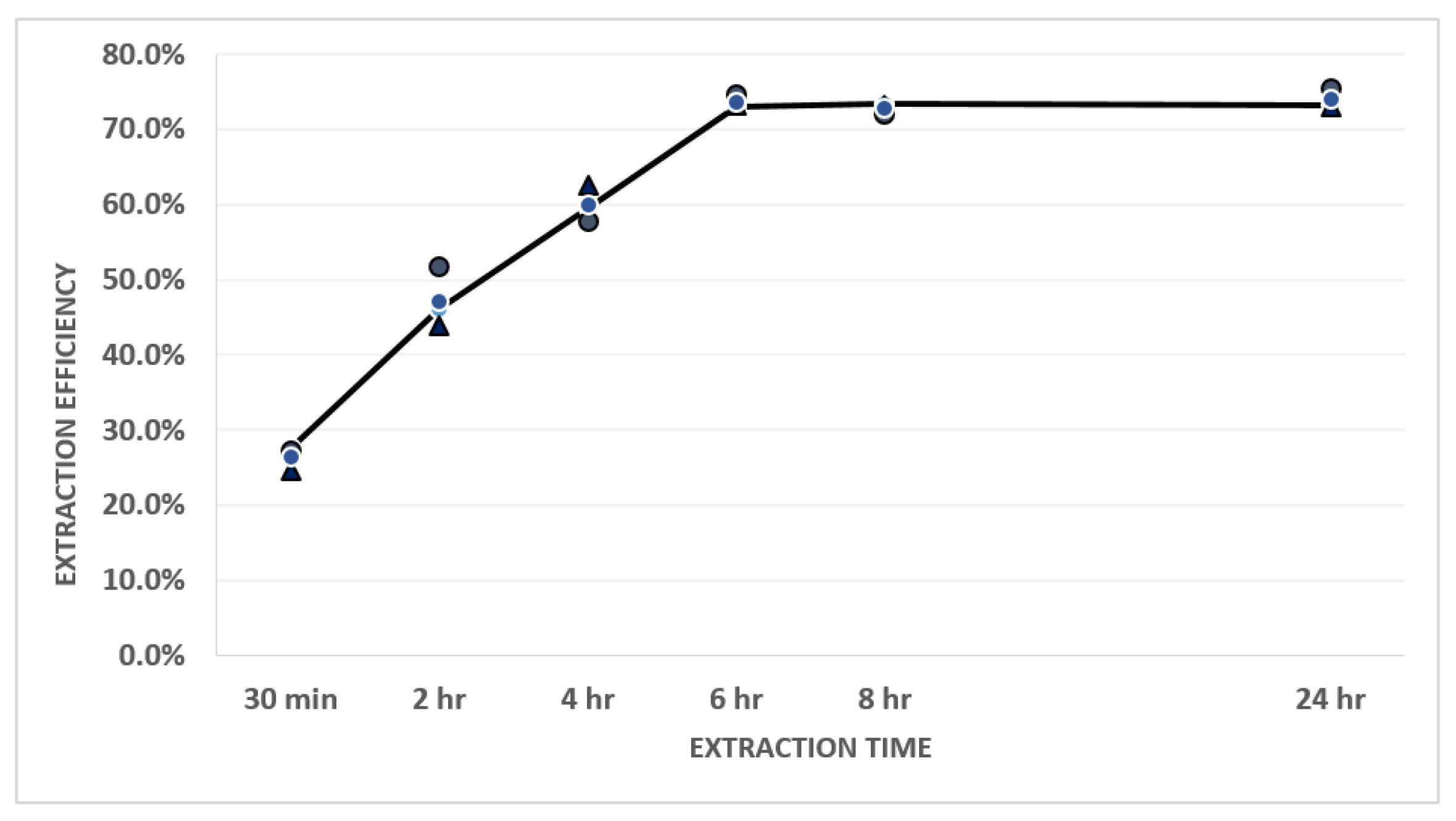
2.2. Studying Extraction Equilibrium
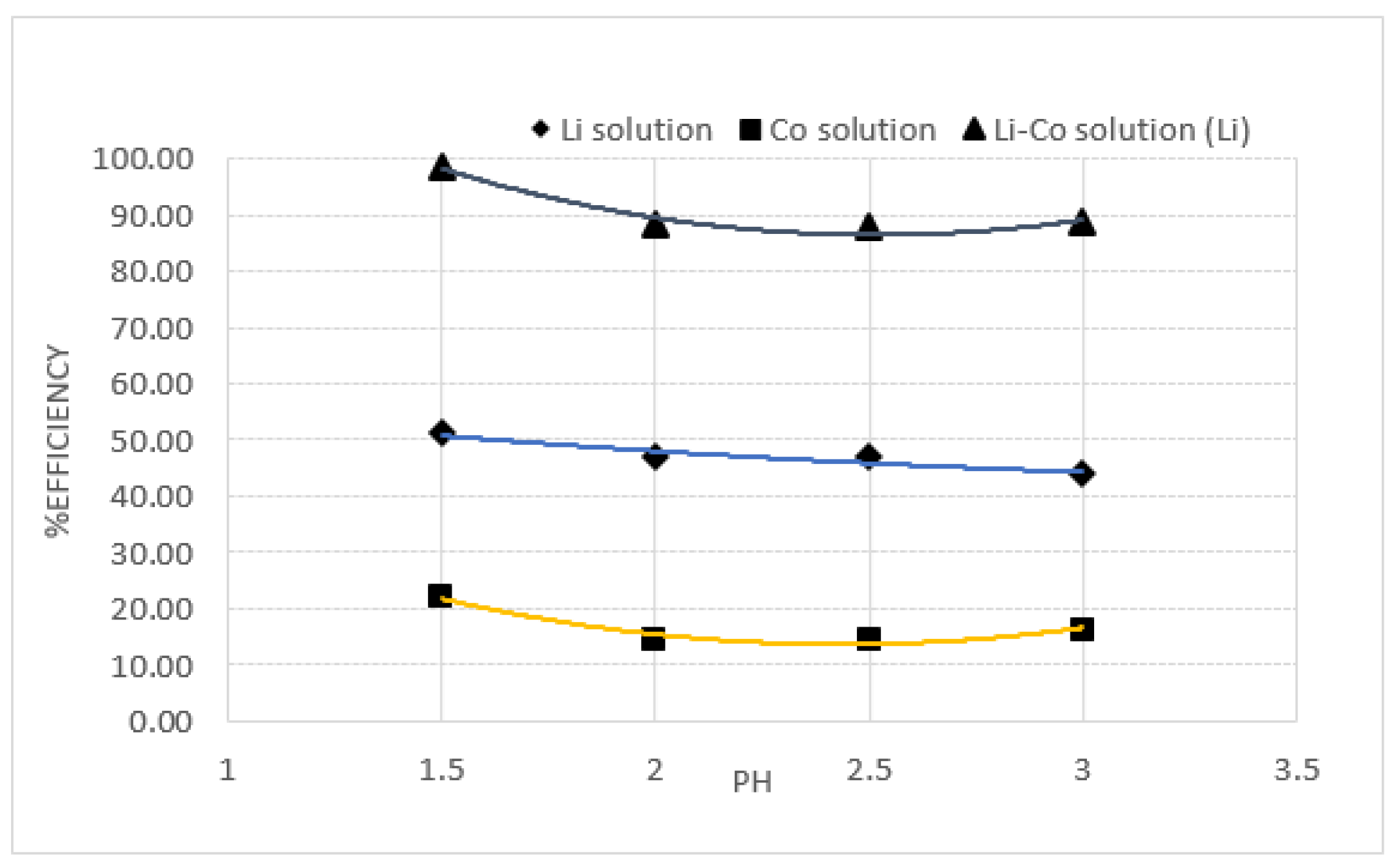
2.3. Effect of pH
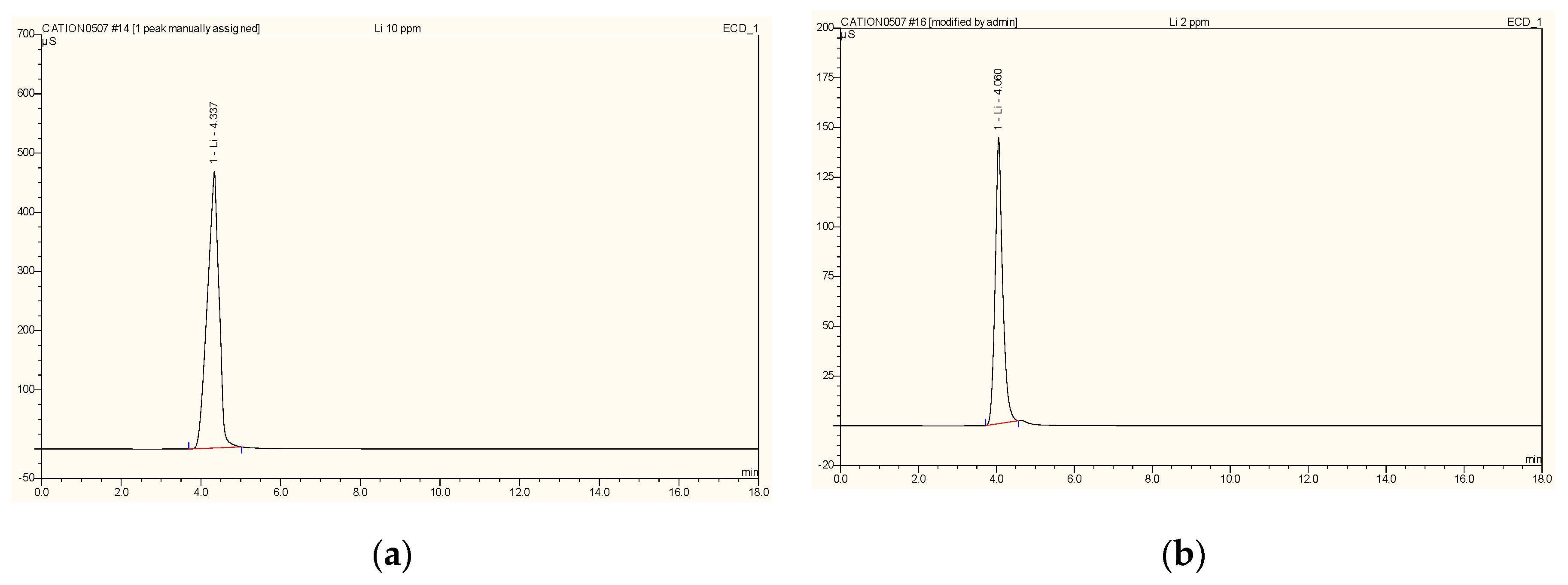
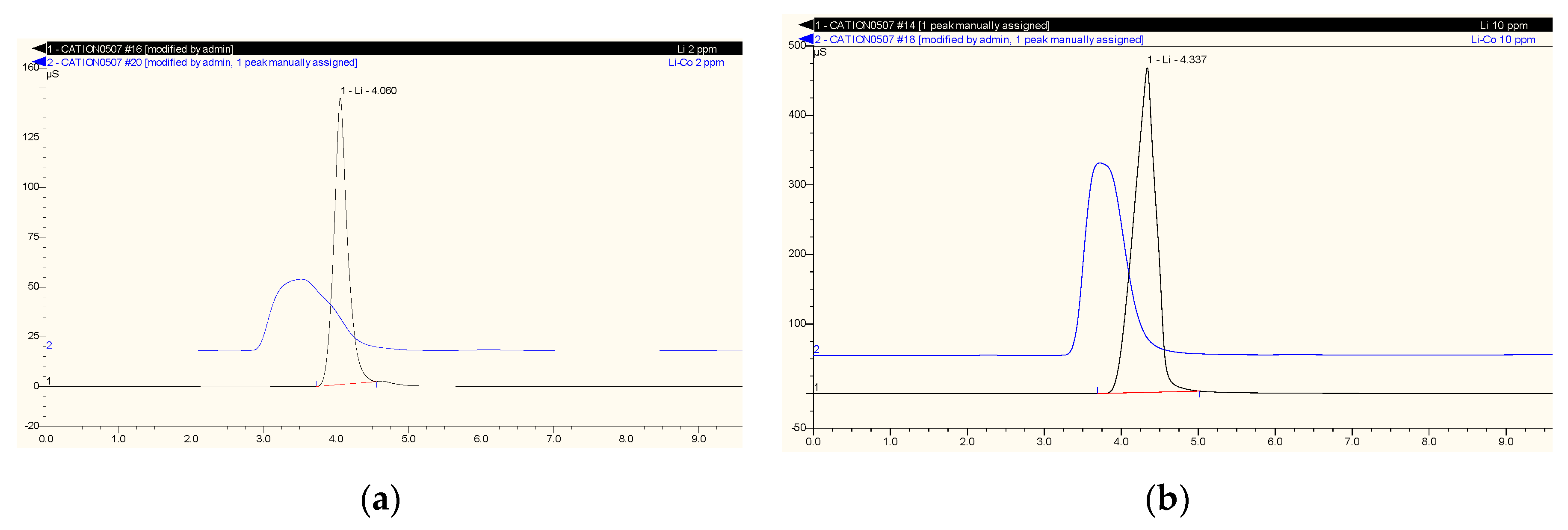
2.4. Studying Extraction Selectivity
2.5. Effect of Initial Concentration of Lithium
3. Results
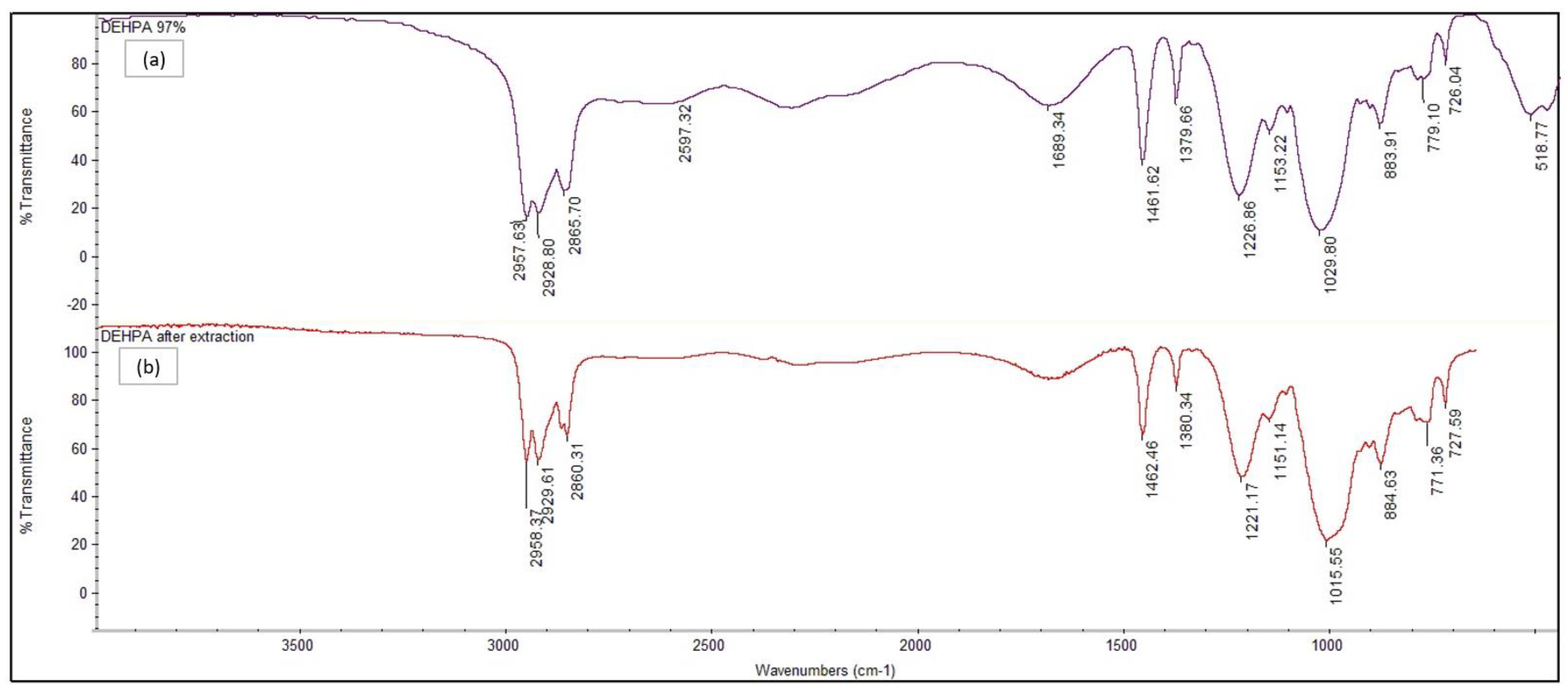
3.1. Result of Extraction Efficiency
3.2. Result of Extraction Equilibrium
3.3. Result of pH Effect
3.4. Result of Initial Concentration Effect
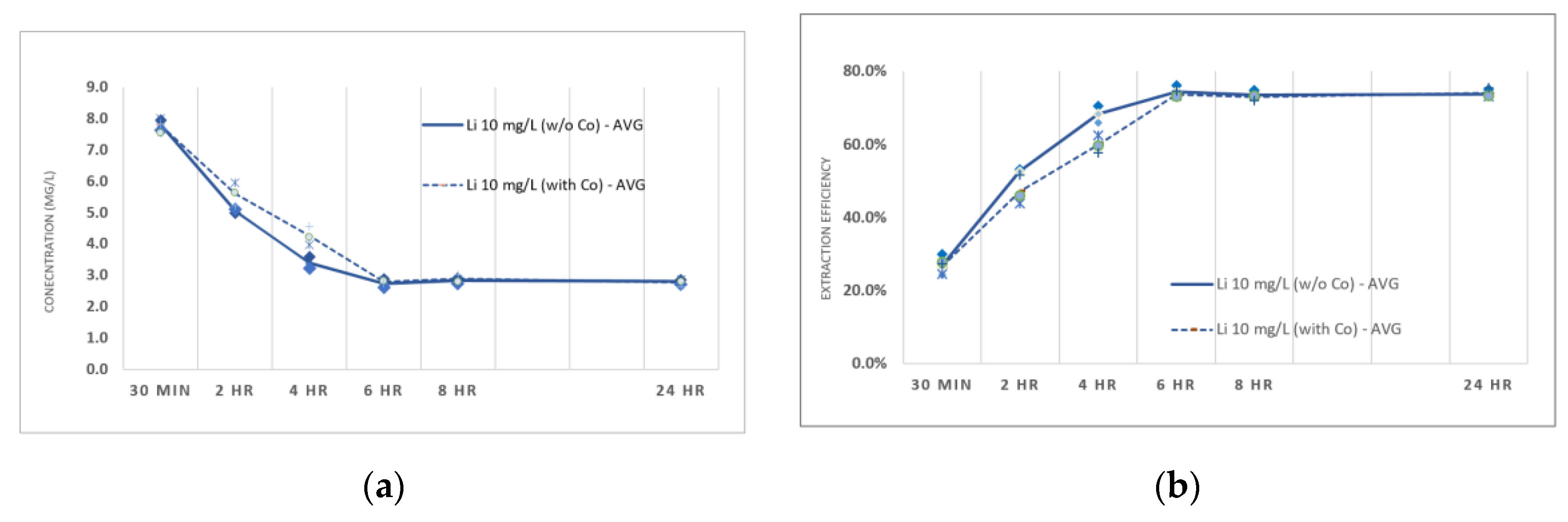
3.5. Effect of Co-Ion on Extraction Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Global Market for Lithium-Ion Batteries—Forecast, Trends & Opportunities 2014–2020. 2014. Available online: https://www.marketresearch.com/product/sample-8323376.pdf (accessed on 1 November 2018).
- Navigant Consulting. The Global Market for Lithium Ion Batteries for Vehicles is Expected to Total $221 Billion from 2015 to 2024. Available online: https://guidehouseinsights.com/news-and-views/the-global-market-for-lithium-ion-batteries-for-vehicles-is-expected-to-total-221-billion-from-2015 (accessed on 1 November 2018).
- Dewulf, J.; Vorst, G.V.D.; Denturck, K.; Van Langenhove, H.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. Resources, conservation and recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings. Resour. Conserv. Recycl. 2011, 54, 229–234. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-Ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Jeong, J.; Lee, J.; Lee, G.H.; Sohn, J.S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Manag. 2016, 51, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, E.; Shi, P.; Li, J.; Lu, M. The Recycling Technologies for Spent Lithium Ion Battery; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Dorella, G.; Mansur, M.B. Short communication a study of the separation of cobalt from spent Li-Ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Barnes, C.E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J. Chem. Soc. 1999, 8, 1201. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Chun, S.; Dzyuba, S.V.; Bartsch, R.A. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal. Chem. 2001, 73, 3737–3741. [Google Scholar] [CrossRef] [PubMed]
- Harvianto, G.R.; Kim, S. Solvent Extraction and stripping of lithium ion from aqueous solution and its application to seawater. Rare Met. 2016, 35, 948–953. [Google Scholar] [CrossRef]
- Swain, B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: A review. J. Chem. Technol. Biotechnol. 2016, 91, 2549–2562. [Google Scholar] [CrossRef]
- El-Nadi, Y.A. Solvent extraction and its applications on ore processing and recovery of metals: Classical approach. Sep. Purif. Rev. 2017, 46, 195–215. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Habaki, H.; Nakamura, K.; Egashira, R. Extraction equilibrium of valuable metals from NdFeB permanent magnet using carboxylic acid as extractant. J. Chem. Eng. Jpn. 2017, 50, 610–617. [Google Scholar] [CrossRef]
- Kinugasa, T.; Nishibara, H.; Murao, Y.; Kawamura, Y.; Watanabe, K.; Takeuchi, H. Equilibrium and kinetics of lithium extraction by a mixture of LIX54 and TOPO. J. Chem. Eng. Jpn. 1994, 27, 815–818. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; In, G.; Choi, J.M. Chemical equilibrium and synergism for solvent extraction of trace lithium with thenoyltrifluoroacetone in the presence of trioctylphosphine oxide. Bull. Korean Chem. Soc. 2003, 24, 1495–1500. [Google Scholar]
- El-Nadi, Y.A. Lanthanum and neodymium from Egyptian monazite: Synergistic extractive separation using organophosphorus reagents. Hydrometallurgy 2012, 119, 23–29. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Liquid–liquid extraction of europium (III) and other trivalent rare-earth ions using a non-fluorinated functionalized ionic liquid. Dalton Trans. 2014, 43, 1862–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma Aldrich Product Catalog. Bis(2-ethylhexyl) Phosphate. CAS no 237825. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/237825?lang=en®ion=TH (accessed on 1 January 2020).
- Jian, G.; Guo, J.; Wang, X.; Sun, C.; Zhou, Z.; Yu, L. Study on separation of cobalt and lithium salts from waste mobile-phone batteries. Procedia Environ. Sci. 2012, 16, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Virolainen, S.; Fallah, M.; Laitinen, A.; Sainio, T. Solvent extraction fractionation of Li-Ion battery leachate containing Li, Ni, and Co. Sep. Purif. Technol. 2017, 179, 274–282. [Google Scholar] [CrossRef]
- Nascimento, M.; Valverde, B.M.; Ferreira, F.A.; Gomes, R.D.C.; Soares, P.S.M. Separation of rare earths by solvent extraction using DEHPA. Rev. Esc. Minas 2015, 68, 427–434. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waengwan, P.; Eksangsri, T. Recovery of Lithium from Simulated Secondary Resources (LiCO3) through Solvent Extraction. Sustainability 2020, 12, 7179. https://doi.org/10.3390/su12177179
Waengwan P, Eksangsri T. Recovery of Lithium from Simulated Secondary Resources (LiCO3) through Solvent Extraction. Sustainability. 2020; 12(17):7179. https://doi.org/10.3390/su12177179
Chicago/Turabian StyleWaengwan, Pattamart, and Tippabust Eksangsri. 2020. "Recovery of Lithium from Simulated Secondary Resources (LiCO3) through Solvent Extraction" Sustainability 12, no. 17: 7179. https://doi.org/10.3390/su12177179



