Abstract
Stabilization of slopes subject to landslide by measures with low impact, such as those of bioengineering, is a topic of interest. The use of scarcely studied alpine pioneer plants could contribute to innovation in soil bioengineering and restoration ecology but to use them, knowledge of the ex situ germinability of their seeds is fundamental. This research analysed the germinability of seeds of nine alpine pioneer species (Papaver aurantiacum, Rumex scutatus, Tofieldia calyculata, Pulsatilla alpina, Silene glareosa, Adenostyles alpina, Dryas octopetala, Laserpitium peucedanoides and Laserpitium krapfii) treated with water, gibberellic acid (GA3) and/or calcium carbonate at room temperature. The seeds had different responses to the treatments: Laserpitium peucedanoides, L. krapfii and Silene glareosa showed difficulty in germinating (germination < 2.5%), while Dryas octopetala had good germination (39–61%) regardless of treatment. GA3 significantly increased the seed germination rate of Papaver aurantiacum, Pulsatilla alpina, Rumex scutatus and Tofieldia calyculata, while the addition of calcium carbonate made the seeds of Rumex scutatus and Tofieldia calyculata germinate more quickly. Results are discussed focusing on the perspectives of using alpine pioneer species in future soil bioengineering work for slopes stabilization and restoration, and on the actions that stakeholders should take to make this happen.
1. Introduction
The stabilization of mountain slopes is a topic of great interest both for land managers and technicians (engineers) as well as researchers working in this sector, as many mountain areas are increasingly affected by landslides or other phenomena of hydrogeological instability [1,2]. For the Alps, this is due both to climate change, whose effects are apparent in increasingly frequent extreme weather phenomena [3,4], and to the abandonment of good land management practices due to the continuous depopulation of mountain areas, a phenomenon that has intensified in recent decades [5,6,7,8] and that is still in progress today. As is well known, the Italian territory is moreover particularly subject to landslides, and consequently, soil conservation takes on a fundamental role in the design of suitable works for the protection of inhabited areas and natural attractions in order to avoid, as much as possible, natural disasters [9]. Although screes slopes and debris are not the primary kind of hydrogeological risk [10], recent imbalances due to climate changes, with intensified precipitation, caused considerable disruption. Very often the areas subject to this kind of risk are of touristic and naturalistic interest, in particular in the dolomitic massifs. For example, in 2016, a storm of rain and hail moved the screes of Mount Pelmo, belonging to the Zoldo Dolomites (Belluno), making impassable part of the forest road that climbs from Tiera towards Rifugio Venezia and several points of path No. 480, which leads to the Val d’Arcia; these are areas of important touristic flow and were very frequented at the moment of the disaster (August 2016).
Engineering interventions are often difficult in these sites, and the landscape impact they can provoke can have foreseeable consequences on touristic flow. In order to fight land degradation and, more generally, to comply with the objectives of the Europe 2020 strategy [11], the European Union has drawn up a “Roadmap to a resource efficient Europe” [12], which emphasizes the importance of investment in natural capital, for example, through the creation of Green Infrastructures [13,14]. Green infrastructures also include soil bioengineering work understood as low-impact measures for soil stabilization using living plants (or parts thereof) as stabilising materials in combination with other materials such as stones, soil, timber, steel, etc. [15,16,17,18,19]. Soil bioengineering techniques for slope stabilization, compared to traditional ones (hydraulics), have the objective not only of stabilizing the soil but also of minimizing impact on the environment and landscape of the area where they are used [18,20,21] compatibly with project priorities.
Living plants are then an important tool in bioengineering and slope stabilization. Studies of slope geomorphology and vegetation initially developed along separate routes [22,23,24]. Most research was conducted by biologists, who frequently ascribed vegetation distribution on slopes to ground instability caused by unspecified geomorphic processes. Meanwhile, geomorphologists studying slope dynamics seldom correlated them with plant patterns. Some earth scientists “explained” slope stability with hypothetical vegetation influences, and plants were alleged to decrease or even stop debris descent [25,26,27,28]. This close connection between landforms and plants coined new terms such as phytogeomorphology [29] and ecogeomorphology [30], which stresses further the ecological connections between biology and geomorphology and focuses on the tight coupling among geological, pedological, biological and ecological processes [31].
Individual plants and plant communities play a crucial role since the plant species used in soil bioengineering works must be able to combine the technical result, soil reinforcement, with an ecological one that minimizes the impact on ecosystems. This perspective, [32], recently recommended a series of good practices for technicians who design and implement soil bioengineering works, as well as highlighting some critical issues that should be addressed by researchers in order to improve the success of such works. Among these, there is the biotechnical and ecological study of “new” pioneer plant species to be used in soil bioengineering which, in addition to stabilizing the soil, could accelerate the spontaneous vegetation successions [33,34] hence respecting the mechanisms and processes that regulate ecosystems.
Instead, in soil bioengineering works for slope stabilization (and in restoration projects), herbaceous species are often introduced through hydroseeding of seed mixtures without paying much attention to their floristic composition and/or seed origin [20,32,35]. Sometimes, in fact, in those countries where the law allows it, commercial mixtures are used. On the one hand, they are extremely cheap and available in large quantities, but on the other, they have a limited number of species (a dozen at the most) whose seeds, apart from anything else, can be of low quality and come from populations thousands of kilometres away from the intervention areas. Therefore, these mixtures, in addition to producing genetic pollution [36], may be poorly suited for the environmental conditions of the areas in which they are sown. Furthermore, the most common species of commercial mixtures are those of fertile meadow communities (Molinio-Arrhenatheretea), such as Dactylis glomerata L., Lolium perenne L., Phleum pratense L., Poa pratensis L., Festuca rubra L., Festuca pratensis Huds., Trifolium pratense L. and Trifolium repens L, which are easy to grow and whose seeds are easy to harvest. Because of their ecological requirements, these species are unlikely to grow in the mountainous areas of the Alps (over 1200 m a.s.l.), especially on soils poor in nutrients, with little organic substance and rich in skeleton. Recently, in [32], it was shown that the use of a commercial seed mixture in a landslide area of the Alps which had undergone soil stabilization work proved to be inefficient as only three species (two of which with a reduced number of individuals) out of twelve were present two years after sowing. Furthermore, according to [37], some species (such as Festuca rubra) present in commercial mixtures, if introduced in alpine areas affected by restoration interventions, can compete with the native species, and may delay successful natural alpine vegetation recovery.
In order to avoid these criticalities, these mixtures should then consist of native seeds of alpine pioneer species [38] able to germinate on debris. Unfortunately, the greatest problem is that today, on the market, it is difficult, if not impossible, to find such mixtures, especially in quantities that can be used over large areas [39]. The study of the ecological characteristics of the alpine pioneer species, as well as that of their biotechnical characteristics, could contribute to solving this problem. These studies require the collaboration of researchers from various scientific sectors as well as a step by step approach. One of the first “steps” to focus on is to understand if the seeds of alpine pioneer species, collected in their natural environment, are vital ex situ, i.e., able to germinate spontaneously or with the help of phytostimulants.
Taking these factors into consideration, this research aims to analyse the germinability of the seeds of nine alpine pioneer species (perennial herbs and dwarf shrubs) typical of communities established on limestone screes or on thin soils (present on more or less consolidated screes) in order to understand whether, and how, it is possible to germinate their seeds ex situ in a sterile condition and with an easy protocol. The germination is in fact the first step in a production procedure. Various germination tests were conducted in the laboratory using calcium carbonate based treatments (in order to simulate the chemical characteristics of calcareous screes) and gibberellic acid (phytostimulant) in order to assess the effects that these substances have on the seeds of the plants studied and to identify the method which allows maximum seed germination in the shortest time.
In particular, the aim was to inquire if seeds of little-known alpine plants growing on calcareous screes answer differently to standardised treatments based on the use of calcium carbonate (a substance characterizing their habitat) or if the well-known plant hormone gibberellic acid can increase their germinability. The results of the tests are discussed focusing on the prospects for the use of these species in future soil bioengineering work and on innovative actions that should be taken in this sector, at least in Europe. A preliminary study is useful to start to hypothesize the use of these plants in soil bioengineering, which has not often been contemplated until today.
2. Materials and Methods
2.1. Plant Material and Sampling Areas
Nine perennial alpine species were considered in this research: Papaver aurantiacum Loisel., Rumex scutatus L., Tofieldia calyculata (L.) Wahlenb., Pulsatilla alpina subsp. austroalpina D.M. Moser, Silene glareosa Jord. subsp. glareosa, Adenostyles alpina (L.) Bluff et Fingerh., Dryas octopetala L., Laserpitium peucedanoides L. and Laserpitium krapfii subsp. gaudinii (Moretti) Thell. These plants are herbs and dwarf shrubs that are part of plant communities that grow on calcareous screes (Thlaspion rotundifolii and Petasition paradoxii phytosociological alliances) and/or on thin and skeleton-rich alkaline soils (Caricion firmae, Seslerion caeruleae, Caricion austroalpinae alliances) [40,41,42,43]. There is no information regarding seeds germination of these plants in natural environment. Their seeds were collected in August and September 2018 in two sampling areas of the Southern Alps (Italy): Mount Cavallo/Mount Pegherolo group (Orobie Bergamasche Regional Park, Latitude: 46°02′ N, Longitude: 9°41′ E) [44] and Brenta Dolomites (Adamello-Brenta Natural Park, Latitude: 46°09′ N, Longitude: 10°50′ E). In both areas, the mature seeds were collected from plants growing on limestone screes at 1.600–2.200 m a.s.l. altitudinal range. Plant identification was carried out using Pignatti’s dichotomous keys [43]. The seeds of the same species collected in the two sampling areas were uniformly mixed, air dried, cleaned and stored in darkness at 4 °C for six months, after which germination tests were performed. The scientific names of species are according to “Flora d’Italia” [43].
2.2. Germination Tests
Seeds were sterilised in 15% sodium hypochlorite solution for 5 min [45], then rinsed with distilled water and finally transferred to sterile Petri dishes to avoid the attack of pathogens able to compromise the germination. Three Whatman No. 3 filter paper discs were placed in each Petri dish with 25 seeds placed on top to permit an easy visual control of the germination of each seed. Each Petri dish contained the seeds of a single species and underwent one of the following treatments:
- Addition of 5 mL of distilled water (control);
- Addition of 1 g of CaCO3 and 5 mL of distilled water;
- Addition of 5 mL of a solution containing 100 mg L−1 of gibberellic acid (GA3);
- Addition of 1 g of CaCO3 and 5 mL of a solution containing 100 mg L−1 of GA3.
Petri dishes were hermetically sealed with parafilm to prevent evaporation. Four replicas were performed for each treatment; therefore 400 seeds of each species were used for the germination tests.
The concentration of GA3 solution used in these tests is the same as that used in research by [46] and which allowed the greatest seed germination (more than 90%) of two basophilous species endemics in the Italian Alps. The CaCO3 concentration used is the amount that guarantees that water is saturated with carbonates at room temperature (18 °C) and forms a carbonate layer in the Petri dish, a simulation of the chemical conditions of water and substrate of limestone screes.
Seeds were incubated for 50 days in a germination chamber (FDM-Series C) in the following environmental conditions: 12/12 h light/dark cycle at 23/18 °C respectively. Every two days the Petri dishes were re-randomised [47] and seeds showing radicle emergence were recorded as “germinated” and removed from the plates.
2.3. Data Analysis
At the end of the germination tests, the germination percentage (GRP) and germination speed coefficient (GSP) were calculated according to [48]. In detail, GRP was calculated as follows:
where ni is the number of germinated seeds in the i time, k is the last day of germination evaluation and N is the total number of seeds in each experimental unit. GSP was calculated using this formula:
where Gi is the number of seeds germinated in the i time, k is the last day of germination evaluation and Xi is the number of days from sowing. Both GRP and GPS are expressed in percentage.
For each species a one-way ANOVA test was used to evaluate the effect of the treatments on GRP and GSP. The assumptions of normality of group data and homogeneity of variances had been verified using the Shapiro–Wilk test and Levene’s test respectively. When significant (p < 0.05) effects existed, differences were tested by Tukey’s post-hoc test at 95% confidence level. Finally, the GRP and GSP data of the treatments of each species were analysed using Principal Component Analysis (PCA) in order to highlight the main variables that differentiate them. GRP, GSP and ANOVA were calculated/performed using the “GerminaR” package [48] of R 3.5.2 software [49]. PCA was performed using the “vegan” package [50] of R.
3. Results
At the end of the germination tests, a fair number of the seeds of each species had germinated except for Silene glareosa, Laserpitium peucedanoides and Laserpitium krapfii. Since only few seeds germinated (less than 2.5% considering all the treatments) for these three species, they were excluded from the statistical analyses. Figure 1 shows the cumulative germination lines of the seeds of the other six species undergoing different treatments. The graphs show that the germination lines are different from one species to another and between various treatments. In particular, some species (Adenostyles alpina, Rumex scutatus and Tofieldia calyculata) present, at least for some treatments, germination lines that tend to increase gradually, while other species (Dryas octopetala, Papaver aurantiacum and Pulsatilla alpina) present lines similar to sigmoid curves. The graphs in Figure 1 show that the various species, at the end of the tests, have different germination percentages: only Papaver aurantiacum, Dryas octopetala and Tofieldia calyculata have germination curves of more than 50%. Moreover, for some species (Papaver aurantiacum, Pulsatilla alpina, Rumex scutatus and Tofieldia calyculata) there would seem to be a considerable effect on germination due to GA3.
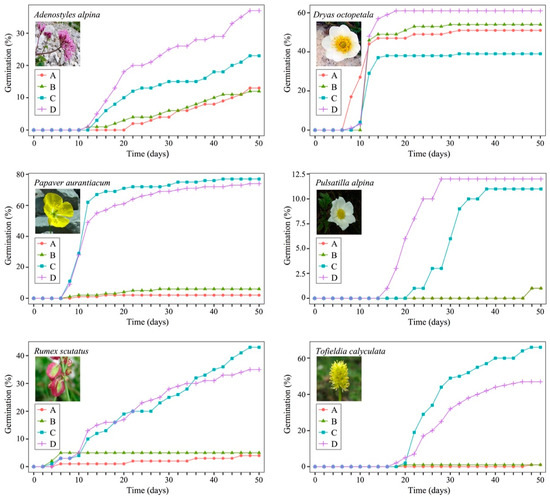
Figure 1.
Germination lines for tested species (excluding Laserpitium krapfii, L. peucedanoides and Silene glareosa) given various treatments: A, water (control); B, water and CaCO3; C, GA3 solution; D, CaCO3 and GA3 solution. The A and B lines of treatments of Pulsatilla alpina are overlapped.
In Table 1, the one-way ANOVA results show that for most species treatment has a significant effect on GRP and GSP. Only for Dryas octopetala does the type of treatment have no significantly different effect on GRP, nor does it have a significantly different effect on GSP for Adenostyles alpina and Papaver aurantiacum.

Table 1.
One-way ANOVA results of main effects (germination percentage (GRP) and germination speed coefficient (GSP)) of treatments. Key: *, significant (p < 0.05).
Figure 2 shows the GRP values of each treatment of each species and the results of Tukey’s post-hoc test. From the graphs, it can be observed that there is a significant difference between the treatments with GA3 (treatment C and D) and without GA3 (A and B) for Papaver aurantiacum, Pulsatilla alpina, Rumex scutatus and Tofieldia calyculata.
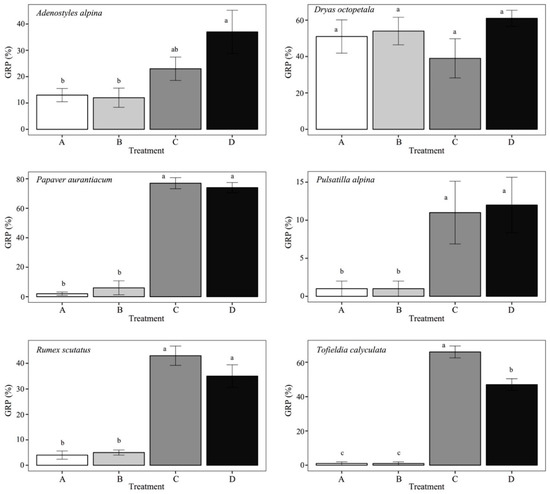
Figure 2.
Bar graphs with germination percentage (GRP) for each treatment: A, water (control); B, water and CaCO3; C, GA3 solution; D, CaCO3 and GA3 solution. Different letters above bars indicate significant differences (p < 0.05) among treatments.
In all these cases, the treatments with GA3 significantly increase GRP, except for the case of Dryas octopetala. Only for Tofieldia calyculata is there a difference between treatments C and D with a greater GRP in C, while for Adenostyles alpina the treatments without GA3 (A and B) are significantly different only compared to D, which is the treatment with the highest GRP value. For no species is GRP significantly different between the control (A) and the treatment with the addition of only CaCO3 (B).
Figure 3 shows the GSP values of each treatment of each species and the results of Tukey’s post-hoc test. Dryas octopetala is the only species that has significantly higher GSP values with treatment A. Rumex scutatus has, instead, significantly higher GSP with treatment B. The same can also be seen for Tofieldya caliculata, although in this case, treatment B led to the germination of very few seeds (Figure 1). Only for Pulsatilla alpina is there a significant difference between the C and D treatments with GSP, which is higher in D.
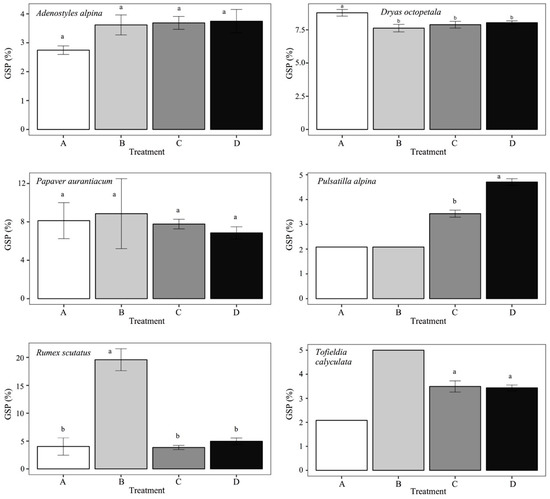
Figure 3.
Bar graphs with a germination speed coefficient (GSP) for each treatment: A, water (control); B, water and CaCO3; C, GA3 solution; D, CaCO3 and GA3 solution. Different letters above bars indicate significant differences (p < 0.05) among treatments. The error is null for those treatments where the error bar is not indicated.
Finally, Figure 4 shows the PCA biplot in which the species have been grouped according to treatment. Group D is the one with the lowest dispersion of points, which suggests that for this treatment the seeds of the various species responded in a similar manner. The PCA biplot suggests a direct correlation between GRP and GA3 and between GSP and CaCO3, since along the x axis (PC1), GRP increases with GA3 treatment, while along the y axis (PC2), GSP increases with CaCO3 treatment.
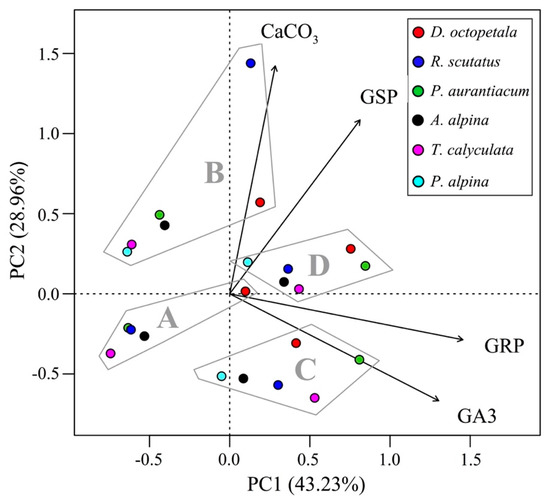
Figure 4.
PCA biplot of the species grouped according to germination treatment: A, water (control); B, water and CaCO3; C, GA3 solution; D, CaCO3 and GA3 solution. The first two axes (PC1 and PC2) explain 72.19% of the total variance in the dataset. Key: GRP, germination percentage; GSP, germination speed coefficient; GA3, gibberellic acid; CaCO3, calcium carbonate.
4. Discussion
The analysis of the germination test results shows that the nine species considered, despite having the same ecological requirements and living in the same environments, responded to the tests very differently. While the seeds of some did not germinate (or very few germinated) with no treatment, others had different responses (as regards GRP and GSP) to the various treatments. This is in agreement with [51] who, after analysing 23 key herbaceous species for use in European habitat restoration, found that differences exist among species in their responsiveness to various germination cues.
The difficulty in germinating the seeds of Silene glareosa, Laserpitium krapfii and L. peucedanoides suggests greater difficulty in producing seedlings of these species, which could affect their potential use in soil bioengineering and/or restoration ecology. On the contrary, Dryas octopela was the only species among those studied whose seeds showed good and rapid germination regardless of the type of treatment. Even the seeds of Adenostyles alpina are able to germinate with the addition of water only but had a much lower germination rate (13%), less than a third compared to that of Dryas octopetala (51%) for the same treatment. As regards the other four species (Papaver aurantiacum, Pulsatilla alpina, Rumex scutatus and Tofieldia calyculata), the percentage of germination in water, as well as that in water and CaCO3, was extremely low. However, these species showed a good response, in terms of GRP, to treatments with GA3, demonstrating that this plant hormone is necessary for their seeds to germinate ex situ. GA3 is one of the main phytohormones to stimulate seed germination of different plants such as Arabidopsis thaliana (L.) Heynh. [52], Primula glaucescens Moretti and Physoplexis comosa (L.) Schur. [46].
Although the species considered are all basiphilous [40], and the seeds were collected from plants present in limestone screes, the addition of only CaCO3 did not produce, for any species, important effects as regards GRP; on the other hand, for some species, it increased GSP values. Notwithstanding this, this effect would not have particular relevance for the production of young plants as the addition of CaCO3 could reduce the germination time by only a few days.
Other actions to increase the germination ability of native seeds would certainly be interesting to investigate with the goal of using them in soil bioengineering and/or restoration works [38,39,53]. In this regard, some treatments with laccase to increase the rate of seed coat degradation [54] and treatment with sulfuric acid followed by manual cleaning [53] apparently increased the germination of orchid and Australian native grasses seeds, respectively, and could also be tested on alpine pioneer species. Low temperatures could also favour the germination of alpine pioneer plant seeds as found in [55].
In this preliminary study, the same concentrations of GA3 and CaCO3 were used for all the considered species to analyse the answer of various alpine species to the same standardized treatments. It should be considered, however, that each species could need different concentrations of GA3 and CaCO3 to maximise the germination of seeds. In fact, in [46] it was demonstrated that the optimum GA3 concentration is different for Primula glaucescens and Physoplexis comosa, which are species belonging to two different families (Primulaceae and Campanulaceae respectively) growing in the same habitat (limestone rocks). The different requirements of each species could be the reason why the seeds of Laserpitium krapfii and L. peucedanoides (the only two species of the Umbelliferae family among those considered in this study) and those of Silene glareosa (Caryophyllaceae) had difficulties germinating. Ad hoc studies could be useful for identifying the optimum concentrations of GA3 and/or CaCO3 to increase or induce the germination of every single species or species belonging to the same genus/family. Likewise, it would be useful and interesting to perform germination tests in laboratory using both sterile substrates and natural soils to evaluate possible differences between the number of germinated seeds in sterile conditions and the number of plants that emerge from the soil. Natural substrates could in fact contain substances able to promote or disadvantage the germination or pathogens (as fungi or bacteria) able to compromise the survival of young plants even before their emergence from the soil. Performing germination tests in laboratory (under controlled conditions) and in the field (environmental conditions) would also be interesting for understanding if, for each species, there are differences in germination performances due to environmental conditions such as climate (for example different temperature and humidity ranges) and/or soil (for example the presence of specific microorganisms as bacteria and/or fungi). These studies, although very complex due to the number of variables to consider, would certainly help to have a better comprehension of propagation and, in general, of the entire lifespan of the alpine species, which still present many aspects that are worth investigating [56] and that could be useful for practical purposes such as bioengineering.
From the results of this research, it is clear that, for most of the species considered, it would not be difficult to germinate the seeds ex situ (in a laboratory/greenhouse) at room temperature (18–23 °C). Although representing a step of fundamental importance, this is not the only aspect to be analysed in order to produce seeds for donor plants to be cultivated ex situ and/or plants to be transplanted in soil bioengineering works. In fact, immediately after seed germination, the question arises of the growth and development of young plants that may not tolerate the environmental conditions in which the seeds germinated. In the case of the production of young plants to be transplanted into the wild, this problem could consist in the length of time needed for the plants to produce a good root system and an equally developed epigeal apparatus. In this period of time, the young plants could be cultivated in climatic chambers able to simulate the climatic conditions of mountain environments and in vases containing limestone gravel to replicate the characteristics of the substrates in which they grow in nature. When producing plants from which to collect the seeds (to be used for the production of additional plants for transplants and/or to produce mixtures of seeds) the issue would be even more complicated as they should be cultivated in environments (climatic chambers, greenhouses and/or fields) that allow these plants to bloom, bear fruit and produce seeds that are healthy (vital) and easy to harvest. However, the effort would be justified by the results.
In fact, many pioneer herbaceous plants of the Alps that colonize screes and/or poorly developed soils are little known or unknown (and used) in soil bioengineering, although they are well known by botanists dealing with flora and/or the study of plant communities (phytosociologists). These species, as well as being fascinating for their beauty and their ability to live in extreme environments [56,57], make up the communities of the early stages of primary vegetation successions [58], the study of which can be extremely useful for restoration of severely damaged habitats [59]. The species of the early stages of primary successions are able to modify the environment (the soil in particular) in which they live, creating the conditions for the growth of other species (herbs, shrubs and trees) which are more demanding and which substitute them, leading to more mature, stratified and stress-tolerant plant communities [20,32,35,41,60,61,62,63,64], that is, those in intermediate/late stages of successions. In particular, some species of the communities that colonize the screes (those of Thlaspietea rotundifolii phytosociological class) are able to stabilize debris (“scree stabilizer”, [65]) and/or accumulate/provide fine sediments and organic substances to the soil (“scree accumulator”, [65]) [66], for example Dryas octopetala L. [64,67]. These species, due to their ability to consolidate debris and improve soil fertility, could be particularly useful in soil bioengineering works for the stabilization of landslide areas, just as they could be equally useful in ecological restoration interventions in areas where anthropogenic disturbance has produced environments with poor soils and rich in debris (such as those that occur following the construction of ski slopes or quarrying). The ability of Dryas octopetala seeds to germinate with the mere addition of distilled water added to the ability of this species to improve soil fertility [68] and to act as an “ecosystem engineer species” [64,67], hence changing its physical environment through its adapted traits and thereby creating habitats for other species [69,70]. This makes it interesting for use in soil bioengineering.
In order to innovate soil bioengineering, alongside purely botanical studies, biomechanical studies should be conducted concerning the ability of the roots (or other hypogeal organs) to stabilize the soil [8,71,72,73]. Such studies, in addition to improving root traction techniques [74], should be carried out to gather more information on plants on which there is very little data, such as those considered in this work. In fact, of the species considered in this research, only for Rumex scutatus is there data on root biomechanical properties. This species, according to [8], has high tensile force (>120 N measured on larger roots) and pronounced elasticity, which make it similar to the larch (Larix decidua) and therefore particularly interesting in soil bioengineering. The same author highlighted an excellent balance between ecological advantages and contribution to soil engineering properties through its roots by Deschampsia cespitosa. This is an herbaceous species of alpine pastures for which, however, there are no data regarding seed germinability ex situ nor regarding cultivation for the production of seeds and/or plants to be used in soil bioengineering or restoration projects. The same is true for Dryas octopetala, whose properties as an “ecosystem engineer species” [64,67] are known, as we exposed above, but for which data are lacking on the mechanical properties of its roots/stems for soil stabilization; moreover, before this research, even the germination potential of its seeds was not known.
Therefore, in coming years, it will be extremely important to obtain biotechnical test data to integrate biological and ecological data (and vice versa) so as to obtain lists of species of which we know the main characteristics useful for the purposes of soil bioengineering. Although such researches would require great effort and collaboration between researchers of various disciplines (biologists and engineers in particular), it should be encouraged by the fact that the market demand for seeds of native plants to be used in restoration projects, also including those of soil bioengineering, is high, at least in Europe [75]. Moreover, among the objectives of the European Union 2020 Biodiversity Strategy, there is the target to restore at least 15% of degraded ecosystems by 2020 and, according to the study performed by [76], the availability of native seeds to use for these objectives still appears to be a critical factor.
To encourage the study of new species to be used in soil bioengineering, in addition to implementing research, regulations are needed that clearly establish which type of seeds/plants should be used in the various types of interventions, thus making the use of autochthonous seeds/plants by those who carry out soil bioengineering operations mandatory. This would certainly create a stimulus for the creation of chains for the production of autochthonous seeds and would eliminate competition with the current low-priced commercial seeds, since the prices of autochthonous seeds are far higher because of high production costs due mainly to current technological tools and lack of knowledge [75,76,77]. Some countries in Europe such as Germany, Austria and Switzerland have adopted a series of regulations governing the production and use of autochthonous seeds, so that in these countries seed zones have been identified (geographical areas within which seeds are to be collected, propagated and sown) [78,79] and in which various seed producers (mostly private bodies) operate. In particular, there are 12 native seed producers in Germany [80], 10 in Austria [81] and 12 in Switzerland [82]. In Italy, where there are still no regulatory instruments governing the use of autochthonous seeds in soil bioengineering and/or restoration projects, there are only 4 companies that produce these plant materials [76] of which three (“Seme Nostrum”, “Centro Flora Autoctona” and “Flora Conservation”) are in the North of Italy. These companies now produce small quantities of native seeds to be used in restoration projects both in the Po Valley, where there are many areas degraded by human activity [83,84,85], and in the hilly and mountainous areas of the Alps. Regarding the production of pioneer plants (and not only) to be used in the Alps, it would be very important to create supranational seed/plant production centres [86] in order to encourage cooperation between European states. This last action could fall within the EU-Strategy of the Alpine Region (EUSALP) [87] and within the aims of the “Native Seed Science, Technology and Conservation” (NASSTEC) training network [39,75].
The study of this kind of pioneer plant use in bioengineering is still to implement as relatively little biogeomorphic research has been carried out at high elevations in the emerging fields of restoration ecology [88], and that which has been done has focused largely [89,90] on the use of vegetation to improve slope stability. The interactions between soil and the organisms that are conducive to decreasing sediment runoff [91] is a great concern on high elevation ski trails [92] and of great importance for the natural, economic and social wellness of high-mountain communities. Avoiding erosion and preserving landscape quality are fundamental both for winter and summer tourism. Further, a multidisciplinary approach considering the role of vegetation and changes due to climatic events for the study of composite debris cones would strongly enhance the capability to investigate natural hazards and assess environmental impacts in mountain regions [93] and to take the right actions.
5. Conclusions
This research assessed the ex situ germination capacity of nine alpine pioneer species in order to start to evaluate the feasibility of reproducing seeds and/or plants in an environment different from the one in which they grow in nature for use in soil bioengineering for slope stabilization and restoration. The relevance and novelty of this research is due to the fact the little-known/used species in soil bioengineering and restoration ecology were considered and that the seeds of each of them, although collected from plants with the same ecological requirements and from the same environment (limestone screes of the Southern Alps), had different responses to standardised germination tests. These differences show how it is important to have a deep knowledge of the biology and ecology of plant species with a view to using them for slope stabilization, starting from the baseline of germination. Additional researches should be addressed to investigating this biodiversity asset and will allow better understanding of the life of alpine plants as well as defining their performances in soil bioengineering and possible applications to improve environmental restoration work.
Author Contributions
Conceptualization, data curation, plant identification, formal analysis, methodology, supervision, visualization, writing—original draft, writing—review and editing: L.G.; visualization, writing—original draft, writing—Review and Editing: V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported supported by FISR-MIUR (“Italian Mountain Lab” project) and Department for Regional Affairs and Autonomies (DARA) of the Italian Presidency of the Council of Ministers (DARA-CRC Ge.S.Di.Mont. agreement).
Acknowledgments
We would like to thank Nicola Pellizzari and Pietro Giupponi for the help provided in collecting seeds and performing germination tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gariano, S.L.; Guzzetti, F. Landslides in a changing climate. Earth Sci. Rev. 2016, 162, 227–252. [Google Scholar] [CrossRef]
- Yuan, X.C.; Wei, Y.M.; Wang, B. Risk management of extreme events under climate change. J. Clean. Prod. 2017, 166, 1169–1174. [Google Scholar] [CrossRef]
- Einhorn, B.; Eckert, N.; Chaix, C.; Ravanel, L.; Deline, P.; Gardent, M.; Boudières, V.; Richard, D.; Vengeon, J.-M.; Schoeneich, G.G.E.P. Climate change and natural hazards in the Alps. J. Alp. Res. 2015, 103, 1–38. [Google Scholar] [CrossRef]
- Kron, W.; Löw, P.; Kundzewicz, Z. Changes in risk of extreme weather events in Europe. Environ. Sci. Policy 2019, 100, 74–83. [Google Scholar] [CrossRef]
- Nordregio. Mountain Areas in Europe: Analysis of Mountain Areas in EU Member States, Acceding and Other European Countries; Commissioned Report by the European Commission–DG Regional Policy; Nordregio: Brussels, Belgium, 2004. [Google Scholar]
- Keenleyside, C.; Tucker, G.M. Farmland Abandonment in the EU: An Assessment of Trends and Prospects Report Prepared for WWF; Institute for European Environmental Policy: London, UK, 2010. [Google Scholar]
- Terres, J.M.; Nisini, L.; Anguiano, E. Assessing the Risk of Farmland Abandonment in the EU. Final Report; Joint Research Centre: Ispra, Italy, 2013. [Google Scholar]
- Cislaghi, A.; Giupponi, L.; Tamburini, A.; Giorgi, A.; Bischetti, G.B. The effects of mountain grazing abandonment on plant community, forage value and soil properties: Observations and field measurements in an alpine area. Catena 2019, 181, 104086. [Google Scholar] [CrossRef]
- Tanzini, M. Fenomeni Franosi e Opere di Stabilizzazione, 2nd ed.; Dario Flaccovio Editore: Palermo, Italy, 2011; pp. 1–35. [Google Scholar]
- Trigila, A.; Iadanza, C.; Bussettini, M.; Lastoria, B. Dissesto Idrogeologico in Italia: Pericolosità e Indicatori di Rischio–Ed. 2018; Rapporti 287/2018; ISPRA: Roma, Italy, 2018; pp. 3–26. [Google Scholar]
- EU Commission. EUROPE 2020 A Strategy for Smart, Sustainable and Inclusive Growth. 2010. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52010DC2020andfrom=it (accessed on 1 March 2020).
- EU Commission. Roadmap to a Resource Efficient Europe. 2011. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011DC0571andfrom=IT (accessed on 1 March 2020).
- EEA. Green Infrastructure and Territorial Cohesion. European Environment Agency; Technical Report 18/2011; EEA: Copenhagen, Denmark, 2011. [Google Scholar]
- Baró, F.; Bugter, R.; Gómez-Baggethun, E.; Kopperoinen, L.; Liquete, C.; Potschin, M. Green Infrastructure. In OpenNESS Ecosystem Service Reference Book; EC FP7 Grant Agreement No. 308428; Potschin, M., Jax, K., Eds.; OpenNESS: Belgium, Brussels, 2015. [Google Scholar]
- Schiechtl, H.M. Sicherungsarbeiten im Landschafsbau; Callwey: Munich, Germany, 1973. [Google Scholar]
- Schiechtl, H.M. Bioengineering for Land Reclamation and Conservation; University of Alberta Press: Edmonton, AB, Canada, 1980. [Google Scholar]
- Schiechtl, H.M. Bioingegneria Forestale-Biotecnica Naturalistica; Edizioni Castaldi: Feltre, Italy, 1991. [Google Scholar]
- Bischetti, G.B.; Di Fi Dio, M.; Florineth, F. On the origin of soil bioengineering. Landsc. Res. 2014, 39, 583–595. [Google Scholar] [CrossRef]
- Studer, R.; Zeh, H. Soil Bioengineering. Construction Type Manual; Hochschulverlag: Zurich, Switzerland, 2014. [Google Scholar]
- Giupponi, L.; Bischetti, G.B.; Giorgi, A. Ecological index of maturity to evaluate the vegetation disturbance of areas affected by restoration work: A practical example of its application in an area of the southern alps. Restor. Ecol. 2015, 23, 635–644. [Google Scholar] [CrossRef]
- Giupponi, L.; Bischetti, G.B.; Giorgi, A. A proposal for assessing the success of soil bioengineering work by analysing vegetation: Results of two case studies in the Italian Alps. Landsc. Ecol. Eng. 2017, 13, 305–318. [Google Scholar] [CrossRef]
- Arques, S. Géodynamique, Colonisation Végétale et Phytodiversité des talus D’éboulis dans le Massif de la Grande Chartreuse (Pre’alpes Fran¸caises du Nord). Caractéristiques Géo-Écologiques et Sensibilité aux Changements Environnementaux. Ph.D. Thesis, Joseph Fourier University, Institut de Géographie Alpine, Grenoble, France, 2006. [Google Scholar]
- Beaudiére, A. Modes de Perception de L’éboulis par les Botanistes; Bulletin de l’Association de Géographes FranÇais: Paris, France, 1983; Volume 491, pp. 25–32. [Google Scholar]
- Pérez, F.L. Geobotanical influence of talus movement on the distribution of caulescent Andean rosettes. Flora 1994, 189, 353–371. [Google Scholar] [CrossRef]
- Behre, C.H. Talus behavior above timber in the Rocky Mountains. J. Geol. 1933, 41, 622–635. [Google Scholar] [CrossRef]
- Rahn, P.H. The relationship between natural forested slopes and angles of repose for sand and gravel. Geol. Soc. Am. 1969, 80, 2123–2128. [Google Scholar] [CrossRef]
- Sigafoos, R. Soil instability in tundra vegetation. Ohio J. Sci. 1951, 51, 281–298. [Google Scholar]
- Smith, J. Some moving soils in Spitsbergen. J. Soil Sci. 1956, 7, 10–21. [Google Scholar] [CrossRef]
- Howard, J.A.; Mitchell, C.W. Phytogeomorphology; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Hupp, C.R.; Osterkamp, W.R.; Howard, A.D. Preface (to Binghamton Symposium volume on Biogeomorphology). Geomorphology 1995, 13, Vii–Viii. [Google Scholar] [CrossRef]
- Phillips, J.D. Soils as extended phenotypes. Geoderma 2009, 149, 143–151. [Google Scholar] [CrossRef]
- Giupponi, L.; Borgonovo, G.; Giorgi, A.; Bischetti, G.B. How to renew soil bioengineering for slope stabilization: Some proposals. Landsc. Ecol. Eng. 2019, 15, 37–50. [Google Scholar] [CrossRef]
- Dierschke, H. Pflanzensoziologie: Grundlagen und Methoden; UTB: Stuttgart, Germany, 1994. [Google Scholar]
- Biondi, E. Phytosociology today: Methodological and conceptual evolution. Plant Biosyst. 2011, 145, 19–29. [Google Scholar] [CrossRef]
- Giupponi, L.; Bischetti, G.B.; Giorgi, A. Vegetation analysis and estimation of forest reconstitution time in protected areas of Val Camonica (Southern Alps) where a commercial mixture of seeds was sown. J. Prot. Mt. Areas Res. Manag. 2017, 9, 22–29. [Google Scholar] [CrossRef]
- Aavik, T.; Edwards, P.J.; Holderegger, R.; Graf, R.; Billeter, R. Genetic consequences of using seed mixtures in restoration: A case study of a wetland plant Lychnis flos-cuculi. Biol. Conserv. 2012, 145, 195–204. [Google Scholar] [CrossRef]
- Hagen, D.; Hansen, T.I.; Graae, B.J.; Rydgren, K. To seed or not to seed in alpine restoration: Introduced grass species outcompete rather than facilitate native species. Ecol. Eng. 2014, 64, 255–261. [Google Scholar] [CrossRef]
- Krautzer, B.; Hacker, E. Soil-Bioengineering: Ecological Restoration with Native Plant and Seed Material Conference. In Proceedings of the Soil-Bioengineering: Ecological Restoration with Native Plant and Seed Material Conference, Liezen, Austria, 5–9 September 2006. [Google Scholar]
- Elzenga, J.T.M.; Bekker, R.M.; Pritchard, H.W. Maximising the use of native seeds in restoration projects. Plant Biol. 2019, 21, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Landolt, E.; Baumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmle, R.W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.H.; Theurillat, J.-P.; et al. Flora Indicativa. Ecological Indicator Values and Biological Attributes of the Flora of Switzerland and the Alps; Haupt Verlag: Bern, Switzerland, 2010. [Google Scholar]
- Pignatti, E.; Pignatti, S. Plant. Life of the Dolomites. Vegetation Structure and Ecology; Springer Nature: Heidelberg/Berlin, Germany, 2014. [Google Scholar]
- Biondi, E.; Blasi, C. Prodromo Della Vegetazione d’Italia. Available online: http://www.prodromo-vegetazione-italia.org (accessed on 1 March 2020).
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italia, 2017. [Google Scholar]
- Giupponi, L.; Giorgi, A. Mount Cavallo Botanical Path: A proposal for the valorization of an area of the Orobie Bergamasche Regional Park (Southern Alps). J. Prot. Mt. Areas Res. Manag. 2017, 9, 5–15. [Google Scholar] [CrossRef]
- Martiónez-Dióaz, E.; Martiónez-Saónchez, J.J.; Conesa, E.; Franco, J.A.; Vicente, M.J. Germination and morpho-phenological traits of Allium melananthum, a rare species from south-eastern Spain. Flora 2018, 249, 16–23. [Google Scholar] [CrossRef]
- Cerabolini, B.; De Andreis, R.; Ceriani, R.M.; Pierce, S.; Raimondi, B. (2004) Seed germination and conservation of endangered species from the Italian Alps: Physoplexis comosa and Primula glaucescens. Biol. Conserv. 2004, 117, 351–356. [Google Scholar] [CrossRef]
- Yang, J.; Lovett-Doust, J.; Lovett-Doust, L. Seed germination patterns in green dragon (Arisaema dracontium, Araceae). Am. J. Bot. 1999, 86, 1160–1167. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Benites-Alfaro, O.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2018, 34, 339–346. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Wien, Austria, 2018; Available online: http://www.r-project.org (accessed on 1 March 2020).
- Dixon, P. Vegan, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Del Egido, L.L.; Toorop, P.E.; Lanfermeijer, F.C. Seed enhancing treatments: Comparative analysis of germination characteristics of 23 key herbaceous species used in European restoration programmes. Plant Biol. 2019, 21, 398–408. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Pedrini, S.; Lewandrowski, W.; Stevens, J.C.; Dixon, K.W. Optimising seed processing techniques to improve germination and sowability of native grasses for ecological restoration. Plant Biol. 2019, 21, 415–424. [Google Scholar] [CrossRef]
- Pierce, S.; Spada, A.; Caporali, E.; Ceriani, R.M.; Buffa, G. Enzymatic scarification of Anacamptis morio (Orchidaceae) seed facilitates lignin degradation, water uptake and germination. Plant Biol. 2019, 21, 409–414. [Google Scholar] [CrossRef]
- Schwienbacher, E.; Navarro-Cano, J.A.; Neuner, G.; Erschbamer, B. Correspondence of seed traits with niche position in glacier foreland succession. Plant Ecol. 2012, 213, 371–382. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems; Springer Nature: Heidelberg/Berlin, Germany, 2003. [Google Scholar]
- Giupponi, L.; Giorgi, A. A contribution to the knowledge of Linaria tonzigii Lona, a steno-endemic species of the Orobie Bergamasche Regional Park (Italian Alps). J. Prot. Mt. Areas Res. Manag. 2019, 11, 16–24. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Walker, L.R.; del Moral, R. Lessons from primary succession for restoration of severely damaged habitats. Appl. Veg. Sci. 2008, 12, 56–67. [Google Scholar] [CrossRef]
- Andreis, C.; Caccianiga, M.; Cerabolini, B. Vegetation and environmental factors during primary succession on glacier forelands: Some outlines from the Italian Alps. Plant Biosyst. 2001, 135, 295–310. [Google Scholar] [CrossRef]
- Paiero, P.; Paiero, G. La Vegetazione Rivierasca Alpina. In Atti del 41° Corso di Cultura in Ecologia: Conoscere il Sistema Fiume Nell’ambiente Alpino; D’Agostino, V., Carraro, V., Eds.; Dipartimento TeSAF Università degli Studi di Padova: Padova, Italy, 2005; pp. 28–45. [Google Scholar]
- Caccianiga, M.; Luzzaro, A.; Pierce, S.; Ceriani, R.M.; Cerabolini, B. The functional basis of a primary succession resolved by CSR classification. Oikos 2006, 112, 10–20. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Eichel, J. Vegetation Succession and Biogeomorphic Interactions in Glacier Forelands. In Geomorphology of Proglacial Systems. Geography of the Physical Environment; Heckmann, T., Morche, D., Eds.; Springer Nature: Zürich, Switzerland, 2019; pp. 327–349. [Google Scholar]
- Schröter, C. Das Pflanzenleben der Alpen; Verlag von Albert Raustein: Zürich, Switzerland, 1926. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-MadeHabitats; Springer International Publishing: Zürich, Switzerland, 2017. [Google Scholar]
- Eichel, J.; Corenblit, D.; Dikau, R. (2016) Conditions for feedbacks between geomorphic and vegetation dynamics on lateral moraine slopes: A biogeomorphic feedback window. Earth Surf. Proc. Land. 2016, 41, 406–419. [Google Scholar] [CrossRef]
- Reisigl, H.; Keller, R. Alpenpflanzen im Lebensraum; Spektrum Akademischer Verlag: Heidelberg, Germany, 1994. [Google Scholar]
- Jones, C.G. Ecosystem engineers and geomorphological signatures in landscapes. Geomorphology 2012, 157–158, 75–87. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachack, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Root biomechanical properties during establishment of woody perennials. Ecol. Eng. 2010, 109, 196–206. [Google Scholar] [CrossRef]
- Vergani, C.; Chiaradia, E.A.; Bischetti, G.B. Variability in the tensile resistance of roots in Alpine forest tree species. Ecol. Eng. 2012, 46, 43–56. [Google Scholar] [CrossRef]
- Pal, A.K.; Ahmed, A.; Panday, V.K. Soil Binding Capacity of Different Forage Grasses in Terms of Root. Indian J. Hill Farming 2019, 32, 137–143. [Google Scholar]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Cislaghi, A.; Vergani, C.; Hubble, T.; Phillips, C.; Stokes, A. Methods to measure the mechanical behaviour of tree roots: A review. Ecol. Eng. 2017, 109, 256–271. [Google Scholar] [CrossRef]
- De Vitis, M.; Mondoni, A.; Pritchard, H.W.; Laverack, G.; Bonomi, C. Native Seed Ecology, Production and Policy–Advancing Knowledge and Technology in Europe; MUSE: Trento, Italy, 2018. [Google Scholar]
- De Vitis, M.; Abbandonato, H.; Dixon, K.W.; Laverack, G.; Bonomi, C.; Pedrini, S. The European Native Seed Industry: Characterisation and Perspectives in Grassland Restoration. Sustainability 2017, 9, 1682. [Google Scholar] [CrossRef]
- Schmidt, I.B.; de Urzedo, D.I.; Pina-Rodrigues, F.C.M.; Vieira, D.L.M.; de Rezende, G.M.; Sampaio, A.B.; Junqueira, R.G.P. Community-based native seed production for restoration in Brazil–the role of science and policy. Plant Biol. 2019, 21, 389–397. [Google Scholar] [CrossRef]
- Nevill, P.G.; Tomlinson, S.; Elliott, C.P.; Espeland, E.K.; Dixon, K.W.; Merritt, D.J. Seed production areas for the global restoration challenge. Ecol. Evol. 2016, 6, 7490–7497. [Google Scholar] [CrossRef]
- Durka, W.; Michalski, S.G.; Berendzen, K.W.; Bossdorf, O.; Bucharova, A.; Hermann, J.; Hölzel, N.; Kollmann, J. Genetic differentiation within multiple common grassland plants supports seed transfer zones for ecological restoration. J. Appl. Ecol. 2017, 54, 116–126. [Google Scholar] [CrossRef]
- Prasse, R.; Kunzmann, D.; Schröder, R. Entwicklung und Praktische Umsetzung Naturschutzfachlicher Mindestanforderungen an Einen Herkunftsnachweis fuör Gebietseigenes Wildpflanzensaatgut Krautiger Pflanzen; Institute for Environmental Planning of the Gottfried Wilhelm Leibniz Universitaöt: Hannover, Germany, 2010. [Google Scholar]
- REWISA. Pruöfrichtlinie fuör die Gewinnung und den Vertrieb von Regionalen Wildgraösern und Wildkraöutern (REWISA®). Lehr- und Forschungszentrum fuör Landwirtschaft; Raumberg-Gumpenstein: Irdning, Austria, 2010. [Google Scholar]
- SKEW. Empfehlungen fuör den Anbau und Die Verwendung von Pflanz–Und Saatgut Einheimischer Wildpflanzen. Sekretariat; SKEW: Nyon, Switzerland, 2009. [Google Scholar]
- Giupponi, L.; Corti, C.; Manfredi, P. Onopordum acanthium subsp. acanthium in una ex-discarica della Pianura Padana (Piacenza). Ital. Bot. 2013, 45, 213–219. [Google Scholar]
- Giupponi, L.; Corti, C.; Manfredi, P. The vegetation of the Borgotrebbia landfill (Piacenza, Italy): Phytosociological and ecological characteristics. Plant Biosyst. 2015, 149, 865–874. [Google Scholar] [CrossRef]
- Giupponi, L.; Corti, C.; Manfredi, P.; Cassinari, C. Application of the floristic-vegetational indexes system for the evaluation of the environmental quality of a semi-natural area of the Po Valley (Piacenza, Italy). Plant Sociol. 2013, 50, 47–56. [Google Scholar] [CrossRef]
- Merrit, D.J.; Dixon, K.W. Restoration seed banks-A matter of scale. Science 2011, 332, 424–425. [Google Scholar] [CrossRef]
- Pagliacci, F.; Pavone, P.; Russo, M.; Giorgi, A. Regional structural heterogeneity: Evidence and policy implications for RIS3 in macro-regional strategies. Reg. Stud. 2019, 54, 765–775. [Google Scholar] [CrossRef]
- Pérez, F.L. Phytogeomorphic Influence of Stone Covers and Boulders on Plant Distribution and Slope Processes in High-Mountain Areas. Geogr. Compass 2009, 3, 1774–1803. [Google Scholar] [CrossRef]
- Cammeraat, E.; van Beek, R.; Kooijman, A. Vegetation succession and its consequences for slope stability in SE Spain. Plant Soil 2005, 278, 135–147. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Norris, J.E.; Wint, J. Site investigation for the effects of vegetation on ground stability. J. Geotech. Geol. Eng. 2006, 24, 467–481. [Google Scholar] [CrossRef]
- Giupponi, L.; Pentimalli, D.; Manzo, A.; Panseri, S.; Giorgi, A. Effectiveness of fine root fingerprinting as a tool to identify plants of the Alps: Results of a preliminary study. Plant Biosyst. 2018, 152, 464–473. [Google Scholar] [CrossRef]
- Isselin-Nondedeu, F.; Bédécarrats, A. Influence of alpine plants growing on steep slopes on sediment trapping and transport by runoff. Catena 2007, 71, 330–339. [Google Scholar] [CrossRef]
- Baroni, C.; Armiraglio, S.; Gentili, R.; Carton, A. Landform-vegetation units for investigating the dynamics and geomorphologic evolution of alpine composite debris cones (Valle dell’Avio, Adamello Group, Italy). Geomorphology 2007, 84, 59–79. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).