Disinfection Methods and Survival of SARS-CoV-2 in the Environment and Contaminated Materials: A Bibliometric Analysis
Abstract
1. Introduction
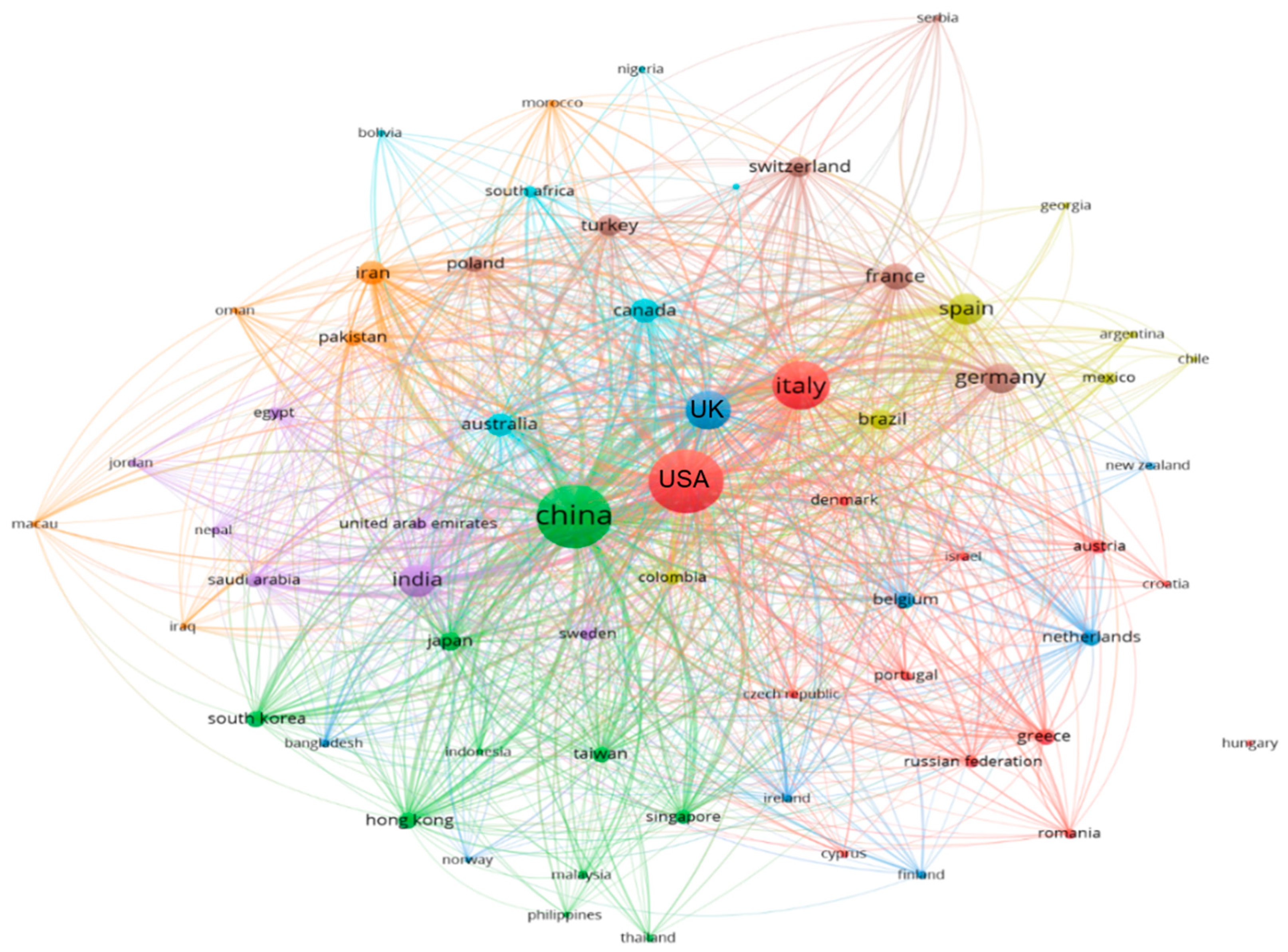
2. Bibliometric Analysis of SARS-CoV-2 in the Literature
3. SARS-CoV-2 in Wastewater
4. Treatment of Hospital Wastewater Contaminated with SARS-CoV-2
5. Disinfection of Sars-Cov-2 in Contaminated Surfaces
6. Disinfection of Disposable Medical Face Masks Contaminated with SARS-CoV-2
7. Survival of SARS-CoV-2 in the Air
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woo, P.C.; Lau, S.K.; Chu, C.M.; Chan, K.H.; Tsoi, H.W.; Huang, Y.; Wong, B.H.; Poon, R.W.; Cai, J.J.; Luk, W.K.; et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rosenfield, E.; Hu, M.; Mi, B. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 2013, 47, 2949–2958. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 4, 1–3. [Google Scholar] [CrossRef]
- Ceustermans, A.; De Clercq, D.; Aertsen, A.; Michiels, C.; Geeraerd, A.; Van Impe, J.; Coosemans, J.; Ryckeboer, J. Inactivation of Salmonella Senftenberg strain W 775 during composting of biowastes and garden wastes. J. Appl. Microbiol. 2007, 103, 53–64. [Google Scholar] [CrossRef]
- Dias, E.; Ebdon, J.; Taylor, H. The application of bacteriophages as novel indicators of viral pathogens in wastewater treatment systems. Water Res. 2018, 129, 172–179. [Google Scholar] [CrossRef]
- Hindson, J. COVID-19: Faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 259. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The Presence of SARS-CoV-2 RNA in Feces of COVID-19 Patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef]
- Spongberg, A.L.; Witter, J.D. Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. J. Sci. Environ. 2008, 397, 148–157. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K.; Mishra, B.K. Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies—A case study of Eastern region of India. Ecotoxicol. Environ. Saf. 2015, 113, 433–438. [Google Scholar] [CrossRef] [PubMed]
- AL-Gheethi, A.A.; Ismail, N.; Lalung, J.; Talib, A.; Kadir, M.O.A. Reduction of Faecal Indicators and Elimination of Pathogens from Sewage Treated Effluents by Heat Treatment. Casp. J. Appl. Sci. Res. 2013, 2, 39–55. [Google Scholar]
- Gomez-Couso, H.; Fontan-Sainz, M.; Sichel, C.; Fernandez-Ibanez, P.; Ares-Mazas, E. Efficacy of the solar water disinfection method in turbid waters experimentally contaminated with Cryptosporidium parvum oocysts under real field conditions. Trop. Med. Int. Health 2009, 14, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Athirah, A.; Al-Gheethi, A.A.S.; Noman, E.A.; Mohamed, R.M.S.R.; Kassim, A.H.M. Centralised and decentralised transport systems for greywater and the application of nanotechnology for treatment processes. In Management of Greywater in Developing Countries; Springer: Cham, Switzerland, 2019; pp. 227–244. [Google Scholar]
- Noman, E.; Al-Gheethi, A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Inactivating pathogenic bacteria in greywater by biosynthesized Cu/Zn nanoparticles from secondary metabolite of Aspergillus iizukae; optimization, mechanism and techno economic analysis. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of metal nanoparticles from fungi and metal salts: Scope and application. Nano Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Kibbee, R.; Örmeci, B. Peracetic acid (PAA) and low-pressure ultraviolet (LP-UV) inactivation of Coxsackievirus B3 (CVB3) in municipal wastewater individually and concurrently. Water Res. 2020, 183, 116048. [Google Scholar] [CrossRef]
- ISO. International Standard Organisation. Aseptic Processing of Health Care Products, Part 1, General Requirements; International Standard ISO 13408-1 1998; International Standard Organisation: Geneva, Switzerland, 1998. [Google Scholar]
- Al-Gheethi, A.A.; Efaq, A.N.; Bala, J.D.; Norli, I.; Abdel-Monem, M.O.; Kadir, M.A. Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl. Water Sci. 2018, 8, 74. [Google Scholar] [CrossRef]
- Trajano, D.; Dias, E.; Ebdon, J.; Taylor, H. Limitations of chlorine disinfection of human excreta: Implications for Ebola disease control. In Proceedings of the 39th WEDC International Conference, Kumasi, Ghana, 11–15 July 2016. [Google Scholar]
- Siddiqi, K.S.; ur Rahman, A.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Magal, P.; Seydi, O.; Webb, G. Understanding Unreported Cases in the 2019-Ncov Epidemic Outbreak in Wuhan, China, and the Importance of Major Public Health Interventions. Biology 2020, 9, 50. [Google Scholar] [CrossRef]
- WHO. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. 2020. Available online: https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (accessed on 3 April 2020).
- Rubio-Romero, J.C.; del Carmen Pardo-Ferreira, M.; García, J.A.T.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef] [PubMed]
- Günter, K.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic–Case study from the Republic of Ireland. Sci. Total Environ. 2020, 725, 138532. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S. Sterilization and disinfection. In Essentials of Neuroanesthesia; Academic Press: Cambridge, MA, USA, 2017; pp. 929–944. [Google Scholar]
- Efaq, A.N.; Rahman, N.N.N.A.; Nagao, H.; Al-Gheethi, A.; Shahadat; Kadir, M.O.A. Supercritical carbon dioxide as non-thermal alternative technology for safe handling of clinical wastes. Environ. Process. 2015, 2, 797–822. [Google Scholar] [CrossRef]
- Jinadatha, C.; Simmons, S.; Dale, C.; Ganachari-Mallappa, N.; Villamaria, F.C.; Goulding, N.; Tanner, B.; Stachowiak, J.; Stibich, M. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. Am. J. Infect. Control 2015, 43, 412–414. [Google Scholar] [CrossRef]
- Katie, O.; Gertsman, S.; Sampson, M.; Webster, R.; Tsampalieros, A.; Ng, R.; Gibson, J.; Lobos, A.-T.; Acharya, N.; Agarwal, A.; et al. Decontaminating N95 masks with Ultraviolet Germicidal Irradiation (UVGI) does not impair mask efficacy and safety: A Systematic Review. J. Hosp. Infect. 2020, 106, 163–175. [Google Scholar]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; La Bruna, A.; Romano, G.L.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Liu, F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J. Hosp. Infect. 2020, 105, 100–101. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio 2015, 6. [Google Scholar] [CrossRef]




| Surface | Survival Time |
|---|---|
| Aerosols | 3 h |
| Plastic | 3 days |
| Stainless steel | 2–5 days |
| Cardboard | 8 h |
| Paper | 5 min to 5 days |
| Glass | 4–5 days |
| Polyvinyl chloride (PVC) | 5 days |
| Silicon rubber | 5 days |
| Surgical gloves (latex) | 5 days |
| Polyfluorotetraethylene (PTFE) | 5 days |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Gheethi, A.; Al-Sahari, M.; Abdul Malek, M.; Noman, E.; Al-Maqtari, Q.; Mohamed, R.; Talip, B.A.; Alkhadher, S.; Hossain, M.S. Disinfection Methods and Survival of SARS-CoV-2 in the Environment and Contaminated Materials: A Bibliometric Analysis. Sustainability 2020, 12, 7378. https://doi.org/10.3390/su12187378
Al-Gheethi A, Al-Sahari M, Abdul Malek M, Noman E, Al-Maqtari Q, Mohamed R, Talip BA, Alkhadher S, Hossain MS. Disinfection Methods and Survival of SARS-CoV-2 in the Environment and Contaminated Materials: A Bibliometric Analysis. Sustainability. 2020; 12(18):7378. https://doi.org/10.3390/su12187378
Chicago/Turabian StyleAl-Gheethi, Adel, Mohammed Al-Sahari, Marlinda Abdul Malek, Efaq Noman, Qais Al-Maqtari, Radin Mohamed, Balkis A. Talip, Sadeq Alkhadher, and Md. Sohrab Hossain. 2020. "Disinfection Methods and Survival of SARS-CoV-2 in the Environment and Contaminated Materials: A Bibliometric Analysis" Sustainability 12, no. 18: 7378. https://doi.org/10.3390/su12187378
APA StyleAl-Gheethi, A., Al-Sahari, M., Abdul Malek, M., Noman, E., Al-Maqtari, Q., Mohamed, R., Talip, B. A., Alkhadher, S., & Hossain, M. S. (2020). Disinfection Methods and Survival of SARS-CoV-2 in the Environment and Contaminated Materials: A Bibliometric Analysis. Sustainability, 12(18), 7378. https://doi.org/10.3390/su12187378









