Effect of 1-Methyl Cyclopropane and Modified Atmosphere Packaging on the Storage of Okra (Abelmoschus esculentus L.): Theory and Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. 1-MCP Application
2.4. Modified Atmosphere Packaging (MAP)
2.5. Fruit Quality Parameters
2.6. Statistical Analysis
3. Results & Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finger, F.L.; Della-Justina, M.E.; Casali, V.W.D.; Puiatti, M. Temperature and modified atmosphere affect the quality of okra. Sci. Agric. 2008, 65, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Olivera, D.F.; Mugridge, A.; Chaves, A.R.; Mascheroni, R.H.; Viña, S.Z. Quality attributes of Okra (Abelmoschus esculentus L. Moench) pods as affected by cultivar and fruit size. J. Food Res. 2012, 1, 224. [Google Scholar] [CrossRef] [Green Version]
- Saleem, A.M.; Amjad, M.; Ziaf, K.; Sahi, S.T. Characterization of okra (Abelmoschus esculentus) genotypes for fruit firmness, other horticultural traits and heritability studies. Int. J. Agric. Biol. 2018, 20, 345–352. [Google Scholar] [CrossRef]
- Al-Wandawi, H. Chemical Composition of Seeds of Two Okra Cultivars. J. Agric. Food Chem. 1983, 31, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; El-Gizawy, A.M.; El-Bassiouny, R.E.L.; Ali, H.M. Effects of anti-coloring agents on blackening inhibition and maintaining physical and chemical quality of fresh-cut okra during storage. Ann. Agric. Sci. 2013, 58, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Hussein, J.; Ilesanmi, J.; Filli, K.; Sanusi, M. Effects of Drying Methods on the Chemical Properties of Okra (Abelmoschus esculentus L. Moench) Slices. Curr. J. Appl. Sci. Technol. 2018, 26, 1–10. [Google Scholar] [CrossRef]
- Gemede, H.F. Nutritional Quality and Health Benefits of Okra (Abelmoschus esculentus): A Review. J. Food Process Technol. 2015, 6. [Google Scholar] [CrossRef]
- Woolfe, M.L.; Chaplin, M.F.; Otchere, G. Studies on the mucilages extracted from okra fruits (Hibiscus esculentus L.) and baobab leaves (Adansonia digitata L.). J. Sci. Food Agric. 1977, 28, 519–529. [Google Scholar] [CrossRef]
- Sharma, D.K.; Jain, V.K.; Jain, R.; Sharma, N. Postharvest study of okra [Abelmoschus esculentus (L.) moench] fruits and phytopathological effect of associated micro flora. Intl. J. Innov. Res. Rev. 2013, 1, 27–34. [Google Scholar]
- Liu, J.; Yuan, Y.; Wu, Q.; Zhao, Y.; Jiang, Y.; John, A.; Wen, L.; Li, T.; Jian, Q.; Yang, B. Analyses of quality and metabolites levels of okra during postharvest senescence by 1H-high resolution NMR. Postharvest Biol. Technol. 2017, 132, 171–178. [Google Scholar] [CrossRef]
- Dhall, R.K.; Sharma, S.R.; Mahajan, B.V.C. Development of post-harvest protocol of okra for export marketing. J. Food Sci. Technol. 2014, 51, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.R.; Balasubramanian, S. Qualitative and textural changes in fresh okra pods (hibiscus esculentus L.) under modified atmosphere packaging in perforated film packages. Food Sci. Technol. Int. 2009, 15, 131–138. [Google Scholar] [CrossRef]
- Ribeiro, F.C.S.; Silva, T.P.; Neves, L.L.M.; Finger, F.L. Hydrothermal treatment minimizes the effects of refrigeration in okra fruits. Hortic. Bras. 2017, 35, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Taain, D.A.; Jasim, A.M.; Al-Hij, M.J.H.H. A study of storage behavior of okra fruits. Int. J. Farming Allied Sci. 2014, 3, 760–766. [Google Scholar]
- Carvajal, F.; Martinez, C.; Jamilena, M.; Garrido, D. Differential response of zucchini varieties to low storage temperature. Sci. Hortic. 2011, 130, 90–96. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Pardossi, A.; Tognoni, F.; Guidi, L. Physiological basis of sensitivity to enzymatic browning in ‘lettuce’,‘escarole’and ‘rocket salad’when stored as fresh-cut products. Food Chem. 2007, 104, 209–215. [Google Scholar] [CrossRef]
- Boontongto, N.; Srilaong, V.; Uthairatanakij, A.; Wongs-Aree, C.; Aryusuk, K. Effect of methyl jasmonate on chilling injury of okra pod. Acta Hortic. 2007, 746, 323–327. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesis, E.; Ackerman, M.; Ben-Arie, R.; Feygenberg, O.; Feng, X.; Apelbaum, A.; Goren, R.; Prusky, D. Ethylene involvement in chilling injury symptoms of avocado during cold storage. Postharvest Biol. Technol. 2002, 24, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell-wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- In, B.C.; Strable, J.; Binder, B.M.; Falbel, T.G.; Patterson, S.E. Morphological and molecular characterization of ethylene binding inhibition in carnations. Postharvest Biol. Technol. 2013, 86, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Candan, A.P.; Graell, J.; Larrigaudiere, C. Postharvest quality and chilling injury of plums: Benefits of 1-methylcyclopropene. Span. J. Agric. Res. 2011, 9, 554. [Google Scholar] [CrossRef] [Green Version]
- JIA, X.; WANG, W.; DU, Y.; TONG, W.; WANG, Z.; Gul, H. Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears. J. Integr. Agric. 2018, 17, 693–703. [Google Scholar] [CrossRef]
- Huang, S.; Li, T.; Jiang, G.; Xie, W.; Chang, S.; Jiang, Y.; Duan, X. 1-Methylcyclopropene reduces chilling injury of harvested okra (Hibiscus esculentus L.) pods. Sci. Hortic. 2012, 141, 42–46. [Google Scholar] [CrossRef]
- Tan, C.K.; Ali, Z.M.; Ismail, I.; Zainal, Z. Effects of 1-methylcyclopropene and modified atmosphere packaging on the antioxidant capacity in pepper “kulai” during low-temperature storage. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faasema, J.; Alakali, J.S.; Abu, J.O. Effects of storage temperature on 1-methylcyclopropene-treated mango (mangnifera indica) fruit varieties. J. Food Process. Preserv. 2014, 38, 289–295. [Google Scholar] [CrossRef]
- Massolo, J.F.; Concellón, A.; Chaves, A.R.; Vicente, A.R. 1-Methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2011, 59, 10–15. [Google Scholar] [CrossRef]
- Sultan, M.; Miyazaki, T.; Mahmood, M.H.; Khan, Z.M. Solar assisted evaporative cooling based passive air-conditioning system for agricultural and livestock applications. J. Eng. Sci. Technol. 2018, 13, 693–703. [Google Scholar]
- Mahmood, M.H.; Sultan, M.; Miyazaki, T. Significance of Temperature and Humidity Control for Agricultural Products Storage: Overview of Conventional and Advanced Options. Int. J. Food Eng. 2019, 15. [Google Scholar] [CrossRef]
- Nath, A.; Deka, B.C.; Singh, A.; Patel, R.K.; Paul, D.; Misra, L.K.; Ojha, H. Extension of shelf life of pear fruits using different packaging materials. J. Food Sci. Technol. 2012, 49, 556–563. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Song, B.; Li, J.; Shang, Z.; Guan, J. Combined effects of 1-MCP and MAP on the fruit quality of pear (Pyrus bretschneideri Reld cv. Laiyang) during cold storage. Sci. Hortic. 2013, 164, 544–551. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Romero, D.; Guillén, F.; Castillo, S.; Valero, D. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
- Sultan, M.; Miyazaki, T. Energy-Efficient Air-Conditioning Systems for Nonhuman Applications. In Refrigeration; Ekren, O., Ed.; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Hardenburg, R.E.; Watada, A.E.; Wang, C.Y. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; US Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 1986.
- Perkins-Veazie, P.; Collins, J.K. Cultivar, packaging, and storage temperature differences in postharvest shelf life of okra. Horttechnology 1992, 2, 350–352. [Google Scholar] [CrossRef] [Green Version]
- Babarinde, G.O.; Fabunmi, O.A. Effects of packaging materials and storage temperature on quality of fresh okra (Abelmoschus Esculentus) fruit. Agric. Trop. Subtrop. 2009, 42, 151–156. [Google Scholar]
- Baxter, L.; Waters, L. Controlled atmosphere effects on physical changes and ethylene evolution in harvested okra. HortScience 1990, 25, 92–95. [Google Scholar] [CrossRef]
- Saltveit, M.E. A summary of CA requirements and recommendations for vegetables. In Proceedings of the VIII International Controlled Atmosphere Research Conference, Rotterdam, The Netherlands, 10 March 2003; Volume 600, pp. 723–727. [Google Scholar]
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Dadalı, G.; Kılıç Apar, D.; Özbek, B. Color change kinetics of okra undergoing microwave drying. Dry. Technol. 2007, 25, 925–936. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; Association of Official Agricultural Chemists AOAC International: Rockville, MD, USA, 2010; ISBN 0935584676. [Google Scholar]
- Öztürk, N. Phenolic composition and antioxidant activity of the different extracts from Thymus longicaulis C Presl. subsp. longicaulis var. longicaulis and T. longicaulis C. Presl. subsp. longicaulis var. subisophyllus growing in Turkey. Pak. J. Pharm. Sci. 2015, 28, 465–472. [Google Scholar] [PubMed]
- Phornvillay, S.; Pongprasert, N.; Wongs-Aree, C.; Uthairatanakij, A.; Srilaong, V. Exogenous putrescine treatment delays chilling injury in okra pod (Abelmoschus esculentus) stored at low storage temperature. Sci. Hortic. 2019, 256, 108550. [Google Scholar] [CrossRef]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Delay of avocado (Persea americana) fruit ripening by 1-methylcyclopropene and wax treatments. Postharvest Biol. Technol. 2003, 28, 247–257. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z. 1-Methylcyclopropene application and modified atmosphere packaging affect ethylene biosynthesis, fruit softening, and quality of “Tegan Blue” Japanese plum during cold storage. J. Am. Soc. Hortic. Sci. 2008, 133, 290–299. [Google Scholar] [CrossRef]
- Indore, H.D.; Garande, V.K.; Dhumal, S.S.; Patgaonkar, D.R.; Patil, V.S.; Sonawane, P.N. Effect of Packaging Materials and Storage Conditions on Shelf Life and Quality of Okra*. Int. J. Adv. Res. 2016, 4, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Gong, X.; Jing, W.; Peng, Z.; Li, J. Quality Change of Postharvest Okra at Different Storage Temperatures. J. Food Eng. Technol. 2018, 7, 43. [Google Scholar]
- Bashir, H.A.; Abu-Goukh, A.-B.A. Compositional changes during guava fruit ripening. Food Chem. 2003, 80, 557–563. [Google Scholar] [CrossRef]
- Adom, K.K.; Dzogbefia, V.P.; Ellis, W.O.; Simpson, B.K. Solar drying of Okra—effects of selected package materials on storage stability. Food Res. Int. 1996, 29, 589–593. [Google Scholar] [CrossRef]
- El-Mahdy, A.R.; El-Sebaiy, L.A. Preliminary studies on the mucilages extracted from Okra fruits, Taro tubers, Jew’s mellow leaves and Fenugreek seeds. Food Chem. 1984, 14, 237–249. [Google Scholar] [CrossRef]
- Gernah, D.I.; Daagema, A.A. Effect of storage conditons on some physico-chemical and microbiological properties of fresh okra (Abelmoschus esculenta) fruits. Curr. Res. J. Biol. Sci. 2012, 4, 444–448. [Google Scholar]
- Cheng, Y.; Dong, Y.; Yan, H.; Ge, W.; Shen, C.; Guan, J.; Liu, L.; Zhang, Y. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 2012, 135, 415–422. [Google Scholar] [CrossRef]
- Baxter, L.; Waters, L. Chemical changes in okra stored in air and controlled atmosphere. J. Am. Soc. Hortic. Sci. 2019, 115, 452–454. [Google Scholar] [CrossRef]
- Dou, H.; Jones, S.; Ritenour, M. Influence of 1-MCP application and concentration on post-harvest peel disorders and incidence of decay in citrus fruit. J. Hortic. Sci. Biotechnol. 2005, 80, 786–792. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; Van Doorn, W.G. Effect of modified atmosphere packaging on chilling-induced peel browning in banana. Postharvest Biol. Technol. 2004, 31, 313–317. [Google Scholar] [CrossRef]
- Li, X.; Yun, J.; Fan, X.; Xing, Y.; Tang, Y. Effect of 1-methylcyclopropene and modified atmosphere packaging on chilling injury and antioxidative defensive mechanism of sweet pepper. Afr. J. Biotechnol. 2011, 10, 6581–6589. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, M.; Wang, J.; Jiang, C.-Z.; Wang, Q. Application of exogenous ethylene inhibits postharvest peel browning of ‘Huangguan’pear. Front. Plant Sci. 2017, 7, 2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.W.A.; Jahangir, M.; Qaisar, M.; Khan, S.A.; Mahmood, T.; Saeed, M.; Farid, A.; Liaquat, M. Storage stability of kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules 2015, 20, 22645–22661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Atta-Aly, M.A. Effect of high temperature on ethylene biosynthesis by tomato fruit. Postharvest Biol. Technol. 1992, 2, 19–24. [Google Scholar] [CrossRef]
- Blankenship, S.M. 1-Methyl-cyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Villalobos Acuña, M.G.; Biasi, W.V.; Mitcham, E.J.; Holcroft, D. Fruit temperature and ethylene modulate 1-MCP response in “Bartlett” pears. Postharvest Biol. Technol. 2011, 60, 17–23. [Google Scholar] [CrossRef]
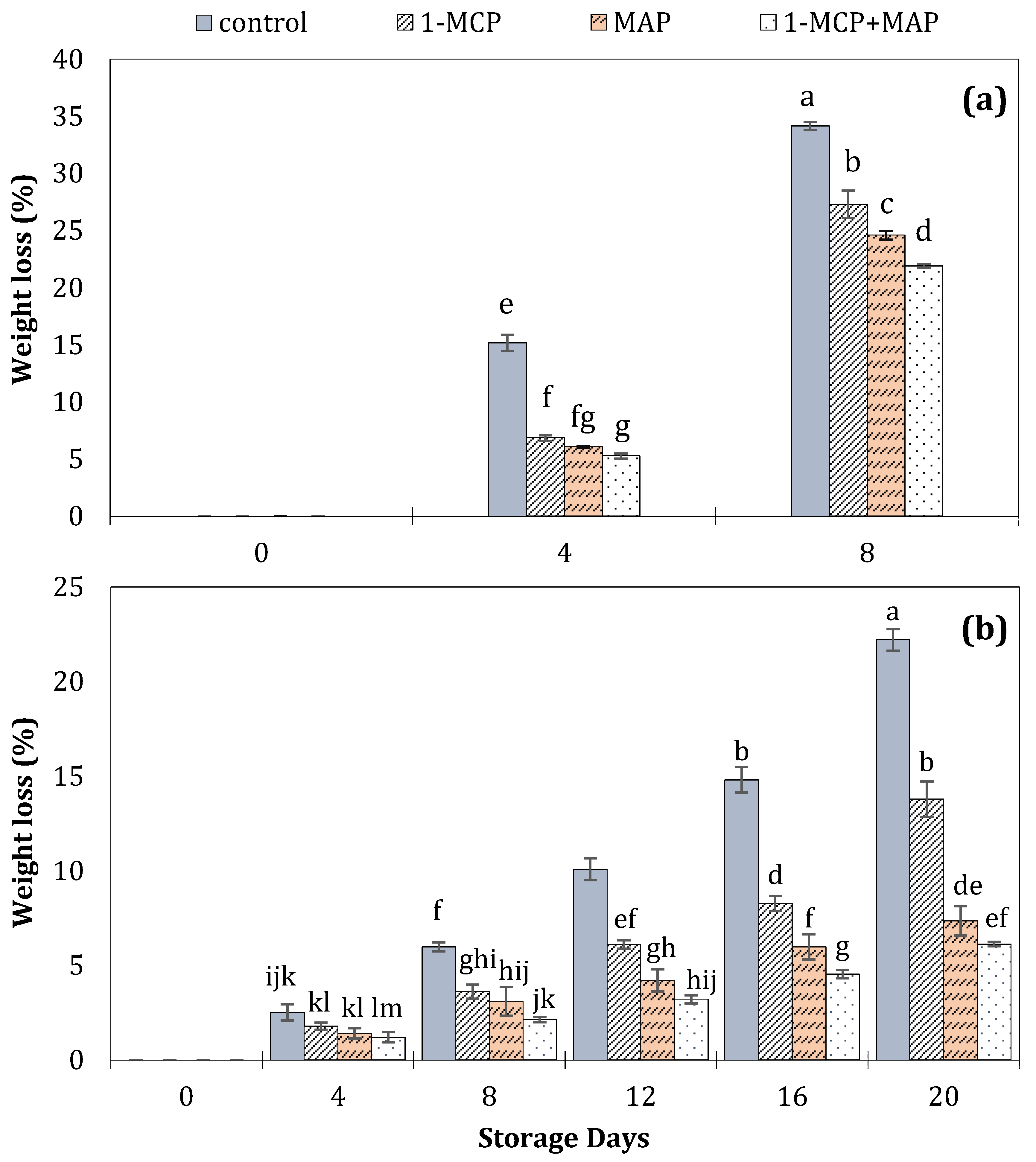

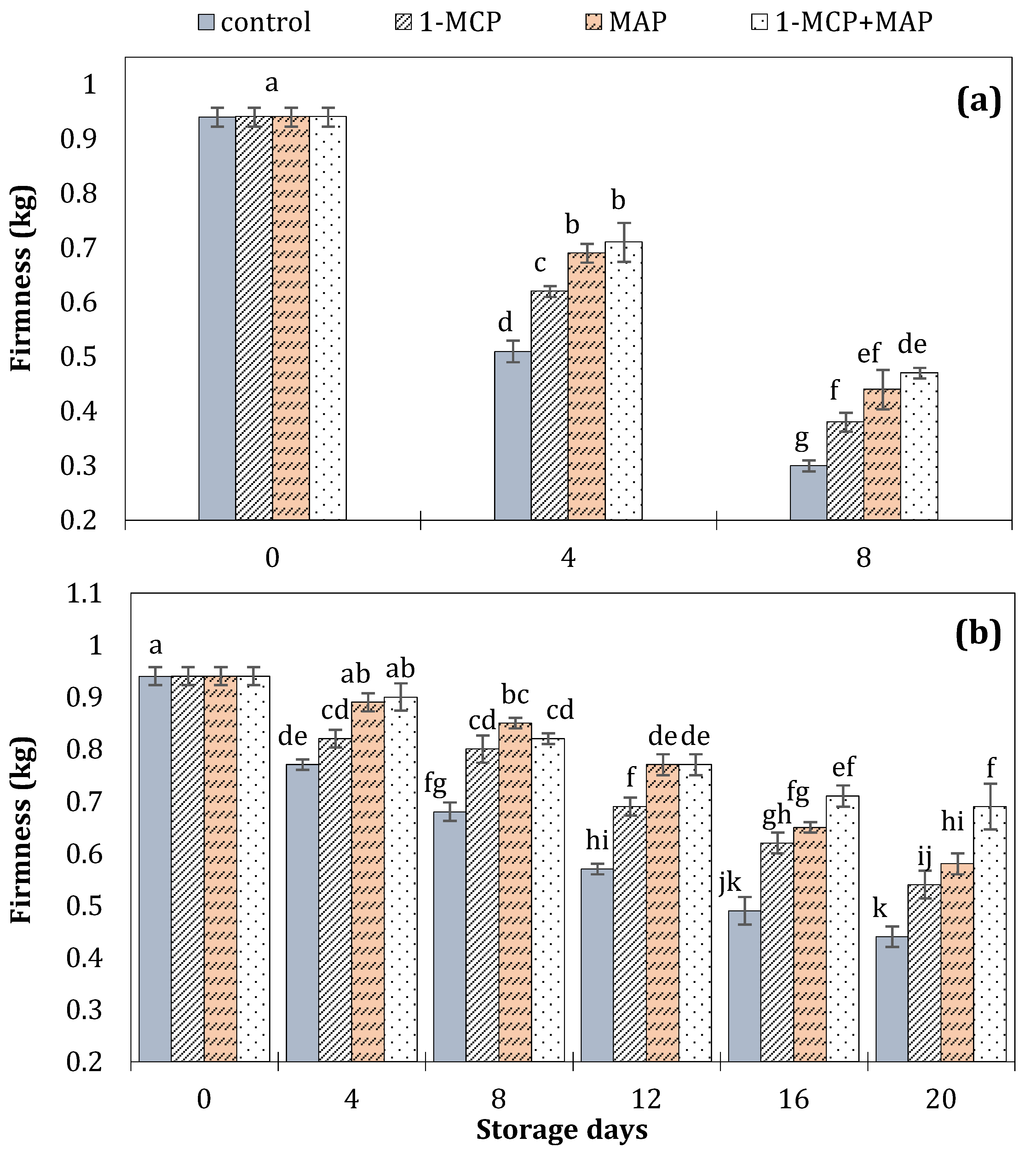
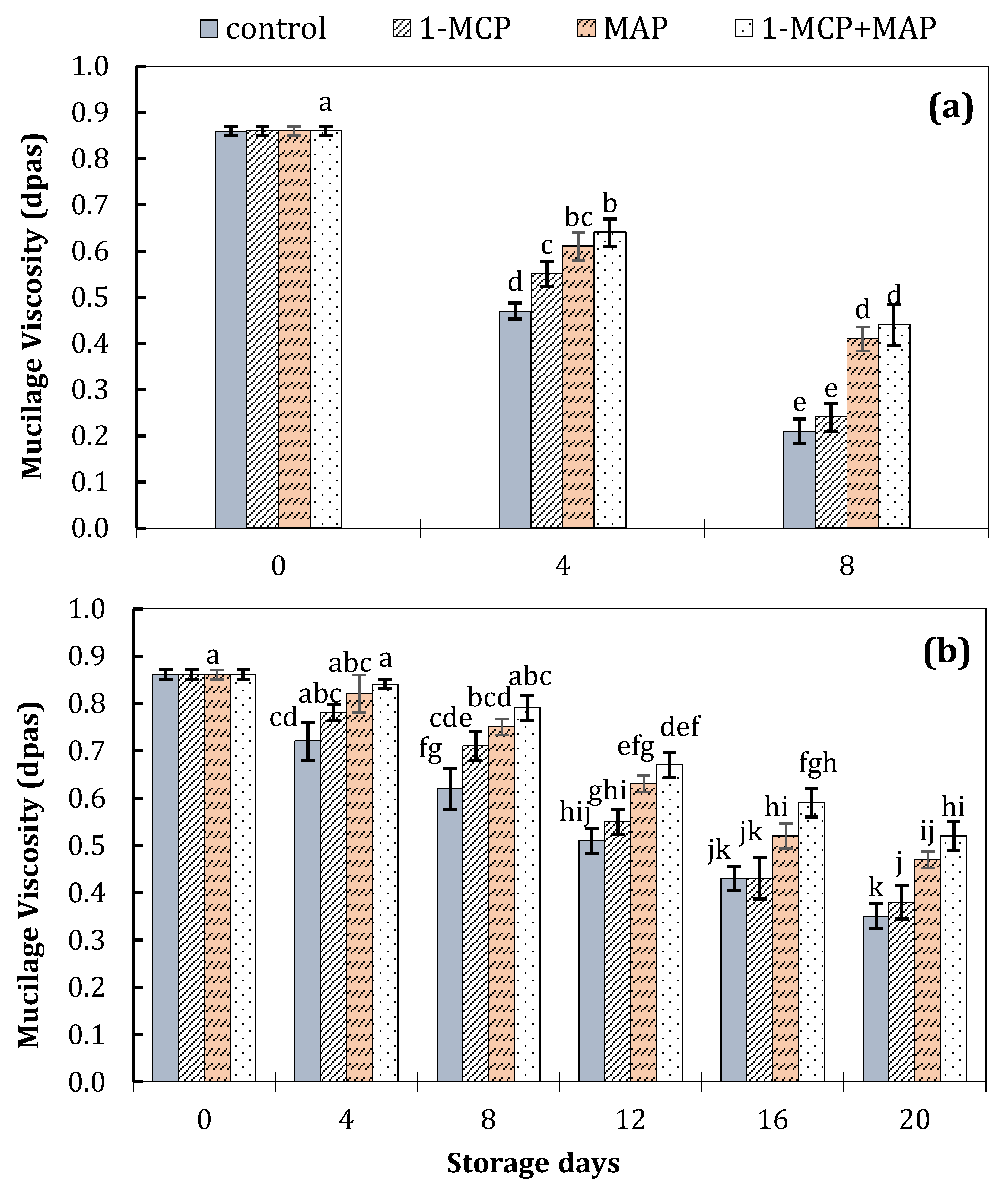
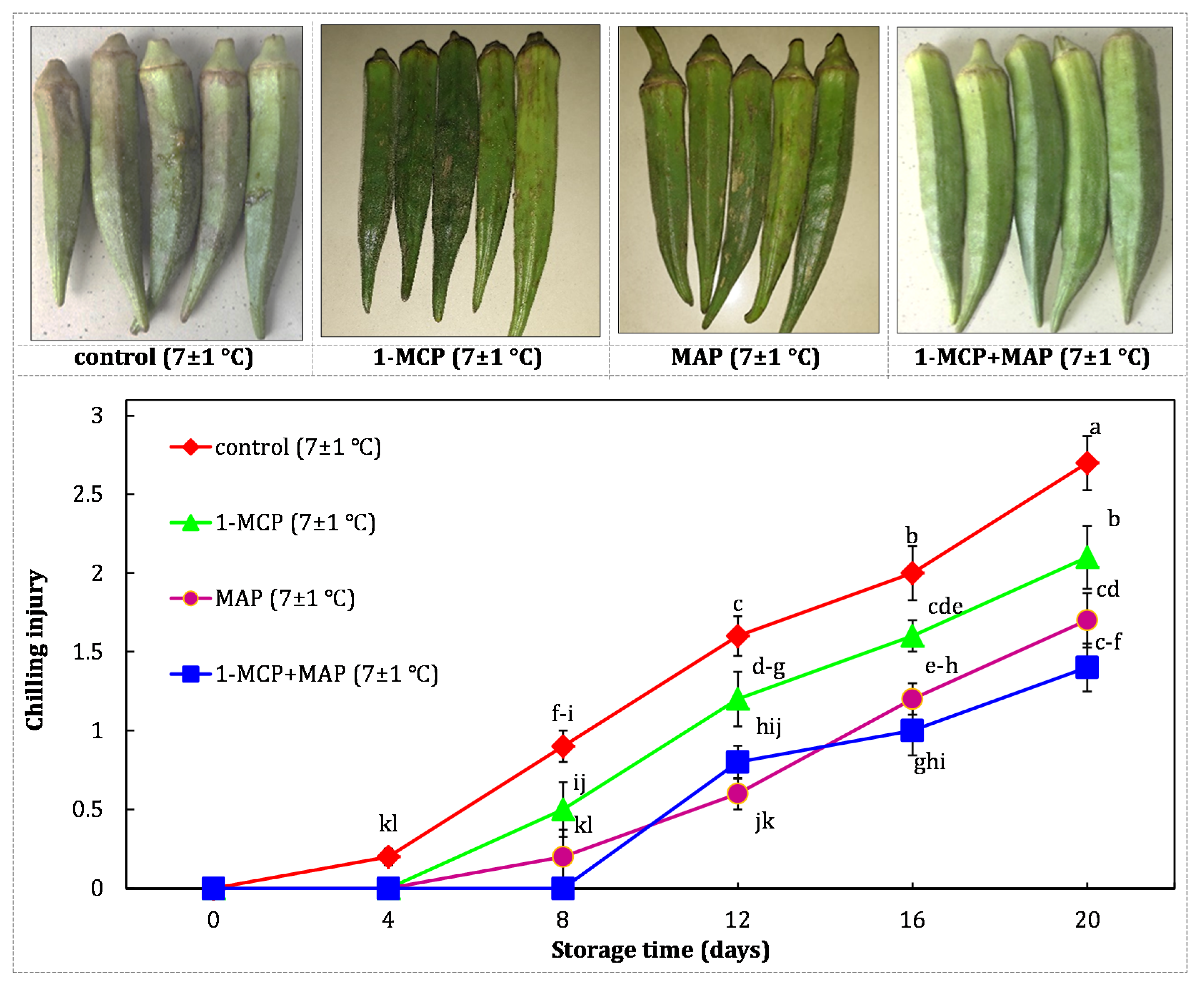


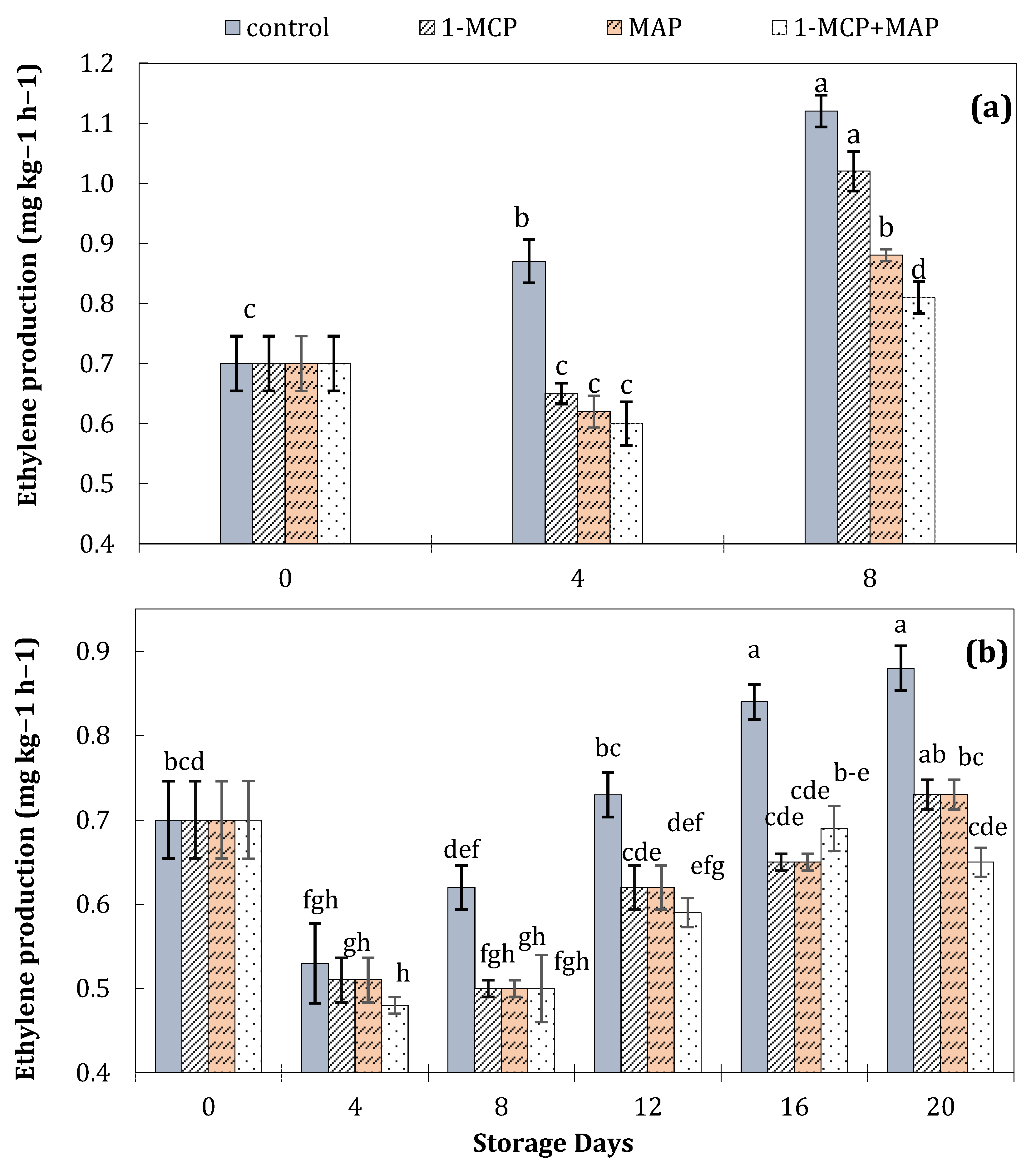
| Technique | Storage Conditions | Storage Period | Ref. | ||
|---|---|---|---|---|---|
| Temperature | Relative Humidity | ||||
| Packaging | HDPE (High density Polyethylene) | 12.5 °C and 3 °C | 80 ± 5% | 8 days | [35] |
| Polyvinyl chloride (PVC) | 5 °C, 10 °C and 25 °C | 85–95% | 7–10 days | [1] | |
| LDPE (low density Polyethylene) | 15 ± 2 °C and 28 ± 2 °C | 9 days | [36] | ||
| Polypropylene O2 (6.3–8.4%) CO2 (10.7–11.8%) | 15 °C | 75% | 9 days | [12] | |
| Control Atmosphere | 5% O2 + 10% CO2 | 11 ± 1 °C | 90–93% | 12 days | [37] |
| 4–10% CO2 | 7–12 °C | 90–95% | [38] | ||
| Temp. | Days | Ascorbic Acid | Ethylene | Firmness | Hue Angle | Moisture Content | Respiration Rate | Weight | BI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Days p-values | 0.00 1.00 | - | - | - | - | - | - | - | - | - |
| Ascorbic acid | −0.62 0.00 | −0.63 0.00 | - | - | - | - | - | - | - | - |
| Ethylene | −0.39 0.00 | −0.41 0.00 | 0.75 0.00 | - | - | - | - | - | - | - |
| Firmness | −0.59 0.00 | −0.67 0.00 | 0.99 0.00 | 0.69 0.00 | - | - | - | - | - | - |
| Hue angle | −0.59 0.00 | −0.53 0.00 | 0.94 0.00 | 0.90 0.00 | 0.91 0.00 | - | - | - | - | - |
| Moisture content | −0.61 0.00 | −0.56 0.00 | 0.97 0.00 | 0.85 0.00 | 0.95 0.00 | 0.99 0.00 | - | - | - | - |
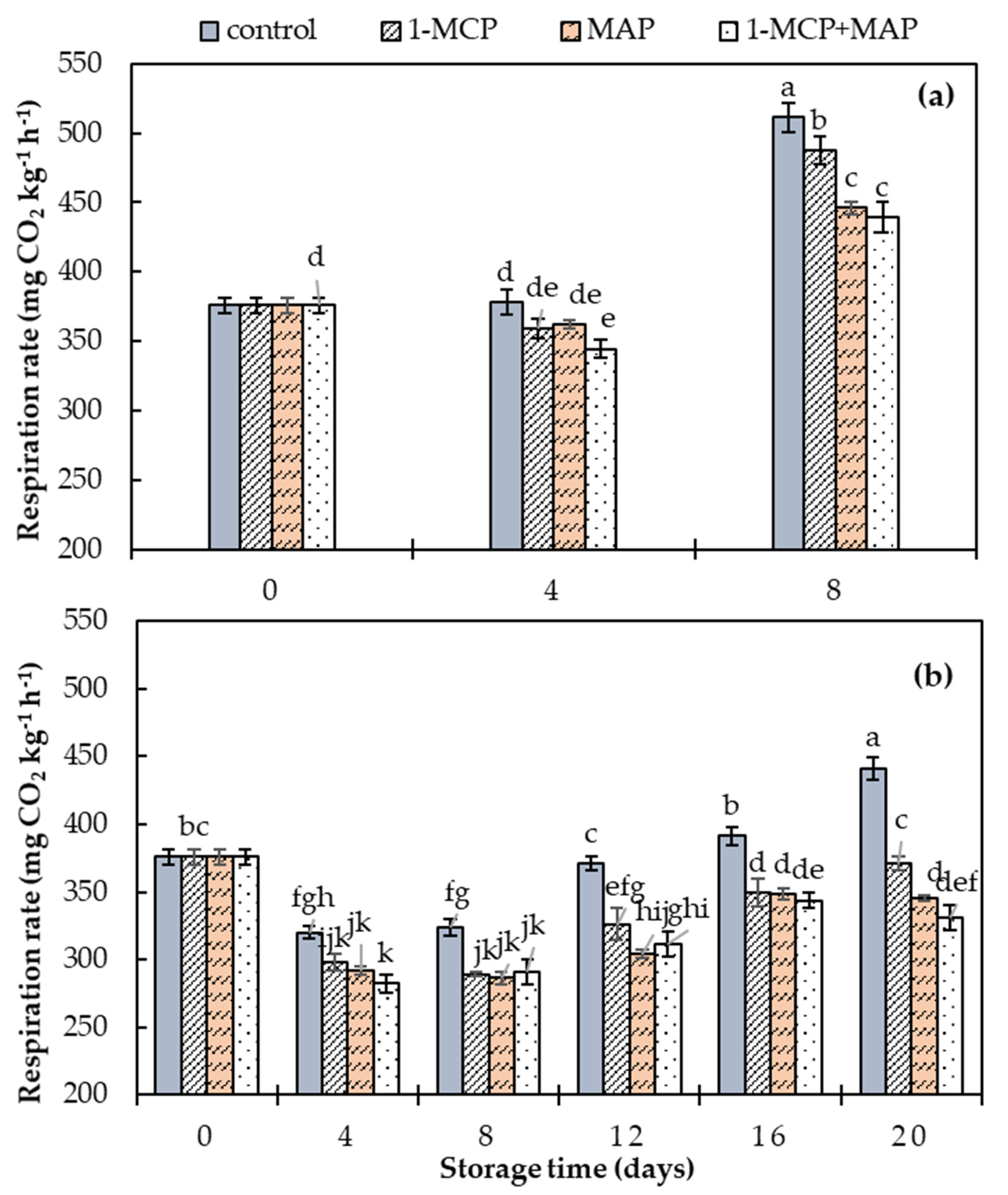
| Respiration rate | −0.42 0.00 | −0.48 0.00 | 0.81 0.00 | 0.98 0.00 | 0.77 0.00 | 0.94 0.00 | 0.90 0.00 | - | - | - |
| Weight loss | 0.03 0.69 | 0.09 0.25 | 0.04 0.63 | 0.65 0.00 | −0.03 0.67 | 0.35 0.00 | 0.23 0.00 | 0.58 0.00 | - | - |
| Mucilage viscosity | −0.55 0.00 | −0.72 0.00 | 0.97 0.00 | 0.65 0.00 | 0.99 0.00 | 0.87 0.00 | 0.92 0.00 | 0.73 0.00 | −0.08 0.30 | |
| BI | −0.24 0.00 | −0.20 0.01 | 0.48 0.00 | 0.91 0.00 | 0.41 0.00 | 0.73 0.00 | 0.64 0.00 | 0.88 0.00 | 0.88 0.00 | |
| ∆E | −0.006 0.94 | 0.13 0.09 | 0.07 0.39 | 0.68 0.00 | −0.01 0.86 | 0.38 0.00 | 0.27 0.00 | 0.60 0.00 | 0.97 0.00 | 0.89 0.00 |
| Treatments | Storage Time (Days) | Ambient Temperature (27 ± 2 °C) | Cold Store (7 ± 1 °C) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ascorbic Acid (mg/100 g) | Hue Angle | Total Change in Color (∆E) | Browning Index (BI) | Ascorbic Acid (mg/100 g) | Hue Angle | Total Change in Color (∆E) | Browning Index (BI) | ||
| Control | 0 | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 i | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 s |
| 4 | 14.2 ± 0.43 c | 113.23 ± 0.34 e,f | 6.20 ± 0.06 d | 58.91 ± 0.04 e | 19.9 ± 0.15 b | 118.21 ± 0.32 a,b | 1.06 ± 0.06 ab | 38.14 ± 0.03 n | |
| 8 | 8.1 ± 0.56 f | 107.86 ± 0.43 h | 14.15 ± 0.04 a | 128.72 ± 0.05 a | 18.2 ± 0.30 d,e | 116.75 ± 0.36 c,d,e | 3.09 ± 0.05 d,e | 45.84 ± 0.02 j | |
| 12 | - | - | - | - | 15.8 ± 0.36 g | 114.02 ± 0.40 g,h | 6.31 ± 0.05 h,j | 60.98 ± 0.07 d | |
| 16 | - | - | - | - | 13.6 ± 0.34 h | 113.59 ± 0.44 h | 7.28 ± 0.06 j,k | 65.98 ± 0.09 b | |
| 20 | - | - | - | - | 11.2 ± 0.37 i | 111.11 ± 0.67 i | 8.78 ± 0.07 k | 78.29 ± 0.03 a | |
| 1-MCP | 0 | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 i | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 s |
| 4 | 16.2 ± 0.49 b | 115.21 ± 0.50 c,d | 4.84 ± 0.07 e | 52.28 ± 0.093 f | 19.5 ± 0.27 b,c | 118.60 ± 0.08 a | 0.40 ± 0.03 a,b | 35.52 ± 0.08 r | |
| 8 | 10.4 ± 0.56 e | 109.91 ± 0.65 g | 12.43 ± 0.05 b | 110.66 ± 0.04 b | 19.2 ± 0.15 b,c,d | 117.87 ± 0.44 a,b | 0.58 ± 0.13 a,b | 37.24 ± 0.04 a,b | |
| 12 | - | - | - | - | 17.6 ± 0.29 e,f | 115.84 ± 0.34 d,e,f | 3.53 ± 0.05 d,e | 48.43 ± 0.04 g | |
| 16 | - | - | - | - | 15.7 ± 0.14 g | 114.34 ± 0.14 f,g | 5.36 ± 0.05 f,g,h | 56.81 ± 0.12 e | |
| 20 | - | - | - | - | 15.3 ± 0.30 g | 114.14 ± 0.58 g,h | 6.85 ± 0.04 i,j,k | 62.39 ± 0.03 c | |
| MAP | 0 | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 i | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 s |
| 4 | 16.4 ± 0.36 b | 116.13 ± 0.30 b,c | 3.82 ± 0.05 f | 48.93 ± 0.06 g | 21.7 ± 0.17 a | 118.93 ± 0.30 a | 0.14 ± 0.05 a | 34.24 ± 0.06 t | |
| 8 | 12.1 ± 0.26 d | 112.33 ± 0.42 f | 9.34 ± 0.08 c | 80.19 ± 0.23 c | 20.1 ± 0.20 b | 117.85 ± 0.42 a,b | 0.66 ± 0.04 a,b | 36.71 ± 0.03 a,b | |
| 12 | - | - | - | - | 18.8 ± 0.17 c,d | 116.14 ± 0.47 c,f | 2.46 ± 0.05 c,d,e | 42.85 ± 0.04 l | |
| 16 | - | - | - | - | 17.1 ± 0.26 f | 115.32 ± 0.43 e,f,g | 3.80 ± 0.02 e,f,g | 47.50 ± 0.03 h | |
| 20 | - | - | - | - | 16.9 ± 0.30 f | 115.17 ± 0.45 e,f,g | 4.90 ± 0.03 g,h,i | 50.19 ± 0.04 f | |
| 1-MCP + MAP | 0 | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 i | 21.6 ± 0.42 a | 118.82 ± 0.49 a | - | 34.72 ± 0.03 s |
| 4 | 16.9 ± 0.21 b | 116.82 ± 0.53 b | 3.58 ± 0.07 g | 46.46 ± 0.05 h | 22.3 ± 0.25 a | 118.88 ± 0.41 a | 0.24 ± 0.03 a | 34.29 ± 0.07 t | |
| 8 | 12.8 ± 0.29 d | 113.91 ± 0.52 d,e | 9.28 ± 0.05 c | 78.51 ± 0.03 d | 21.4 ± 0.43 a | 118.42 ± 0.42 a,b | 1.06 ± 0.05 a,b,c | 37.49 ± 0.03 o | |
| 12 | - | - | - | - | 19.2 ± 0.45 b,c,d | 117.22 ± 0.42 a,b,c | 1.63 ± 0.06 b,c,d | 39.68 ± 0.05 m | |
| 16 | - | - | - | - | 18.7 ± 0.51 c,d | 115.96 ± 0.49 b,c,d | 3.09 ± 0.05 a,b,c | 44.00 ± 0.07 k | |
| 20 | - | - | - | - | 18.2 ± 0.41 d,e | 115.93 ± 0.49 d,e,f | 3.46 ± 0.07 b,c,d | 46.12 ± 0.03 i | |
| Technology | Storage Conditions | Effect(s) | Ref. | |
|---|---|---|---|---|
| Temperature | Humidity | |||
| Methyl Jasmonate coating | 4 °C | 90–95% | Reduced electrolyte leakage, color change, and chilling injury | [17] |
| Putrescine (Put) dipping | 4 °C | Minimized chilling injury stress and activity of polyphenol oxidase and peroxidase enzymes | [43] | |
| PVC film wrapping | 5 °C and 10 °C | 95% | Reduced chilling injury, weight loss, decay, and rate of chlorophyll degradation | [1] |
| 1-MCP | 7 °C | 80–85% | Alleviated chilling injury stress with lower membrane permeability and lipid peroxidation | [24] |
| Hot water dipping | 5 °C | 90 ± 2% | Reduced chilling injury disorder and oxidative stress | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, R.; Ashraf, H.; Sultan, M.; Babu, I.; Yasmin, Z.; Nadeem, M.; Asghar, M.; Shamshiri, R.R.; Ibrahim, S.M.; Ahmad, N.; et al. Effect of 1-Methyl Cyclopropane and Modified Atmosphere Packaging on the Storage of Okra (Abelmoschus esculentus L.): Theory and Experiments. Sustainability 2020, 12, 7547. https://doi.org/10.3390/su12187547
Kanwal R, Ashraf H, Sultan M, Babu I, Yasmin Z, Nadeem M, Asghar M, Shamshiri RR, Ibrahim SM, Ahmad N, et al. Effect of 1-Methyl Cyclopropane and Modified Atmosphere Packaging on the Storage of Okra (Abelmoschus esculentus L.): Theory and Experiments. Sustainability. 2020; 12(18):7547. https://doi.org/10.3390/su12187547
Chicago/Turabian StyleKanwal, Rabia, Hadeed Ashraf, Muhammad Sultan, Irrum Babu, Zarina Yasmin, Muhammad Nadeem, Muhammad Asghar, Redmond R. Shamshiri, Sobhy M. Ibrahim, Nisar Ahmad, and et al. 2020. "Effect of 1-Methyl Cyclopropane and Modified Atmosphere Packaging on the Storage of Okra (Abelmoschus esculentus L.): Theory and Experiments" Sustainability 12, no. 18: 7547. https://doi.org/10.3390/su12187547
APA StyleKanwal, R., Ashraf, H., Sultan, M., Babu, I., Yasmin, Z., Nadeem, M., Asghar, M., Shamshiri, R. R., Ibrahim, S. M., Ahmad, N., Imran, M. A., Zhou, Y., & Ahmad, R. (2020). Effect of 1-Methyl Cyclopropane and Modified Atmosphere Packaging on the Storage of Okra (Abelmoschus esculentus L.): Theory and Experiments. Sustainability, 12(18), 7547. https://doi.org/10.3390/su12187547










