The COVID-19 Pandemic Lockdowns and Changes in Body Weight among Polish Women. A Cross-Sectional Online Survey PLifeCOVID-19 Study
Abstract
:1. Introduction
2. Materials and Methods
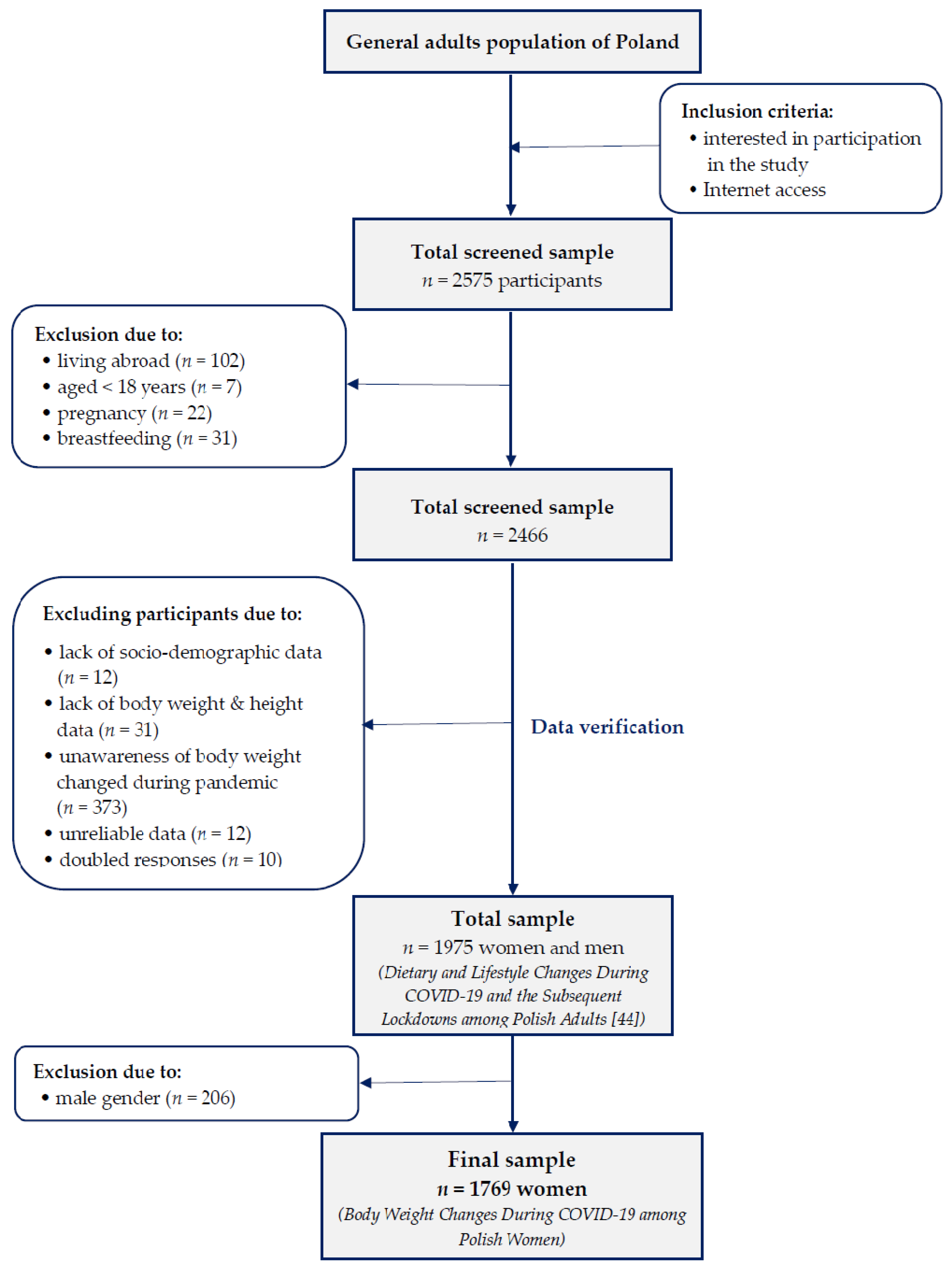
2.1. Study Design and Sample Collection
2.2. Applied Questionnaire
2.2.1. Dietary Data
2.2.2. Diet Quality
2.2.3. Lifestyle Data
2.2.4. Socioeconomic Data
2.3. Anthropometric Data
2.4. Statistical Analysis
3. Results
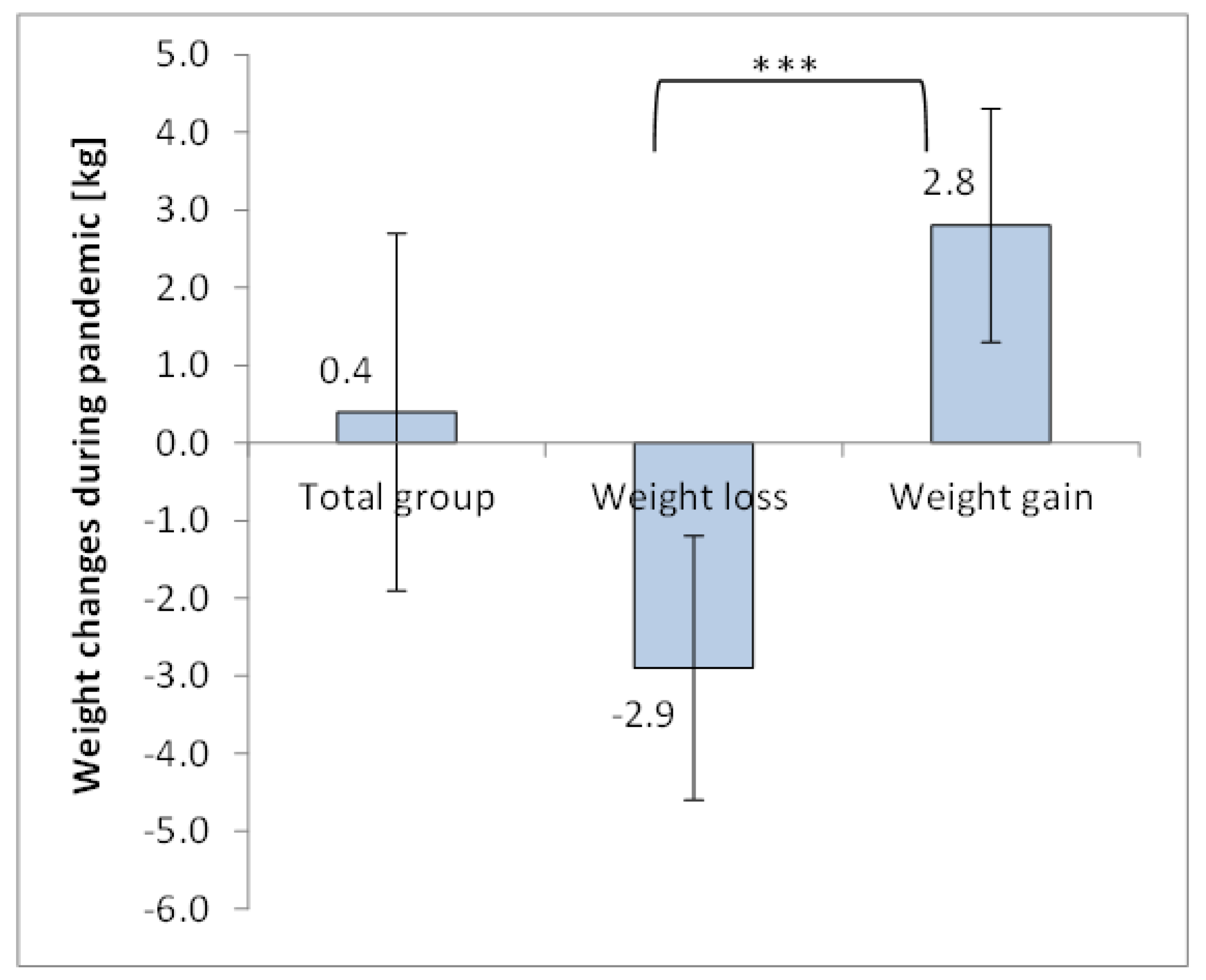
3.1. Body Weight and BMI Changes
3.2. Factors Associated with Body Weight Changes
4. Discussion
Strengths and Limitations
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hakovirta, M.; Denuwara, N. How COVID-19 Redefines the Concept of Sustainability. Sustainability 2020, 12, 3727. [Google Scholar] [CrossRef]
- Pradhan, P.; Kropp, J.P. Interplay between Diets, Health, and Climate Change. Sustainability 2020, 12, 3878. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Prevalence of Overweight among Adults, BMI ≥ 25, Age-standardized. Estimates by WHO Region. 2017. Available online: https://apps.who.int/gho/data/view.main.GLOBAL2461A?lang=en (accessed on 2 June 2020).
- Poznańska, A.; Rabczenko, D.; Wojtyniak, B. Selected life style-related health RSC factors. In Health Stastus of Polish Population–In Short; Wojtyniak, B., Goryński, P., Eds.; National Institute of Public Health–National Institute of Hygiene: Warsaw, Poland, 2018; pp. 227–239. ISBN 978-83-65870-16-2. [Google Scholar]
- World Health Organization. Obesity and Overweight. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 June 2020).
- Zamri, L.A.; Appannah, G.; Zahari Sham, S.Y.; Mansor, F.; Ambak, R.; Mohd Nor, N.S.; Aris, T. Weight Change and Its Association with Cardiometabolic Risk Markers in Overweight and Obese Women. J. Obes. 2020. [Google Scholar] [CrossRef]
- Castillo-Martinez, L.; Lopez-Alvarenga, J.C.; Villa, A.R.; Gonzalez-Barranco, J. Menstrual cycle length disorders in 18-to 40-year-old obese women. Nutrition 2003, 19, 317–320. [Google Scholar] [CrossRef]
- Lumsden, M.A.; Hor, K. Impact of obesity on the health of women in midlife. Obstet. Gynaecol. 2015, 17, 201–208. [Google Scholar] [CrossRef]
- Douchi, T.; Kuwahata, R.; Yamamoto, S.; Oki, T.; Yamasaki, H.; Nagata, Y. Relationship of upper body obesity to menstrual disorders. Acta. Obstet. Gynecol. Scand. 2002, 81, 147–150. [Google Scholar] [CrossRef]
- Huttunen, R.; Syrjanen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. The impact of obesity and metabolic syndrome on vaccination success. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 86–97. [Google Scholar]
- Lee, A.; Cardel, M.; Donahoo, W.T. Social and Environmental Factors Influencing Obesity. Endotext.Org. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278977/ (accessed on 2 July 2020).
- Magkos, F.; Tetens, I.; Gjedsted Bügel, S.; Felby, C.; Rønnow Schacht, S.; Hill, J.O.; Ravussin, E.; Astrup, A. The Environmental Foodprint of Obesity. Obesity 2020, 28, 73–79. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Nutrition Landscape Information System (NLiS). 2017. Available online: https://apps.who.int/nutrition/landscape/help.aspx?menu=0&helpid=392&lang=EN (accessed on 2 July 2020).
- Lorem, G.F.; Schirmer, H.; Emaus, N. What is the impact of underweight on self-reported health trajectories and mortality rates: A cohort study. Health Qual. Life Outcomes 2017, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yi, S.W.; Sull, J.W.; Hong, J.S.; Jee, S.H.; Ohrr, H. Body mass index and mortality among Korean elderly in rural communities: Kangwha Cohort Study. PLoS ONE 2015, 10, e0117731. [Google Scholar] [CrossRef] [Green Version]
- Pednekar, M.S.; Hakama, M.; Hebert, J.R.; Gupta, P.C. Association of body mass index with all-cause and cause-specific mortality: Findings from a prospective cohort study in Mumbai (Bombay), India. Int. J. Epidemiol. 2008, 37, 524–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvaget, C.; Ramadas, K.; Thomas, G.; Vinoda, J.; Thara, S.; Sankaranarayanan, R. Body mass index, weight change and mortality risk in a prospective study in India. Intern. J. Epidemiol. 2008, 37, 990–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, X.; An, R. Body weight status and onset of cognitive impairment among U.S. middle-aged and older adults. Arch. Gerontol. Geriatr. 2015, 60, 394–400. [Google Scholar] [CrossRef]
- Wu, S.; Wang, R.; Zhao, Y.; Ma, X.; Wu, M.; Yan, X.; He, J. The relationship between self-rated health and objective health status: A population-based study. BMC Public Health 2013, 13, 320. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Q.; Pang, G.; Lin, L.; Origasa, H.; Wang, Y.; Di, J.; Shi, M.; Fan, C.; Shi, H. Association between Body Mass Index and Health-Related Quality of Life: The “Obesity Paradox” in 21,218 Adults of the Chinese General Population. PLoS ONE 2015, 10, e0130613. [Google Scholar] [CrossRef]
- Andersen, A.E.; Ryan, G.L. Eating disorders in the obstetric and gynecologic patient population. Obstet. Gynecol. 2009, 114, 1353–1367. [Google Scholar] [CrossRef]
- Boutari, C.; Pappas, P.D.; Mintziori, G.; Nigdelis, M.P.; Athanasiadis, L.; Goulis, D.G.; Mantzoros, C.S. The effect of underweight on female and male reproduction. Metab. Clin. Exp. 2020, 107, 154229. [Google Scholar] [CrossRef]
- Harpsøe, M.C.; Nielsen, N.M.; Friis-Møller, N.; Andersson, M.; Wohlfahrt, J.; Linneberg, A.; Nohr, E.A.; Jess, T. Body mass index and risk of infections among women in the Danish national birth cohort. Am. J. Epidemiol. 2016, 183, 1008e17. Available online: http://aje.oxfordjournals.org/content/183/11/1008.abstract (accessed on 5 July 2020). [CrossRef]
- Ladabaum, U.; Mannalithara, A.; Myer, P.A.; Singh, G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am. J. Med. 2014, 127, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.L.; Bentham, G.; Day, P.; Kingham, S. Associations between neighborhood environmental characteristics and obesity and related behaviours among adult New Zealanders. BMC Public Health 2014, 14, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angkurawaranon, C.; Jiraporncharoen, W.; Chenthanakij, B.; Doyle, P.; Nitsch, D. Urban environments and obesity in Southeast Asia: A systematic review, meta-analysis and meta-regression. PLoS ONE 2014, 9, e113547. [Google Scholar] [CrossRef] [PubMed]
- Burgoine, T.; Forouhi, N.G.; Griffin, S.J.; Wareham, N.J.; Monsivais, P. Associations between exposure to takeaway food outlets, takeaway food consumption, and body weight in Cambridgeshire, UK: Population based, cross sectional study. BMJ 2014, 348, g1464. [Google Scholar] [CrossRef] [Green Version]
- Escoto, K.; French, S.; Harnack, L.; Toomey, T.L.; Hannan, P.J.; Mitchell, N.R. Work hours, weight status, and weight–related behaviours: A study of metro transit workers. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- Kirkegaard, H.; Stovring, H.; Rasmussen, K.; Abrams, B.; Sørensen, T.I.A.; Nohr, E.A. How do pregnancy—Related weight changes and breastfeeding relate to maternal weight and BMI–adjusted waist circumference 7 y after delivery? Results from a path analysis. Am. J. Clin. Nutr. 2014, 99, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Chaput, J.P.; Pérusse, L.; Després, J.P.; Tremblay, A.; Bouchard, C. Findings from the Quebec Family Study on the Etiology of Obesity: Genetics and Environmental Highlights. Curr. Obes. Rep. 2014, 3, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Siahpush, M.; Huang, T.T.; Sikora, A.; Tibbits, M.; Shaikh, R.A.; Singh, G.K. Prolonged financial stress predicts subsequent obesity: Results from a prospective study of an Australian national sample. Obesity 2013, 22, 616–621. [Google Scholar] [CrossRef]
- Kubzansky, L.; Bordelois, P.; Jun, H.; Roberts, A.L.; Cerda, M.; Bluestone, N.; Koenen, K.C. The weight of traumatic stress: A prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry 2014, 71, 44–51. [Google Scholar] [CrossRef]
- Jeffery, S.; Karla, H. Marital status, marital history, body weight, and obesity. Marriage Fam. Rev. 2011, 47, 474–504. [Google Scholar] [CrossRef]
- Lassetter, J.H.; Clark, L.; Morgan, S.E.; Brown, L.B.; VanServellen, G.; Duncan, K.; Hopkins, E.S. Health literacy and obesity among native Hawaiian and Pacific Islanders in the United States. Public Health Nurs. 2015, 32, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinŕ, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Ponzo, V.; Rosato, R.; Scumaci, E.; Goitre, I.; Benso, A.; Belcastro, S.; Crespi, C.; De Michieli, F.; Ghigo, E.; et al. Changes in Weight and Nutritional Habits in Adults with Obesity during the “Lockdown” Period Caused by the COVID-19 Virus Emergency. Nutrients 2020, 12, 2016. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Molina-Montes, E.; Verardo, V.; Artacho, R.; García-Villanova, B.; Guerra-Hernández, E.J.; Ruíz-López, M.D. Changes in Dietary Behaviours during the COVID-19 Outbreak Confinement in the Spanish COVIDiet Study. Nutrients 2020, 12, 1730. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M.A.; Balanza-Martınez, V. Natural environments, ancestral diets, and microbial ecology: Is there a modern “paleo-deficit disorder”? Part II. J. Physiol. Anthropol. 2015, 34, 9. [Google Scholar] [CrossRef] [Green Version]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Górnicka, M.; Drywień, M.E.; Zielinska, M.A.; Hamułka, J. Dietary and Lifestyle Changes During COVID-19 and the Subsequent Lockdowns among Polish Adults: A Cross-Sectional Online Survey PLifeCOVID-19 Study. Nutrients 2020, 12, 2324. [Google Scholar] [CrossRef]
- Jarosz, M. Pyramid of Healthy Nutrition and Physical Activity for adults. National Center for Nutritional Education. 2019. Available online: https://ncez.pl/abc-zywienia-/zasady-zdrowego-zywienia/piramida-zdrowego-zywienia-i-aktywnosci-fizycznej-dla-osob-doroslych (accessed on 5 June 2020). (In Polish).
- Branca, F.; Nikogosian, H.; Lobstein, T. World Health Organization. Regional Office for Europe. In The Challenge of Obesity in the WHO European Region and the Strategies for Response; WHO Regional Office for Europe: Copenhagen, Denmark, 2007; ISBN 9789289014083. [Google Scholar]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of early health risk: Simpler and more predictive than using a matrix based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef] [Green Version]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.M.; DeClercq, V.; Cui, Y.; Forbes, C.; Grandy, S.; Keats, M.; Parker, L.; Sweeney, E.; Dummer, T.J.B. Fruit and vegetable intake and body adiposity among populations in Eastern Canada: The Atlantic Partnership for Tomorrow’s Health Study. BMJ Open 2018, 8, 18060. [Google Scholar] [CrossRef] [Green Version]
- Lal, A.; Peeters, A.A.; Brown, V.; Nguyen, P.; Quynh Tran, H.N.; Nguyen, T.; Tonmukayakul, U.; Sacks, G.; Calache, H.; Martin, J.; et al. The Modelled Population Obesity-Related Health Benefits of Reducing Consumption of Discretionary Foods in Australia. Nutrients 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, J.-A.S.; Galindo-Fraga, A.; Ortiz-Hernández, A.A.; Gu, W.; Hunsberger, S.; Galán-Herrera, J.-F.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Beigel, J.H. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respi. Viruses. 2019, 13, 3–9. [Google Scholar] [CrossRef]
- Bell, R.; Pliner, P.L. Time to eat: The relationship between the number of people eating and meal duration in three lunch settings. Appetite 2003, 41, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Abdulnour, J.; Doucet, E.; Brochu, M.; Lavoie, J.-M.; Strychar, I.; Rabasa-Lhoret, R.; Prud’homme, D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: A Montreal-Ottawa New Emerging Team group study. Menopause 2012, 19, 760–767. [Google Scholar] [CrossRef]
- Duncan, M.J.; Vandelanotte, C.; Caperchione, C.; Christine Hanley, C.; Mummery, W.K. Temporal trends in and relationships between screen time, physical activity, overweight and obesity. BMC Public Health 2012, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Panahi, S.; Tremblay, A. Sedentariness and health: Is sedentary behavior more than just physical inactivity? Front. Public. Heal. 2018, 6, 258. [Google Scholar] [CrossRef] [Green Version]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020, 1–22. [Google Scholar] [CrossRef]
- Magdy Beshbishy, A.; Hetta, H.F.; Hussein, D.E.; Saati, A.A.; Uba, C.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Shah, M.A.; Behl, T.; Batiha, G.-S. Factors Associated with Increased Morbidity and Mortality of Obese and Overweight COVID-19 Patients. Biology 2020, 9, 280. [Google Scholar] [CrossRef]
- Abbade, E.B.; Dewes, H. Behavioral and societal drivers of an obesogenic environment worldwide. Nutr. Food Sci. 2015, 45, 229–241. [Google Scholar] [CrossRef]
- Zienkiewicz, E.; Okonski, M.; Matuszewski, Ł.; Zienkiewicz, T.; Go´zdziewska, M.; Klatka, M. Influence of urbanization level and Gross Domestic Product on the prevalence of adolescent obesity in Poland. Ann. Agric. Environ. Med. 2014, 21, 136–142. [Google Scholar] [PubMed]
- Fox, A.; Feng, W.; Asal, V. What is driving global obesity trends? Globalization or “modernization”? Glob. Health 2019, 15, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brummett, B.H.; Babyak, M.A.; Williams, R.B.; Barefoot, J.C.; Costa, P.T.; Siegler, I.C. NEO personality domains and gender predict levels and trends in body mass index over 14 years during midlife. J. Res. Personal. 2006, 40, 222–236. [Google Scholar] [CrossRef]
- Sutin, A.R.; Ferrucci, L.; Zonderman, A.B.; Terracciano, A. Personality and obesity across the adult life span. J. Pers. Soc. Psychol. 2011, 101, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Magee, C.A.; Heaven, P.C.L. Big-Five personality factors, obesity and 2-year weight gain in Australian adults. J. Res. Personal. 2011, 45, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Jokela, M.; Hintsanen, M.; Hakulinen, C.; Batty, G.D.; Nabi, H.; Singh-Manoux, A.; Kivimäki, M. Association of personality with the development and persistence of obesity: A meta-analysis based on individual-participant data. Obes. Rev. 2012, 14, 315–323. [Google Scholar] [CrossRef]
- Lahti, M.; Räikkönen, K.; Lemola, S.; Lahti, J.; Heinonen, K.; Kajantie, E.; Pesonen, A.-K.; Osmond, C.; Barker., D.J.P.; Eriksson, J.G. Trajectories of physical growth and personality dimensions of the Five-Factor Model. J. Pers. Soc. Psychol. 2013, 105, 154–169. [Google Scholar] [CrossRef]
- Shim, U.; Kim, H.N.; Roh, S.J.; Cho, N.H.; Shin, C.; Ryu, S.; Sung, Y.-A.; Kim, H.L. Personality traits and body mass index in a Korean population. PLoS ONE 2014, 9, e90516. [Google Scholar] [CrossRef] [Green Version]
- Hooker, K.; McAdams, D.P. Personality Reconsidered: A New Agenda for Aging Research. J. Gerontol. Psychol. Sci. 2003, 58, P296–P304. [Google Scholar] [CrossRef] [Green Version]
- Hampson, S.E.; Edmonds, G.W.; Barckley, M.; Goldberg, L.R.; Dubanoski, J.P.; Hillier, T.A. A Big Five Approach to Self-Regulation: Personality Traits and Health Trajectories in the Hawaii Longitudinal Study of Personality and Health. Psychol. Health Med. 2017, 21, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balanzá-Martínez, V.; Atienza-Carbonell, B.; Kapczinski, F.; De Boni, R.B. Lifestyle behaviours during the COVID-19—Time to connect. Acta Psychiatr. Scand. 2020, 141, 399–400. [Google Scholar] [CrossRef] [PubMed]
| Variables | DC | ||
|---|---|---|---|
| Dietary Changes | Vegetables | 0.52 | |
| Fruits | 0.38 | ||
| Whole grains products | 0.32 | ||
| Milk and milk products | −0.14 | ||
| Pulses | 0.22 | ||
| Fish and seafood | 0.18 | ||
| Low-fat meat and/or eggs | −0.04 | ||
| Processed meats | −0.43 | ||
| Fast foods | −0.47 | ||
| Salty snacks | −0.59 | ||
| Confectionery | −0.64 | ||
| Sweetened spreads | −0.35 | ||
| Commercial pastry | −0.55 | ||
| Homemade pastry | −0.34 | ||
| Sweetened cereals and/or cereal bars | −0.34 | ||
| Sugar-sweetened beverages | −0.47 | ||
| Alcohol | −0.32 | ||
| Water | 0.35 | ||
| Explained variance (%) | 15.6 | ||
| Cumulative explained variance (%) | 15.6 | ||
| NLC | PLC | ||
| Lifestyle Changes | Sleep time | 0.42 | 0.80 |
| Screen time | 0.80 | 0.05 | |
| Physical activity | −0.59 | 0.63 | |
| Explained variance (%) | 38.9 | 34.6 | |
| Cumulative explained variance (%) | 38.9 | 73.5 |
| Variables | Total 100% (n = 1769) | Changes in Body Weight | p-Value 1 | ||
|---|---|---|---|---|---|
| Loss 18.1% (n = 320) | Stable 48.3% (n = 854) | Gain 33.6% (n = 595) | |||
| Age | |||||
| <30 years | 29.1 (514) | 36.3 (116) | 28.7 (245) | 25.7 (153) | 0.017 |
| 30–39 years | 45.6 (806) | 43.4 (139) | 44.4 (379) | 48.4 (288) | |
| 40–49 years | 12.0 (212) | 11.3 (36) | 12.8 (109) | 11.3 (67) | |
| 50–59 years | 6.8 (121) | 5.3 (17) | 6.7 (57) | 7.9 (47) | |
| ≥60 years | 6.4 (113) | 3.4 (11) | 7.5 (64) | 6.4 (38) | |
| Educational Level | |||||
| lower | 21.4 (378) | 17.2 (55) | 22.7 (194) | 21.7 (129) | 0.117 |
| higher (university) | 78.6 (1391) | 82.8 (265) | 77.3 (660) | 78.3 (466) | |
| Family Composition | |||||
| living alone | 9.3 (164) | 10.9 (35) | 8.4 (72) | 9.6 (57) | 0.652 |
| living with partner | 21.6 (382) | 21.3 (68) | 21.3 (182) | 22.2 (132) | |
| living with partner and/or children | 58.2 (1030) | 55.0(176) | 59.4 (507) | 58.3 (347) | |
| living with parents or other relatives | 10.9 (193) | 12.8 (41) | 10.9 (93) | 9.9 (59) | |
| Place of Living | |||||
| rural | 15.8 (279) | 15.9 (51) | 16.4 (140) | 14.8 (88) | 0.004 |
| town <50,000 inhabitants | 16.6 (294) | 15.0 (48) | 17.9 (153) | 15.6 (93) | |
| town 50,000–100,000 inhabitants | 12.2 (215) | 9.4 (30) | 11.8 (101) | 14.1 (84) | |
| town 101,000–500,000 inhabitants | 14.4 (254) | 10.9 (35) | 16.2 (138) | 13.6 (81) | |
| town >500,000 inhabitants | 16.1 (285) | 15.6 (50) | 14.4 (123) | 18.8 (112) | |
| urban agglomeration | 25.0 (442) | 33.1(106) | 23.3 (199) | 23.0 (137) | |
| Macroeconomic Region | |||||
| <50% of EU-28 GDP | 17.2 (305) | 13.8 (44) | 20.8 (178) | 13.9 (83) | 0.001 |
| 50–100% of EU-28 GDP | 60.8 (1076) | 59.4 (190) | 59.5 (508) | 63.5 (378) | |
| >100% of EU-28 GDP | 21.9 (388) | 26.9 (86) | 19.7 (168) | 22.5 (134) | |
| Employment Forms during the Pandemic | |||||
| did not work or considerable work time reduction | 46.5 (823) | 42.2 (135) | 48.7 (416) | 45.7 (272) | <0.001 |
| began remote work and/or study | 40.4 (715) | 49.7 (159) | 36.2 (309) | 41.5 (247) | |
| work in the same form as earlier | 13.1 (231) | 8.1 (26) | 15.1 (129) | 12.8 (76) | |
| Difficulties with Food Ability during the Pandemic | |||||
| no | 66.9 (1184) | 63.8 (204) | 69.2 (591) | 65.4 (389) | 0.128 |
| yes | 33.1 (585) | 36.3 (116) | 30.8 (263) | 34.6 (206) | |
| Changes in Total Food Intake during the Pandemic | |||||
| ate less | 14.1 (250) | 42.8 (137) | 9.0 (77) | 6.1 (36) | <0.001 |
| no changes | 50.2 (888) | 43.1 (138) | 67.4 (576) | 29.2 (174) | |
| ate more | 35.7 (631) | 14.1 (45) | 23.5 (201) | 64.7 (385) | |
| Diet Quality Score | |||||
| Mean ± SD | 1.9 ± 1.6 | 2.1 ± 1.5 | 1.9 ± 1.6 | 1.7 ± 1.7 | 0.049 |
| Median | 2.0 | 2.0 a | 2.0 a | 2.0 b | |
| Q1; Q3 | 1.0; 3.0 | 1.0; 3.0 | 1.0; 3.0 | 1.0; 3.0 | |
| BMI Category before the Pandemic | |||||
| underweight | 6.0 (107) | 1.9 (6) | 9.3 (79) | 3.7 (22) | <0.001 |
| normal weight | 61.0 (1079) | 60.0 (192) | 62.8 (536) | 59.0 (351) | |
| overweight | 24.0 (425) | 27.2 (87) | 21.8 (186) | 25.5 (152) | |
| obesity | 8.9 (158) | 10.9 (35) | 6.2 (53) | 11.8 (70) | |
| BMI Category during the Pandemic | |||||
| underweight | 5.8 (103) | 5.0 (16) | 9.3 (79) | 1.3 (8) | <0.001 |
| normal weight | 61.0 (1079) | 68.4 (219) | 62.8 (536) | 54.5 (324) | |
| overweight | 23.2 (411) | 18.1 (58) | 21.8 (186) | 28.1 (167) | |
| obesity | 9.9 (176) | 8.4 (27) | 6.2 (53) | 16.1 (96) | |
| Variables | BMI Category before the Pandemic | p-Value 1 | |||
|---|---|---|---|---|---|
| Underweight 6.0% (n = 107) | Normal 61.0% (n = 1079) | Overweight 24.0% (n = 425) | Obesity 8.9% (n = 158) | ||
| BMI during the Pandemic | |||||
| underweight | 86.9 (93) | 0.9 (10) | - | - | <0.001 |
| normal weight | 13.1 (14) | 95.3 (1028) | 8.7 (37) | - | |
| overweight | - | 3.8 (41) | 85.2 (362) | 5.1 (8) | |
| obesity | - | - | 6.1 (26) | 94.9 (150) | |
| Weight Changes | |||||
| loss | 73.8 (79) | 49.7 (536) | 43.8 (186) | 33.5 (53) | <0.001 |
| stable | 5.6 (6) | 17.8 (192) | 20.5 (87) | 22.2 (35) | |
| gain | 20.6 (22) | 32.5 (351) | 35.8 (152) | 44.3 (70) | |
| Variables | Weight Loss | Weight Gain | ||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Age (Decades) | 0.80 (0.71–0.91) *** | 0.78 (0.69–0.89) *** | 1.07 (0.98–1.17) | 1.03 (0.94–1.14) |
| Educational Level | ||||
| lower | 0.72 (0.53–0.99) * | 0.82 (0.59–1.14) | 1.03 (0.81–1.31) | 1.03 (0.80–1.32) |
| higher (university) | 1 | 1 | 1 | 1 |
| Place of Living | ||||
| rural | 1 | 1 | 1 | 1 |
| town <50,000. | 0.87 (0.57–1.35) | 0.93 (0.60–1.46) | 1.00 (0.71–1.43) | 1.01 (0.70–1.44) |
| town 50,000–100,000 | 0.72 (0.44–1.18) | 0.73 (0.44–1.20) | 1.39 (0.96–2.02) | 1.32 (0.91–1.93) |
| town 101,000–500,000 town >500,000 | 0.71 (0.45–1.14) | 0.68 (0.42–1.10) | 1.02 (0.71–1.46) | 1.01 (0.70–1.46) |
| urban agglomeration | 0.95 (0.62–1.46) | 0.79 (0.49–1.28) | 1.41 (0.99–1.99) | 1.24 (0.84–1.82) |
| rural | 1.41 (0.97–2.05) | 1.22 (0.78–1.92) | 0.97 (0.71–1.35) | 0.84 (0.57–1.23) |
| Macroeconomic Region | ||||
| <50% of EU-28 GDP | 1 | 1 | 1 | 1 |
| 50–100% of EU-28 GDP | 1.27 (0.89–1.82) | 1.22 (0.84–1.79) | 1.45 (1.09–1.92) *** | 1.55 (1.16–2.08) *** |
| >100% of EU-28 GDP | 1.69 (1.13–2.52) ** | 1.22 (0.72–2.05) | 1.41 (1.02–1.96) * | 1.65 (1.08–2.53) * |
| Employment Forms during the Pandemic | ||||
| did not work or considerable work time reduction | 1.55 (0.99–2.42) | 1.53 (0.97–2.41) | 1.01 (0.74–1.37) | 1.00 (0.73–1.38) |
| began remote work and/or study | 2.25 (1.45–3.52) *** | 2.01 (1.27–3.18) ** | 1.08 (0.79–1.47) | 1.14 (0.82–1.57) |
| work in the same form as earlier | 1 | 1 | 1 | 1 |
| BMI before the Pandemic | ||||
| underweight | 0.27 (0.12–0.63) ** | 0.24 (0.10–0.56) *** | 0.54 (0.33–0.87) ** | 0.54 (0.33–0.88) ** |
| normal weight | 1 | 1 | 1 | 1 |
| overweight | 1.19 (0.90–1.58) | 1.39 (1.04–1.86) * | 1.15 (0.91–1.46) | 1.15 (0.90–1.46) |
| obesity | 1.31 (0.88–1.97) | 1.76 (1.15–2.69) ** | 1.65 (1.18–2.32) ** | 1.64 (1.15–2.32) ** |
| Factors | Univariate | Model 1 | Model 2 | |
|---|---|---|---|---|
| β (95% CI) | R2 | β (95% CI) | β (95% CI) | |
| Dietary Changes | −0.316 | 0.10 *** | −0.275 | −0.167 |
| (−0.361–−0.272) *** | (−0.321–−0.230) *** | (−0.212–−0.121) *** | ||
| Negative Lifestyle Changes | 0.182 | 0.03 *** | 0.135 | 0.113 |
| (0.136–0.227) *** | (0.090–0.179) *** | (0.070–0.156) *** | ||
| Positive Lifestyle Changes | −0.126 | 0.02 *** | −0.071 | −0.039 |
| (−0.172–−0.080) *** | (−0.116–−0.026) ** | (−0.082–0.004) | ||
| Diet Quality Score | −0.093 | 0.01 *** | −0.031 | −0.025 |
| (−0.140–−0.047) *** | (−0.075–0.014) | (−0.067–0.017) | ||
| R2 | - | - | 0.12 *** | 0.23 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drywień, M.E.; Hamulka, J.; Zielinska-Pukos, M.A.; Jeruszka-Bielak, M.; Górnicka, M. The COVID-19 Pandemic Lockdowns and Changes in Body Weight among Polish Women. A Cross-Sectional Online Survey PLifeCOVID-19 Study. Sustainability 2020, 12, 7768. https://doi.org/10.3390/su12187768
Drywień ME, Hamulka J, Zielinska-Pukos MA, Jeruszka-Bielak M, Górnicka M. The COVID-19 Pandemic Lockdowns and Changes in Body Weight among Polish Women. A Cross-Sectional Online Survey PLifeCOVID-19 Study. Sustainability. 2020; 12(18):7768. https://doi.org/10.3390/su12187768
Chicago/Turabian StyleDrywień, Małgorzata Ewa, Jadwiga Hamulka, Monika A. Zielinska-Pukos, Marta Jeruszka-Bielak, and Magdalena Górnicka. 2020. "The COVID-19 Pandemic Lockdowns and Changes in Body Weight among Polish Women. A Cross-Sectional Online Survey PLifeCOVID-19 Study" Sustainability 12, no. 18: 7768. https://doi.org/10.3390/su12187768
APA StyleDrywień, M. E., Hamulka, J., Zielinska-Pukos, M. A., Jeruszka-Bielak, M., & Górnicka, M. (2020). The COVID-19 Pandemic Lockdowns and Changes in Body Weight among Polish Women. A Cross-Sectional Online Survey PLifeCOVID-19 Study. Sustainability, 12(18), 7768. https://doi.org/10.3390/su12187768









