Characterization of Inhalable Aerosols from Cosmetic Powders and Sustainability in Cosmetic Products
Abstract
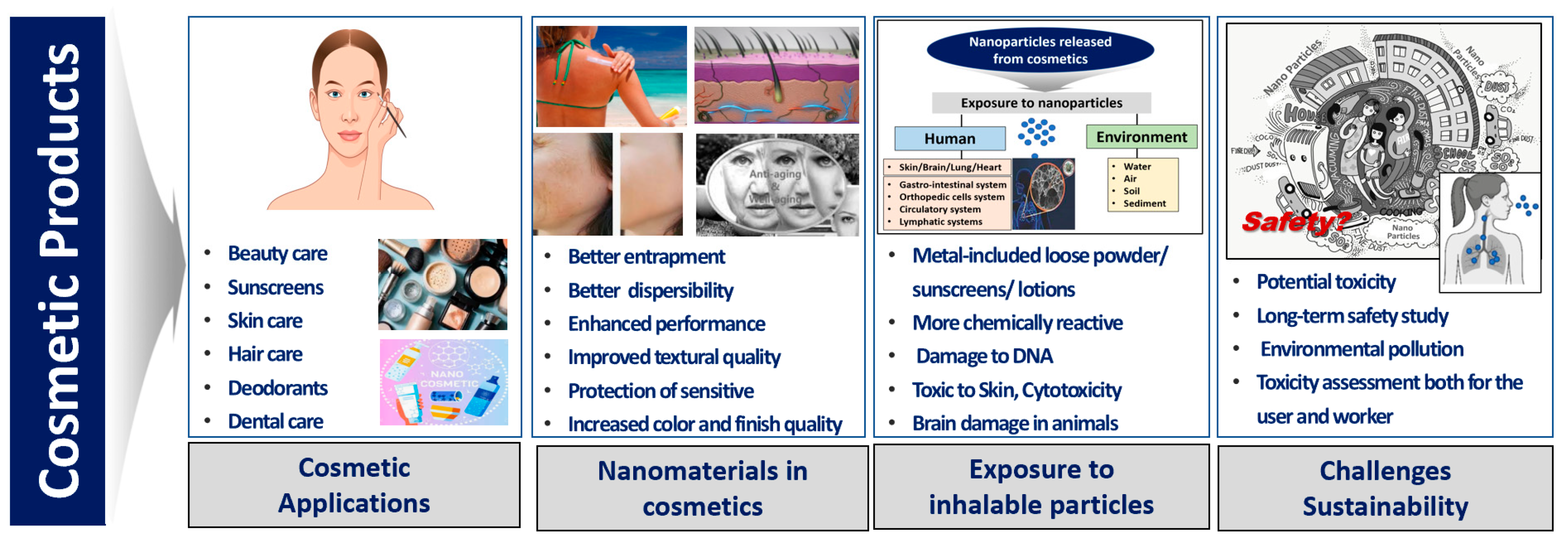
:1. Introduction
2. Materials and Methods
2.1. Tested Cosmetic Powders
2.2. Sample Collection for Inhalable Aerosol from Cosmetic Products
2.3. Morphology and Chemical Composition of Cosmetic Powders
2.4. Quantification of Deposited Doses for Aerosols
2.5. Data Analysis
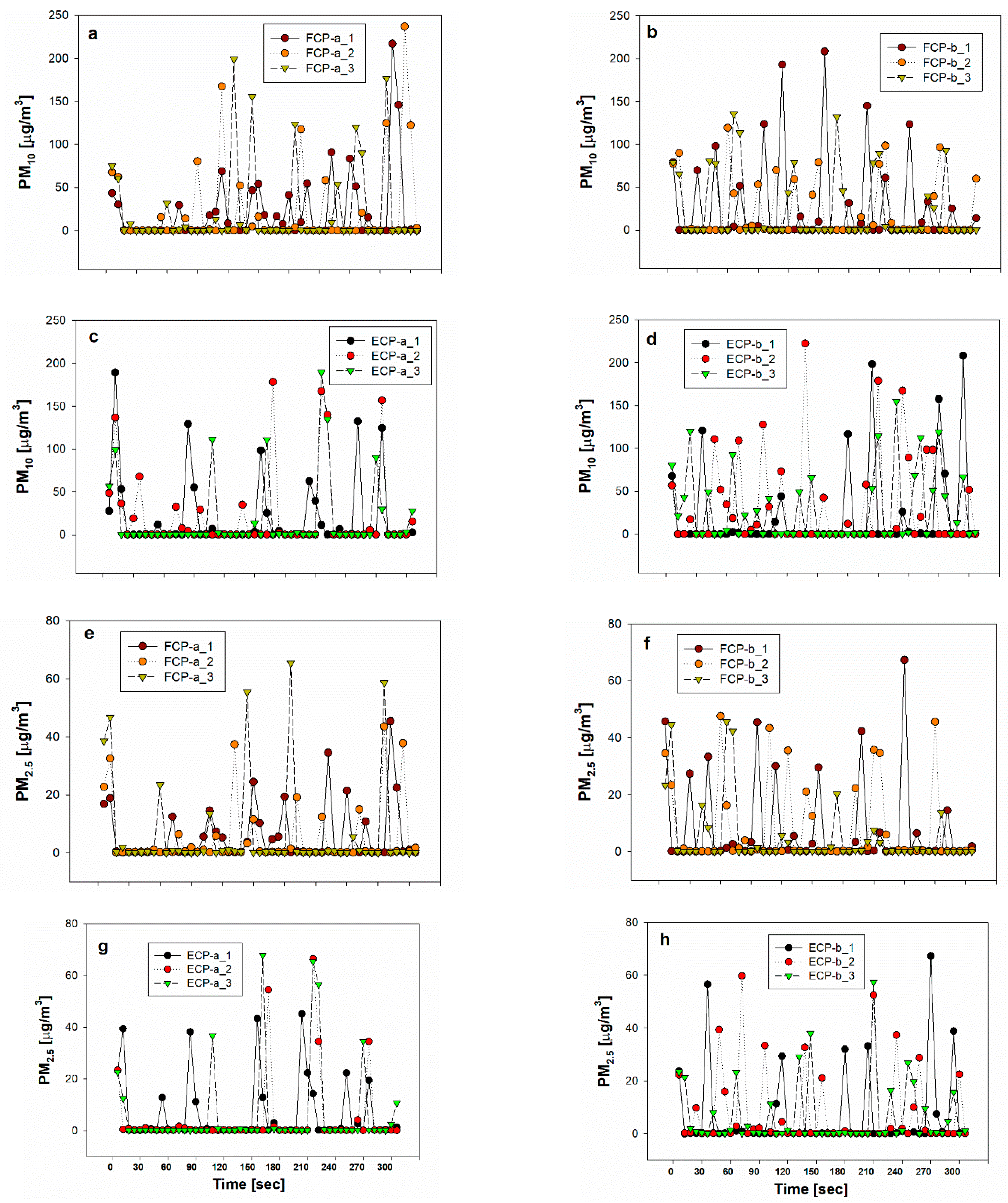
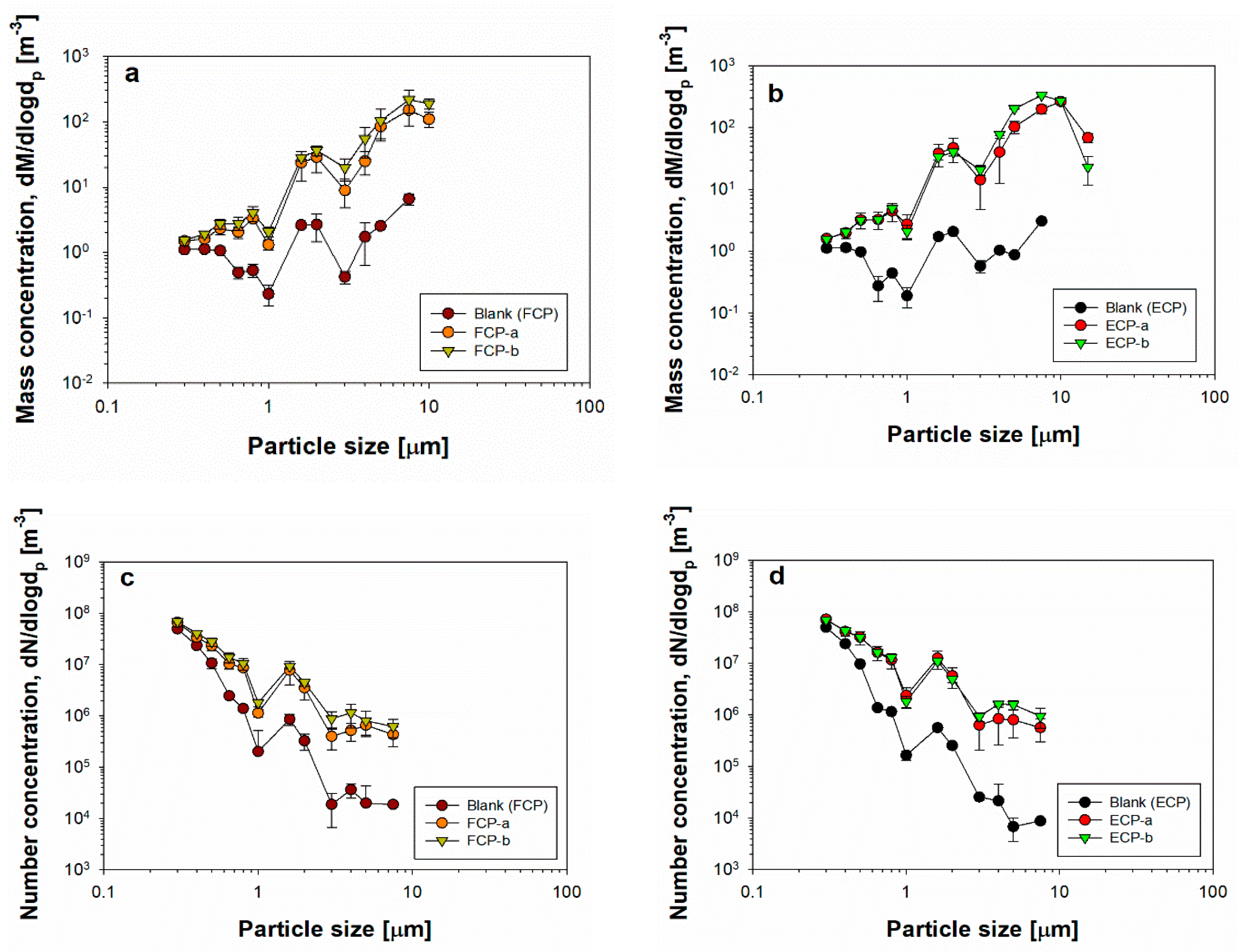
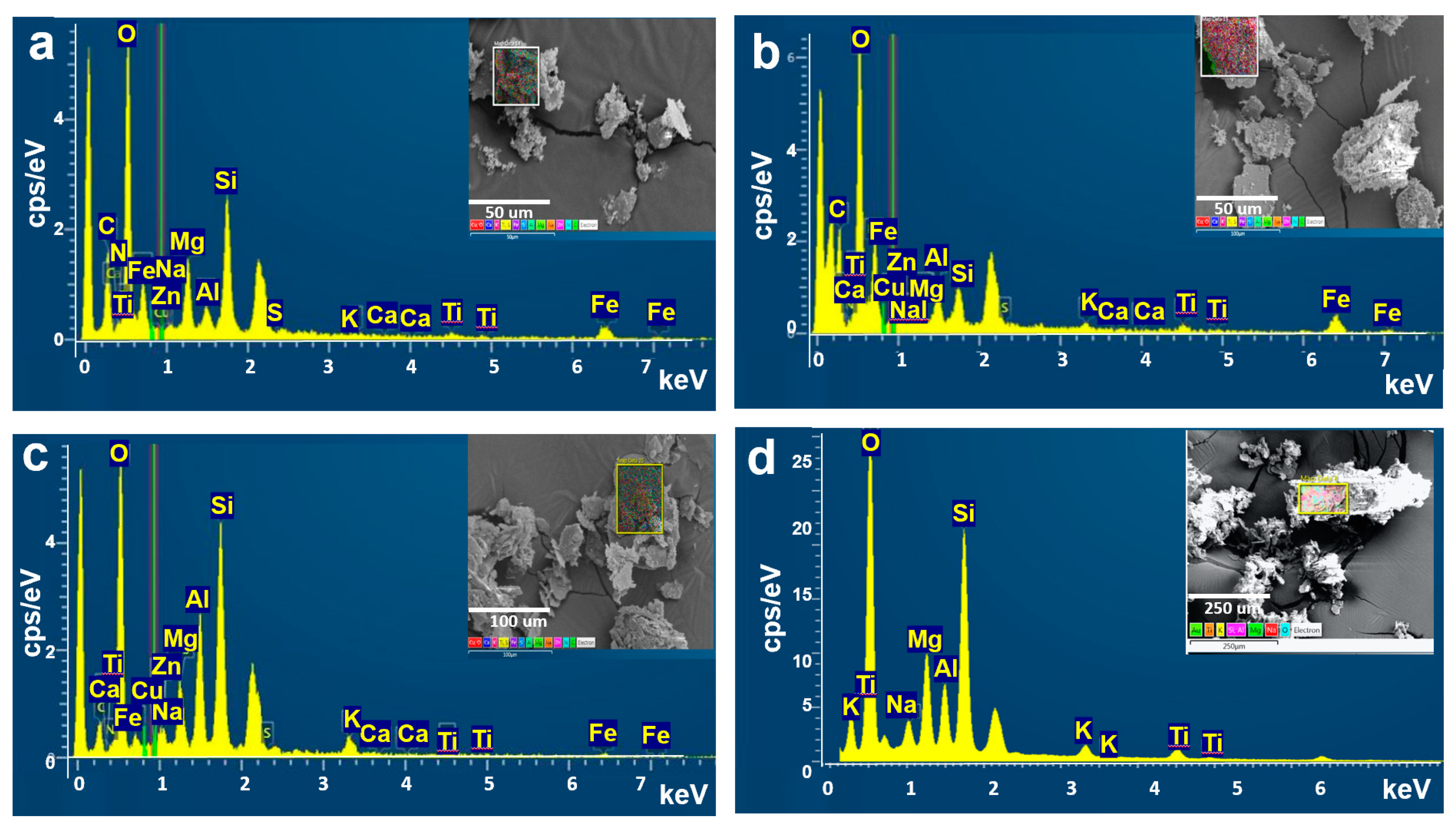
3. Results
3.1. Inhalable Aerosol (PM10, PM2.5) Concentrations from Cosmetic Powders
3.2. Mass and Number Concentrations for Cosmetic Particles
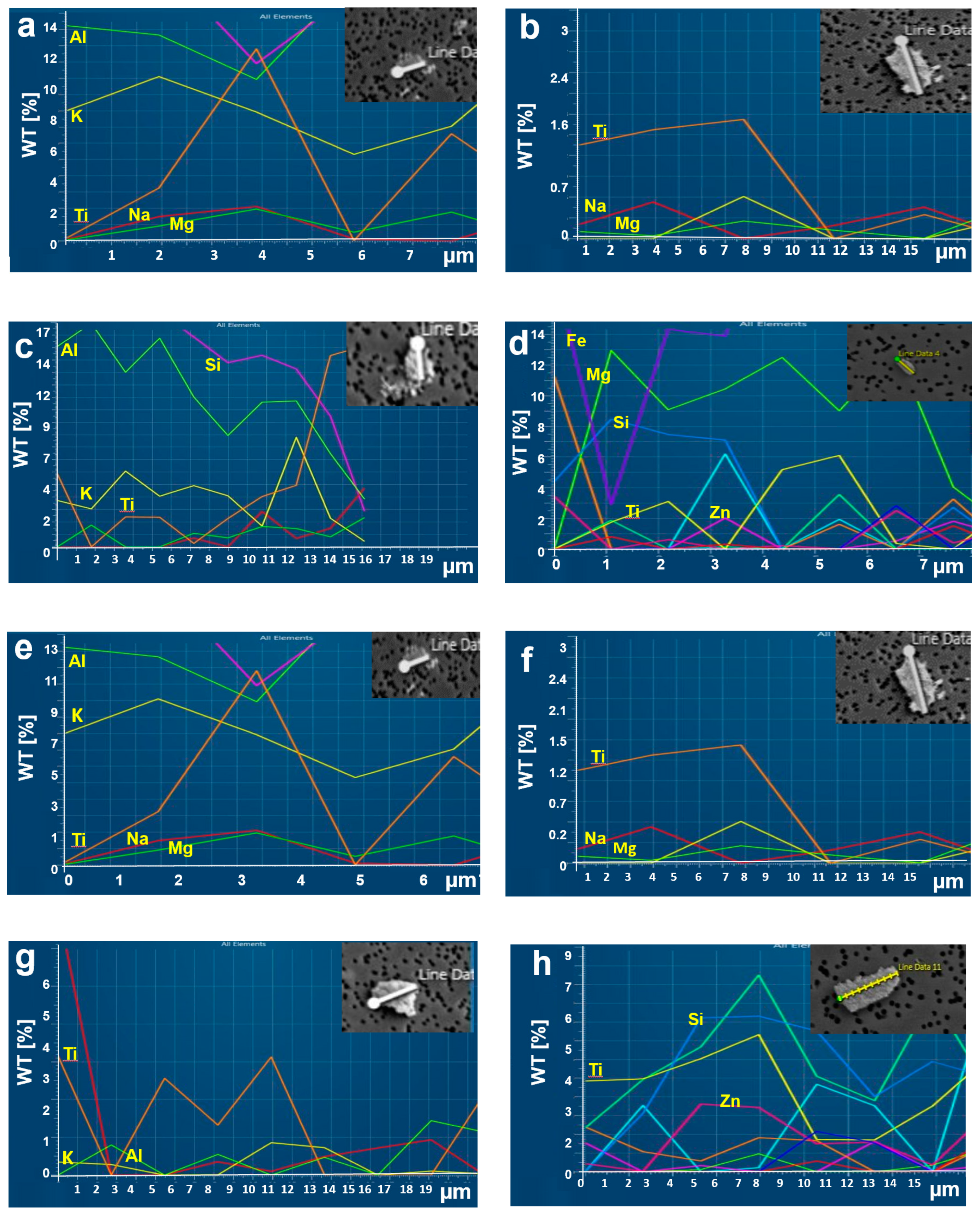
3.3. Characterization of Morphology and Chemical Components
3.4. Characterization of Chemical Components Based on Particle Size
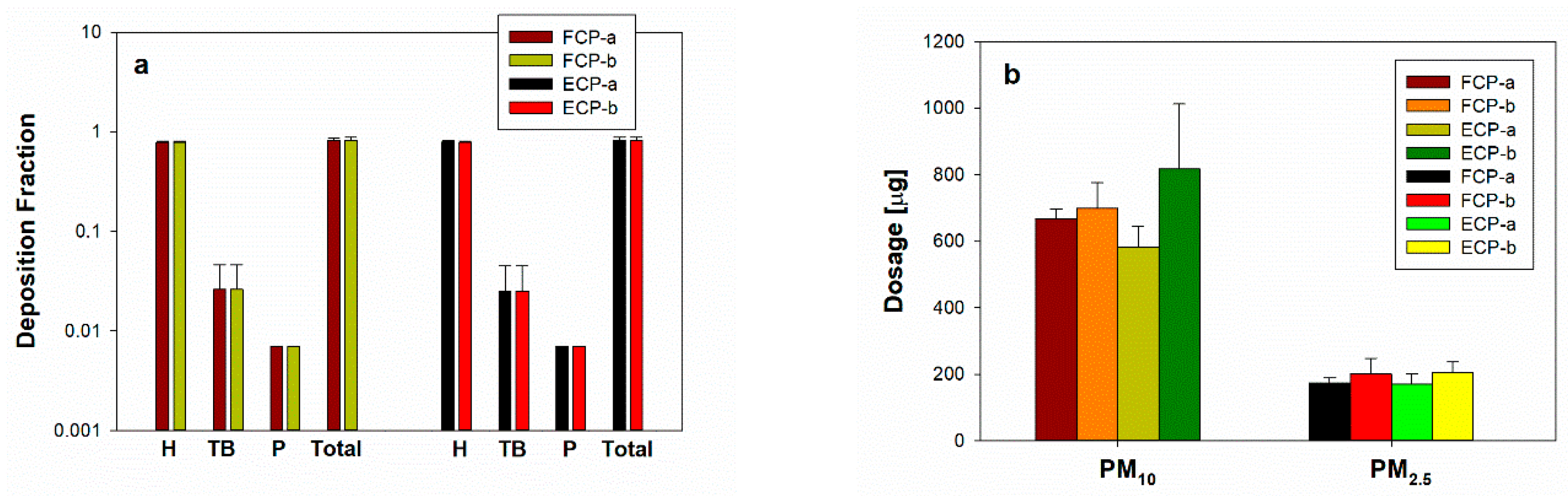
3.5. PM Deposition Fraction and Dosage in Respiratory System
3.6. Sustainability Aspects of Cosmetic Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ficheux, A.S.; Dornic, N.; Bernard, A.; Chevillotte, G.; Roudot, A.C. Probabilistic assessment of exposure to cosmetic products by French children aged 0-3 years. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 94, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.; Dudzina, T.; Delmaar, C.J.; Bakker, M.I.; Russel, F.G.; von Goetz, N.; Scheepers, P.T.; Roeleveld, N. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Berrada, M.P.; Ficheux, A.S.; Guillou, S.; Berge, C.; de Javel, D.; Roudot, A.C.; Ferret, P.J. Consumption and exposure assessment to cosmetic products for children under 2 years old. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 105, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nourmoradi, H.; Foroghi, M.; Farhadkhani, M.; Vahid Dastjerdi, M. Assessment of lead and cadmium levels in frequently used cosmetic products in Iran. J. Environ. Public Health 2013, 2013, 962727. [Google Scholar] [CrossRef] [Green Version]
- Lupkin, S. Women Put an Average of 168 Chemicals on Their Bodies Each Day, Consumer Group Says. Available online: https://abcnews.go.com/Health/women-put-average-168-chemicals-bodies-day-consumer/story?id=30615324 (accessed on 10 August 2020).
- Chow, L. Women Apply an Average of 168 Chemicals on Their Bodies Every Day. Available online: https://www.ecowatch.com/women-apply-an-average-of-168-chemicals-on-their-bodies-every-day-1882041568.html (accessed on 5 September 2020).
- Jones, O.; Sellinger, B. The Chemistry of Cosmetics. Australian Academy of Science. Available online: https://www.science.org.au/curious/people-medicine/chemistry-cosmetics (accessed on 13 August 2020).
- Kumud, M.; Sanju, N. Nanotechnology Driven Cosmetic Products: Commercial and Regulatory Milestones. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 112–121. [Google Scholar] [CrossRef]
- Baan, R.A. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: Recent evaluations by an IARC Monographs Working Group. Inhal. Toxicol. 2007, 19, 213–228. [Google Scholar] [CrossRef]
- CCOHS How Do Particulates Enter the Respiratory System? Available online: https://www.ccohs.ca/oshanswers/chemicals/how_do.html (accessed on 26 August 2020).
- IARC. Carbon Black, Titanium Dioxide, and Talc; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press, International Agency for Research on Cancer: Lyon, France, 2010; Volume 93. [Google Scholar]
- Guan RK, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Alarifi, S.; Ali, D.; Alhadlaq, H.A. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int. J. Nanomed. 2013, 8, 983–993. [Google Scholar]
- Donner, E.M.; Myhre, A.; Brown, S.C.; Boatman, R.; Warheit, D.B. In vivo micronucleus studies with 6 Titanium Dioxide materials (3 pigment-grade & 3 nanoscale) in orally-exposed rats. Regul Toxicol Pharm. 2016, 74, 64–74. [Google Scholar]
- Guichard, Y.S.J.; Darne, C.; Gaté, L.; Goutet, M.; Rousset, D.; Binet, S. Cytotoxicity and genotoxicity of nanosized and microsized Titanium Dioxide and Iron Oxide particles in Syrian Hamster embryo cells. Ann. Occup. Hyg. 2015, 56, 631–644. [Google Scholar]
- Hamzeh, M.; Sunahara, G.I. In vitro cytotoxicity and genotoxicity studies of titanium dioxide (TiO2) nanoparticles in Chinese hamster lung fibroblast cells. Toxicol. In Vitro 2013, 27, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Jomini, S.L.J.; Bauda, P.; Pagnout, C. Modifications of the bacterial reverse mutation test reveals mutagenicity of TiO2 nanoparticles and byproducts from a sunscreen TiO2-based nanocomposite. Toxicol Lett. 2012, 215, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.M.-H.L.; Van Ravenzwaay, B.; Schulz, M.; Wiench, K.; Champ, S.; Oesch, F. Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 2010, 4, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, H.K.; Falk, G.-M.; Catalán, J.; Koivisto, A.J.; Suhonen, S.; Järventaus, H.; Norppa, H. Genotoxicity of inhaled nanosized TiO2 in mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Kobayashi, N.; Ema, M.; Kasamoto, S.; Fukumuro, M.; Takami, S.; Nakanishi, J. In vivo genotoxicity study of titanium dioxide nanoparticles using comet assay following intratracheal instillation in rats. Regul. Toxicol. Pharm. 2012, 62, 1–6. [Google Scholar] [CrossRef]
- Sadiq, R.; Bhalli, J.A.; Yan, J.; Woodruff, R.S.; Pearce, M.G.; Li, Y.; Chen, T. Genotoxicity of TiO2 anatase nanoparticles in B6C3F1 male mice evaluated using Pig-a and flow cytometric micronucleus assays. Mutat. Res. 2012, 745, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.M.; Louro, H.; Antunes, S.; Quarré, S.; Simar, S.; De Temmerman, P.-J.; Silva, M.J. Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicol. In Vitro 2014, 28, 60–69. [Google Scholar] [CrossRef]
- Wang, S.; Hunter, L.A.; Arslan, Z.; Wilkerson, M.G.; Wickliffe, J.K. Chronic exposure to nanosized, anatase titanium dioxide is not cyto- or genotoxic to Chinese hamster ovary cells. Environ. Mol. Mutagen. 2011, 52, 614–622. [Google Scholar] [CrossRef]
- Woodruff, R.S.; Li, Y.; Yan, J.; Bishop, M.; Jones, M.Y.; Watanabe, F.; Chen, T. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. J. Appl. Toxicol. 2012, 32, 934–943. [Google Scholar] [CrossRef]
- Mavon, A.; Miquel, C.; Lejeune, O.; Payre, B.; Moretto, P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol. Physiol. 2007, 20, 10–20. [Google Scholar] [CrossRef]
- Bernardeschi, M.; Guidi, P.; Scarcelli, V.; Frenzilli, G.; Nigro, M. Genotoxic potential of TiO2 on bottlenose dolphin leukocytes. Anal. Bioanal. Chem. 2010, 396, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.F.; Baumgartner, A.; Cemeli, E.; Fletcher, J.N.; Anderson, D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine 2010, 5, 1193–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro 2011, 25, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Sycheva, L.P.; Zhurkov, V.S.; Iurchenko, V.V.; Daugel-Dauge, N.O.; Kovalenko, M.A.; Krivtsova, E.K.; Durnev, A.D. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 726, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Koedrith, P.; Seo, Y.R. Current investigations into the genotoxicity of zinc oxide and silica nanoparticles in mammalian models in vitro and in vivo: Carcinogenic/genotoxic potential, relevant mechanisms and biomarkers, artifacts, and limitations. Int. J. Nanomed. 2014, 9, 271–286. [Google Scholar]
- Liu, X.; Keane, M.J.; Zhong, B.Z.; Ong, T.M.; Wallace, W.E. Micronucleus formation in V79 cells treated with respirable silica dispersed in medium and in simulated pulmonary surfactant. Mutat. Res. 1996, 361, 89–94. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.S.; Wang, H. Cytotoxicity and genotoxicity of ultrafine crystalline SiO2 particulate in cultured human lymphoblastoid cells. Environ. Mol. Mutagen. 2007, 48, 151–157. [Google Scholar] [CrossRef]
- Asharani, P.V.; Mun, L.G.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wichser, A.; Nowack, B.; Wick, P. Cytotoxic effects of nanosilver are highly dependent on the chloride concentration and the presence of organic compounds in the cell culture media. J. Nanobiotechnol. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dobrzyńska, M.M.; Gajowik, A.; Radzikowska, J.; Lankoff, A.; Dušinská, M.; Kruszewski, M. Genotoxicity of Silver and Titanium Dioxide nanoparticles in bone marrow cells of rats in vivo. Toxicology 2014, 315, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.; Zhuravkov, S.; Gapeyev, A.; Plotnikov, V.; Martemianova, I.; Martemianov, D. Comparative study of genotoxicity of Silver and Gold nanoparticles prepared by the electric spark dispersion method. J. Appl. Pharm. Sci. 2017, 7, 35–39. [Google Scholar]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- De Alteriis, E.; Falanga, A.; Galdiero, S.; Guida, M.; Maselli, C.; Galdiero, E. Genotoxicity of gold nanoparticles functionalized with indolicidin towards Saccharomyces cerevisiae. J. Environ. Sci. 2017, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdorster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health Part A 2002, 65, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef]
- Gate, L.; Disdier, C.; Cosnier, F.; Gagnaire, F.; Devoy, J.; Saba, W.; Brun, E.; Chalansonnet, M.; Mabondzo, A. Biopersistence and translocation to extrapulmonary organs of titanium dioxide nanoparticles after subacute inhalation exposure to aerosol in adult and elderly rats. Toxicol. Lett. 2017, 265, 61–69. [Google Scholar] [CrossRef]
- IARC. Titanium Dioxide Group 2B; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press, International Agency for Research on Cancer: Lyon, France, 2006; Volume 9. [Google Scholar]
- OEHHA. Public Hearing to Consider Amendments to the Ambient Air Quality Standards for Particulate Matter and Sulfates; Air Resources Board, California Environmental Protection Agency: Sacramento, CA, USA, 2002.
- Nanosystems, P. Lipid Nanoparticles. Available online: http://www.precisionnanosystems.com/areas-of-interest/formulations/lipid-nanoparticles (accessed on 13 August 2020).
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Dug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Pulicharla, R.; Zolfaghari, M.; Brar, S.K.; Cledon, M.; Drogui, P.; Surampalli, R.Y. Cosmetic Nanomaterials in Wastewater: Titanium Dioxide and Fullerenes. J. Hazard. Toxic. Radioact. Waste 2016, 20, B4014005. [Google Scholar] [CrossRef]
- Al-Bedairy, M.; Alshamsi HA, H. Environmentally Friendly Preparation of Zinc Oxide, Study Catalytic Performance of Photodegradation by Sunlight for Rhodamine B Dye. Eurasian J. Anal. Chem. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhri, N.; Soni, G.C.; Prajapati, S.K. Nanotechnology: An Advance Tool for Nano-cosmetics Preparation. Int. J. Pharm. Res. Rev. 2015, 4, 28–40. [Google Scholar]
- Nanda, S.; Arun, N.; Lohan, S.; Kaur, R.; Singh, B. Nanobiomaterials in Galenic Formulations and Cosmetics; William Andrew: Norwich, NY, USA, 2016; Volume 10. [Google Scholar]
- Borowska, S.; Brzoska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.; Wang, H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat. Res. 2007, 628, 99–106. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Liu, H.; Ma, L.; Zhao, J.; Liu, J.; Yan, J.; Ruan, J.; Hong, F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol. Trace Elem. Res. 2009, 129, 170–180. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Wang, S.; Zhao, X.; Wang, J.; Yan, J.; Ruan, J.; Wang, H.; Hong, F. The mechanism of oxidative damage in the nephrotoxicity of mice caused by nano-anatase TiO2. J. Exp. Nanosci. 2010, 5, 447–462. [Google Scholar] [CrossRef]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarenko, Y.; Zhen, H.; Han, T.; Lioy, P.J.; Mainelis, G. Potential for inhalation exposure to engineered nanoparticles from nanotechnology-based cosmetic powders. Environ. Health Perspect. 2012, 120, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Adawi, H.I.; Newbold, M.A.; Reed, J.M.; Vance, M.E.; Feitshans, I.L.; Bickford, L.R.; Lewinski, N.A. Nano-enabled personal care products: Current developments in consumer safety. NanoImpact 2018, 11, 170–179. [Google Scholar] [CrossRef]
- Environment & Society Portal. Report of the World Commission on Environment and Development: Our Common Future. Available online: http://www.environmentandsociety.org/mml/un-world-commission-environment-and-development-ed-report-world-commission-environment-and (accessed on 27 August 2020).
- Liobikienė, G.; Bernatonienė, J. Why determinants of green purchase cannot be treated equally? The case of green cosmetics: Literature review. J. Clean. Prod. 2017, 162, 109–120. [Google Scholar] [CrossRef]
- Krauter, J. In-Cosmetics 2018: Evonik Focuses on Naturalness. Available online: https://www.cosmeticsdesign-europe.com/Article/2018/10/16/Evonik-hones-in-on-naturals-and-sustainability (accessed on 28 August 2020).
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Cosmetics Europe. Over 97% of Plastic Microbeads already Phased out from Cosmetics–Cosmetics Europe Announces. Available online: https://cosmeticseurope.eu/news-events/over-97-plastic-microbeads-already-phased-out-cosmetics-cosmetics-europe-announces (accessed on 28 August 2020).
- Sahota, A. Sustainability: How the Cosmetics Industry Is Greening Up; Wiley: West Sussex, UK, 2014; Volume 1. [Google Scholar]
- Motta, W.H.; Issberner, L.-R.; Prado, P. Life cycle assessment and eco-innovations: What kind of convergence is possible? J. Clean. Prod. 2018, 187, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M., IV; Manickam, V. Environmental Risk Assessment; Elsevier: Oxford, UK, 2017; Volume 1, pp. 135–150. [Google Scholar]
- United States Environmental Protection Agency. Exposure Factors Handbook; EPA: Washington, DC, USA, 2011.
- Nazarenko, Y.; Zhen, H.; Han, T.; Lioy, P.J.; Mainelis, G. Nanomaterial inhalation exposure from nanotechnology-based cosmetic powders: A quantitative assessment. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol. 2012, 14, 1229–1242. [Google Scholar] [CrossRef]
- Deepthi, Y.; Shiva Nagendra, S.M.; Gummadi, S.N. Characteristics of indoor air pollution and estimation of respiratory dosage under varied fuel-type and kitchen-type in the rural areas of Telangana state in India. Sci. Total Environ. 2019, 650, 616–625. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- EPA. IAQ Standards and Guidlines (EPA and ASHRAE Standard). Available online: https://foobot.io/guides/iaq-standards-and-guidelines.php#:~:text=Air%20quality%20is%20a%20volatile,identify%20and%20reduce%20hazardous%20substances (accessed on 30 September 2020).
- Balásházy, I.; Hofmann, W.; Heistracher, T. Local particle deposition patterns may play a key role in the development of lung cancer. J. Appl. Physiol. 2003, 94, 1719–1725. [Google Scholar] [CrossRef]
| Cosmetic Type | Ingredients | |
|---|---|---|
| Facial Cosmetic Powder | FCP-a | Cruelty-free, Paraben-Free and Gluten-free. Mica, Silica, Phenoxyethanol, Ethylhexylglycerin. Iron Oxides (CI 77499, CI 77492, CI 77491) |
| FCP-b | Talc, Calcium Silicate, Isopropyl Palmitate, Cetyl Acetate, Zinc Stearate, Fragrances, Stearyl Acetate, Imidazolidinyl Urea, Methylparaben, Acetylated Lanolin Alcohol, Oleyl Acetate, Propylparaben, (may contain: Titanium Dioxide, Iron Oxides, Zinc Oxide). | |
| Eyeshadow Cosmetic Powder | ECP-a | Mica, Iron Oxides, Titanium Dioxide. |
| ECP-b | Titanium Dioxide, Zinc Oxide, Iron Oxide, Sericite, Rose Powder, Kosher Grade Rice Powder, Mica, Kaolin Clay. |
| Cosmetics | PM10 [µg] | PM2.5 [µg] | ||||
|---|---|---|---|---|---|---|
| H | TB | P | H | TB | P | |
| FCP-a | 651.02 ± 28.54 | 21.70 ± 0.95 | 5.84 ± 0.26 | 169.27 ± 15.70 | 5.64 ± 0.52 | 1.52 ± 0.14 |
| FCP-b | 680.70 ± 76.11 | 22.69 ± 2.54 | 6.11 ± 0.68 | 195.40 ± 46.05 | 6.51 ± 1.53 | 1.75 ± 0.41 |
| ECP-a | 567.03 ± 60.49 | 18.90 ± 2.02 | 5.09 ± 0.54 | 166.71 ± 29.15 | 5.56 ± 0.97 | 1.50 ± 0.26 |
| ECP-b | 796.56 ± 190.30 | 26.55 ± 6.34 | 7.15 ± 1.71 | 199.49 ± 30.87 | 6.65 ± 1.03 | 1.79 ± 0.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.-J.; Kim, J. Characterization of Inhalable Aerosols from Cosmetic Powders and Sustainability in Cosmetic Products. Sustainability 2020, 12, 8187. https://doi.org/10.3390/su12198187
Oh H-J, Kim J. Characterization of Inhalable Aerosols from Cosmetic Powders and Sustainability in Cosmetic Products. Sustainability. 2020; 12(19):8187. https://doi.org/10.3390/su12198187
Chicago/Turabian StyleOh, Hyeon-Ju, and Jongbok Kim. 2020. "Characterization of Inhalable Aerosols from Cosmetic Powders and Sustainability in Cosmetic Products" Sustainability 12, no. 19: 8187. https://doi.org/10.3390/su12198187
APA StyleOh, H.-J., & Kim, J. (2020). Characterization of Inhalable Aerosols from Cosmetic Powders and Sustainability in Cosmetic Products. Sustainability, 12(19), 8187. https://doi.org/10.3390/su12198187





