Acid-Modified and Unmodified Natural Clay Deposits for In Situ Immobilization and Reducing Phytoavailability of Molybdenum in a Sandy Loam Calcareous Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Preparation, Modification, and Characterization
2.2. Soil Sample Collection, Preparation, and Characterization
2.3. Greenhouse Pot Experiments
2.4. Statistical Analysis
3. Results
3.1. XRD and FTIR Analyses of Activated and Inactivated Clay Deposits
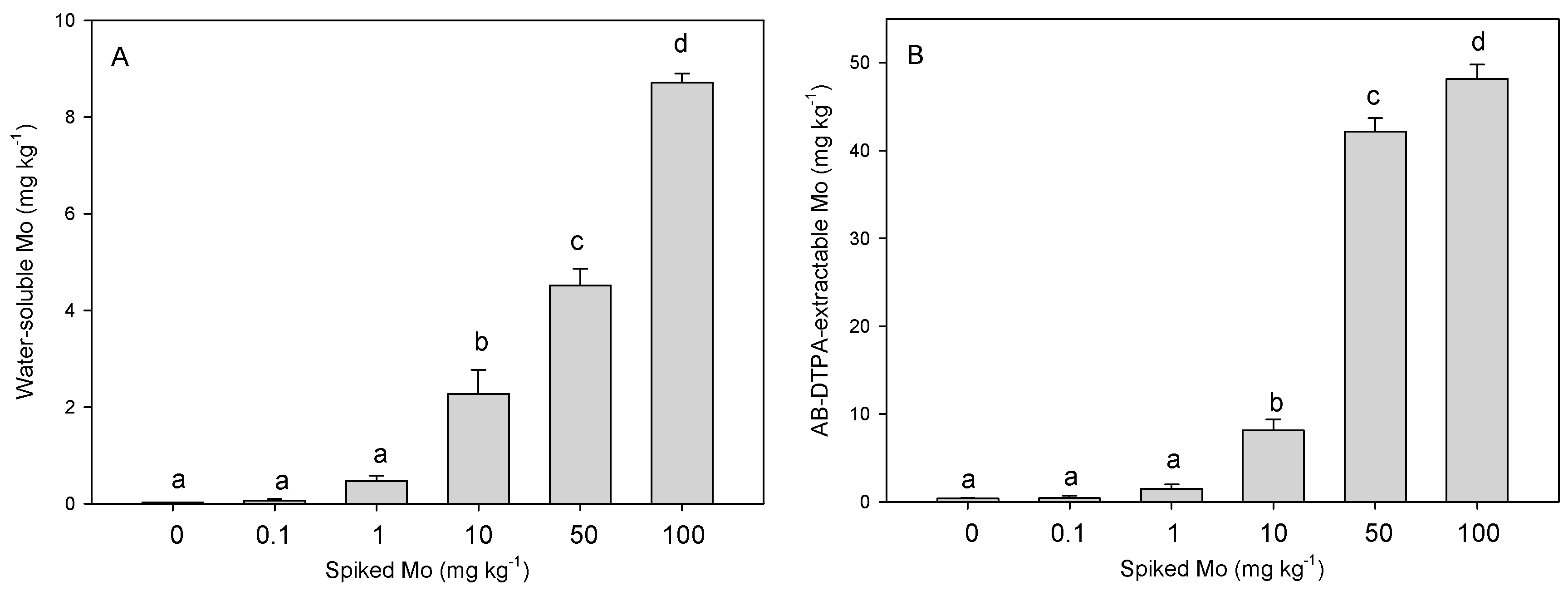
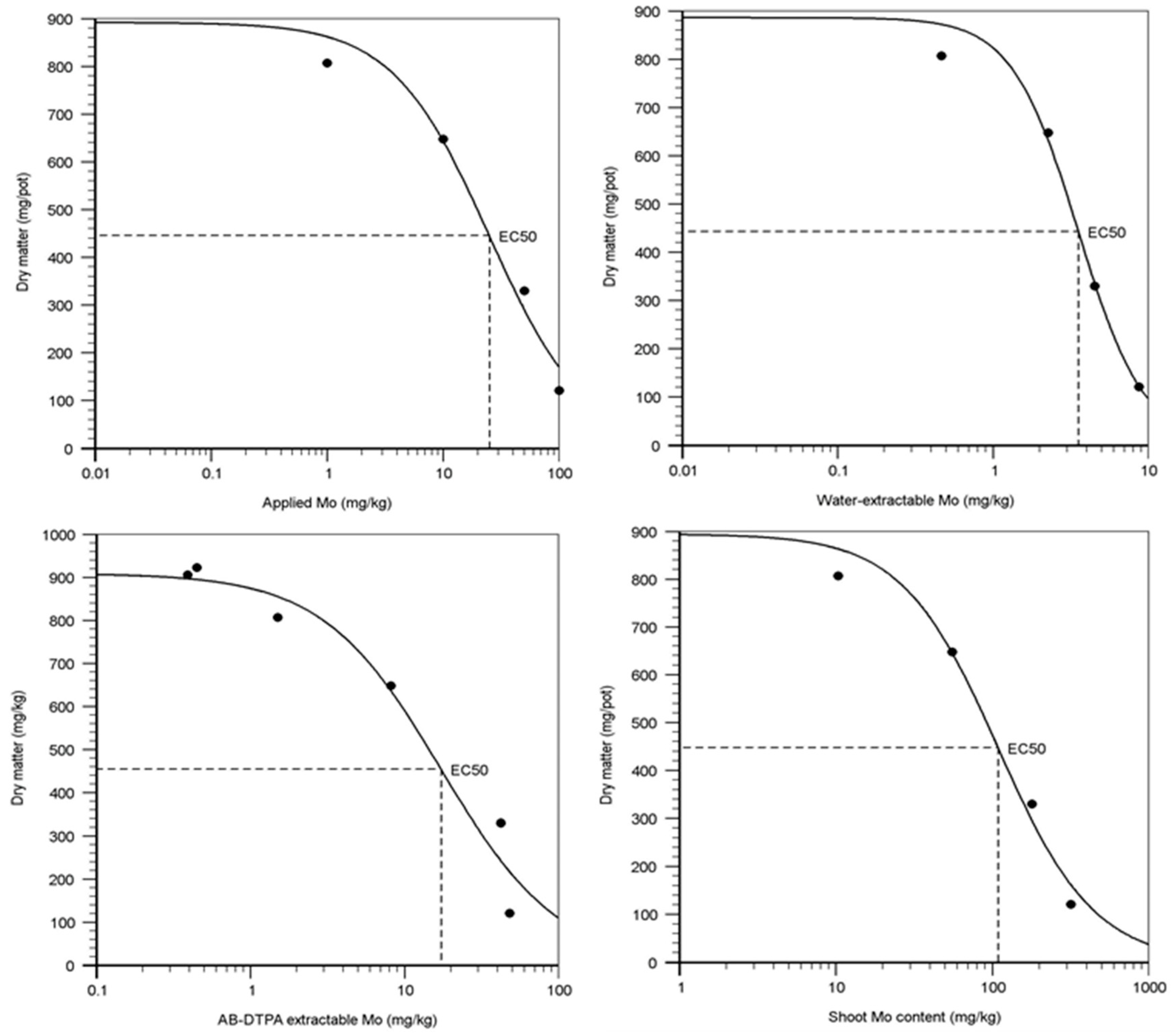
3.2. Effect of Mo Addition on Soil Mo Availability and Plant Toxicity
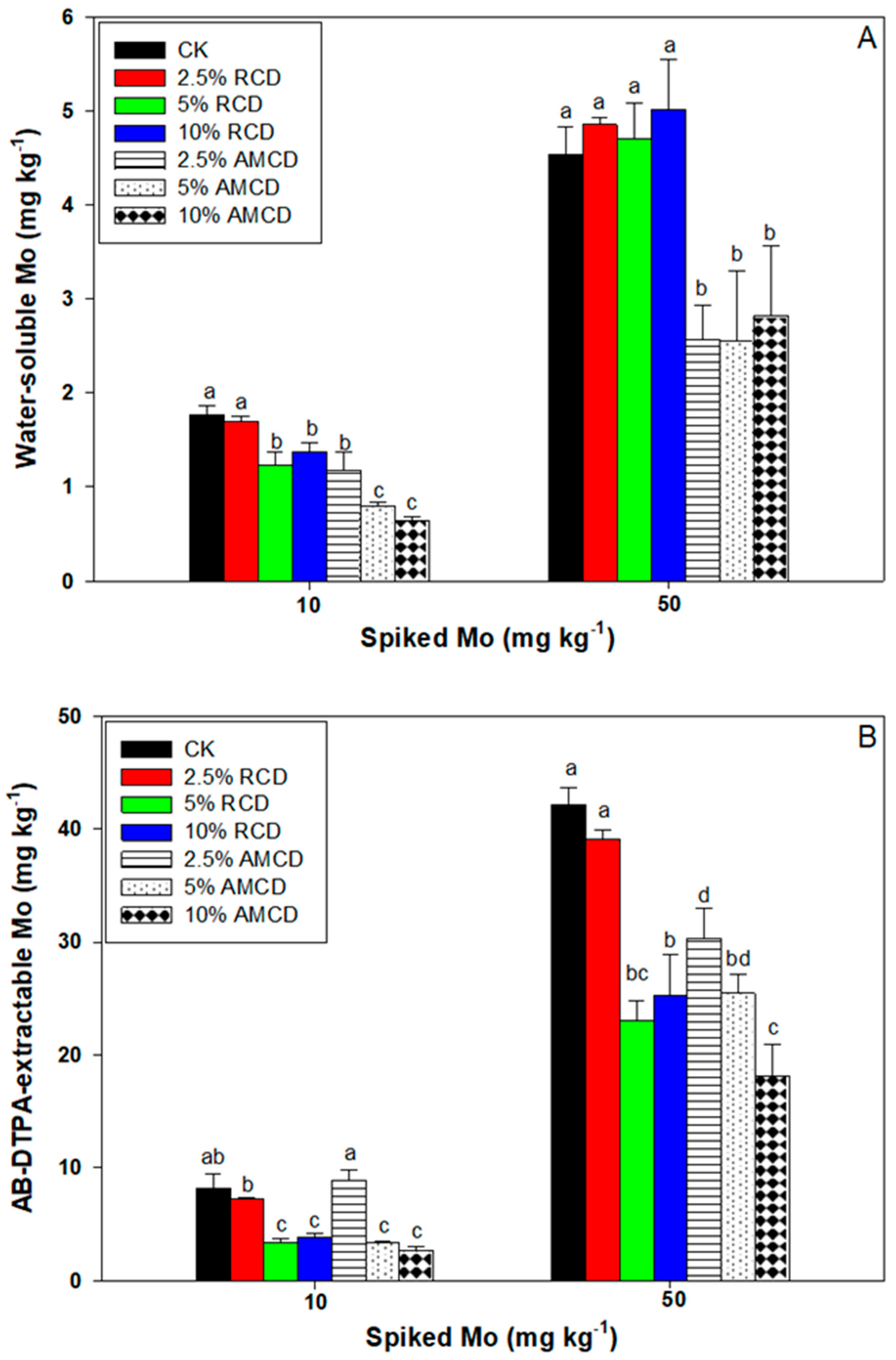
3.3. The Effect of Treatments on Soil Properties and Mo Availability
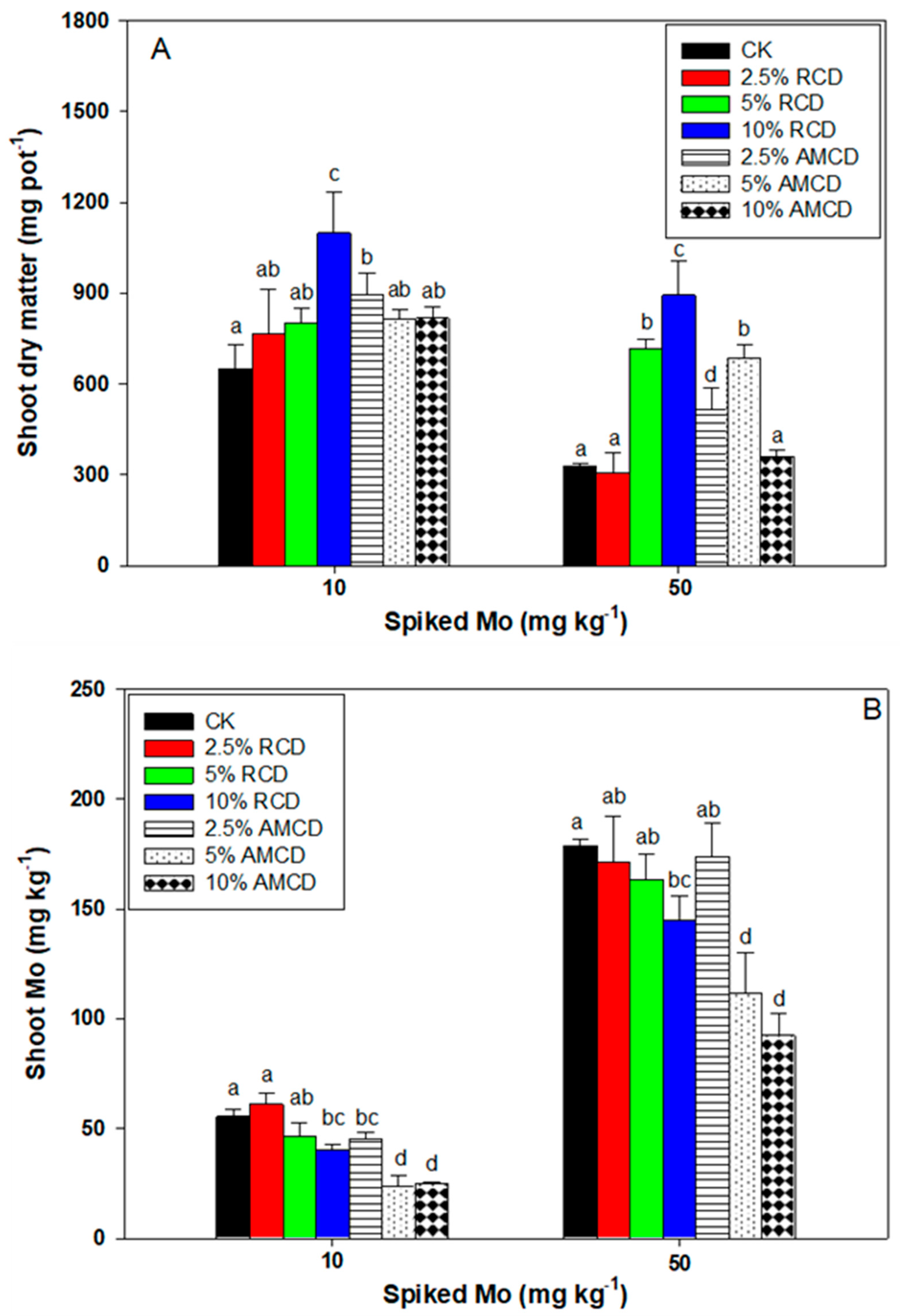
3.4. The Effect of Treatments on Plant Dry Matter and Shoot Mo Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Wabel, M.I.; Sallam, A.S.; Usman, A.R.; Ahmad, M.; El-Naggar, A.H.; El-Saeid, M.H.; Al-Faraj, A.; El-Enazi, K.; Al-Romian, F.A. Trace metal levels, sources, and ecological risk assessment in a densely agricultural area from Saudi Arabia. Environ. Monit. Assess. 2017, 189, 252. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elements Med. Biol. 2005, 19, 125–140. [Google Scholar]
- Buekers, J.; Mertens, J.; Smolders, E. Toxicity of the molybdate anion in soil is partially explained by effects of the accompanying cation or by soil pH. Environ. Toxicol. Chem. 2010, 29, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Bittner, F. Molybdenum metabolism in plants and crosstalk to iron. Front. Plant Sci. 2014, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Kubota, J.; Lazar, V.A.; Simonson, G.H.; Hill, W.W. The Relationship of Soils to Molybdenum Toxicity in Grazing Animals in Oregon. Soil Sci. Soc. Am. J. 1967, 60, 121–131. [Google Scholar] [CrossRef]
- Kumchai, J.; Huang, J.-Z.; Lee, C.-Y.; Chen, F.-C.; Chin, S.-W. Proline partially overcomes excess molybdenum toxicity in cabbage seedlings grown in vitro. Genet. Mol. Res. 2013, 12, 5589–5601. [Google Scholar] [CrossRef]
- McGrath, S.P.; Micó, C.; Zhao, F.J.; Stroud, J.L.; Zhang, H.; Fozard, S. A Predicting molybdenum toxicity to higher plants: Estimation of toxicity threshold values. Environ. Pollut. 2010, 158, 3085–3094. [Google Scholar] [CrossRef]
- McGrath, S.P.; Micó, C.; Curdy, R.; Zhao, F.J. Predicting molybdenum toxicity to higher plants: Influence of soil properties. Environ. Pollut. 2010, 158, 3095–3102. [Google Scholar] [CrossRef]
- Reddy, K.J.; Munn, L.C.; Wang, L. Chemistry and Mineralogy of Molybdenum in Soils. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H.; Godfrey, C. Molybdenum adsorption on oxides, clay minerals and soils. Soil Sci. Soc. Am. J. 1996, 60, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Lahori, A.H.; Zhang, Z.; Shaheen, S.M.; Rinklebe, J.; Guo, Z.; Li, R.; Mahar, A.; Wang, Z.; Ren, C.; Mi, S.; et al. Mono-and co-applications of Ca-bentonite with zeolite, Ca-hydroxide, and tobacco biochar affect phytoavailability and uptake of copper and lead in a gold mine-polluted soil. J. Hazard. Mater. 2019, 374, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Li, H.; Yan, Z.-G.; Zhou, Y.; Bai, L.; Zhang, C.; Wang, X.; Chen, G. In situ immobilisation of toxic metals in soil using Maifan stone and illite/smectite clay. Sci. Rep. 2018, 8, 4618. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. Immobilization of soil copper using organic and inorganic amendments. J. Plant Nutr. Soil Sci. 2015, 178, 112–117. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105–106, 200–206. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Kuzyakov, Y.; Lorenz, K.; Stahr, K. Remediation of a soil contaminated with heavy metals by immobilizing compounds. J. Plant Nutr. Soil Sci. 2006, 169, 205–212. [Google Scholar] [CrossRef]
- Vrinceanu, N.O.; Motelica, D.M.; Calciu, I.; Tanase, V.; Preda, M.; Plopeanu, G.; Ivana, I. Influence of bentonite, dolomite, natural zeolite and manure on heavy metal immobilization in a contaminated soil. AgroLife Sci. J. 2017, 6, 227–234. [Google Scholar]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Neunhäuserer, C.; Berreck, M.; Insam, H. Remediation of Soils Contaminated with Molybdenum using Soil Amendments and Phytoremediation. Water Air Soil Pollut. 2001, 128, 85–96. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Jin, X.; Dong, Y.; Luo, M. Simultaneous reduction of arsenic and cadmium bioavailability in agriculture soil and their accumulation in Brassica chinensis L. by using minerals. Ecotoxicol. Environ. Saf. 2020, 198, 110660. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, A.M.; Choudhary, M.I.; Shalaby, A.A.; Mursi, M.M. Impact of Natural Clay Deposits on Water Movement in Calcareous Sandy Soil. Arid. Land Res. Manag. 2002, 16, 185–193. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Falatah, A.M.; Sheta, A.; Al-Harbi, A.R. Clay Deposits for Water Management of Sandy Soils. Arid. Land Res. Manag. 2004, 18, 171–183. [Google Scholar] [CrossRef]
- Ismail, S.M.; Ozawa, K. Improvement of crop yield, soil moisture distribution and water use efficiency in sandy soils by clay application. Appl. Clay Sci. 2007, 37, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.; Marschner, B. Clay addition to sandy soil: Effect of clay concentration and ped size on microbial biomass and nutrient dynamics after addition of low C/N ratio residue. J. Soil Sci. Plant Nutr. 2016, 16, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Sheta, A.; Al-Omran, A.M.; Falatah, A.M.; Al-Harbi, A.R. Effect of Clay Deposits on Physicochemical and Intermittent Evaporation Characteristics of Torripsamment. Arid. Land Res. Manag. 2006, 20, 295–307. [Google Scholar] [CrossRef]
- Hajjaji, M.; Beraa, A. Chromate adsorption on acid-treated and amines-modified clay. Appl. Water Sci. 2015, 5, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, K.M.; Khoury, H.N.; Tuffaha, R. Mo and Ni Removal from Drinking Water Using Zeolitic Tuff from Jordan. Minerals 2016, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Dehpahlavan, A.; Kalantary, R.R.; Ameri, A.; Farzadkia, M.; Izanloo, H. Application of modified bentonite using sulfuric acid for the removal of hexavalent chromium from aqueous solutions. Environ. Health Eng. Manag. J. 2015, 2, 99–106. [Google Scholar]
- Sallam, A.S.; Al-Zahrani, M.S.; Al-Wabel, M.I.; Al-Farraj, A.S.; Usman, A.R. Removal of Cr(VI) and Toxic Ions from Aqueous Solutions and Tannery Wastewater Using polymerclay Composites. Sustainability 2017, 9, 1993. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.L. Soil Chemical Analysis. Advanced Course, 2nd ed.; Scientific Research: Madison, WI, USA, 1979. [Google Scholar]
- Gjems, O. Studies on clay minerals and clay-mineral formation in soil profiles in Scandinavia–norwegian Forest: Vollebekk, Norway. Meddelelser fra det Norske Skogforsoksvesen 1967, 21, 303–345. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1, 2nd ed.; Klute, A., Ed.; Agronomy 9; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Sparks, D.L. (Ed.) Methods of Soil Analysis. Part 3—Chemical Methods; SSSA Book Series No. 5; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis. Part 3, 3rd ed.; Sparks, D.L., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Hossner, L.R. Dissolution for total elemental analysis. In Methods of Soil Analysis. Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1996; pp. 46–64. [Google Scholar]
- Soltanpour, P.N.; Schwab, A.P. A new soil test for simultaneous extraction of macro and micro nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; University of California: Los Angeles, CA, USA, 1961; Volume 60–61, pp. 150–179. [Google Scholar] [CrossRef] [Green Version]
- Tibco Software Inc. Statistica (Data Analysis Software System), Version 13.5.0 TIBCO Software, Inc. Available online: https://www.tibco.com/products/data-science (accessed on 1 November 2018).
- Duval, B.D.; Natali, S.M.; Hungate, B.A. What Constitutes Plant-Available Molybdenum in Sandy Acidic Soils? Commun. Soil Sci. Plant Anal. 2015, 46, 318–326. [Google Scholar] [CrossRef]
- Axelson, U.; Svavel Till Vall. Molybdenhalter i Grovfoder. HS Rapport nr, 2001, 3/01. Available online: http://hushallningssallskapet.se/wpcontent/uploads/2015/05/2001_3_svavel_till_vall_molybdenhalter_i_grovfoder.pdf (accessed on 18 June 2018).
- Axelson, U. Kopparbrist—Diagnoshjälpmedel och Lämpliga Växtodlingsåtgärder SLF Project. H0733451. 2011. Available online: http://www.lantbruksforskning.se/projektbanken/kopparbrist-diagnoshjalpmedel-ochlampliga-vaxtodl (accessed on 18 June 2018).
- Goldberg, S.; Lesch, S.M.; Suarez, D.L. Predicting Molybdenum Adsorption by Soils Using Soil Chemical Parameters in the Constant Capacitance Model. Soil Sci. Soc. Am. J. 2002, 66, 1836–1842. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Niu, D.; Ren, Y.; Fu, H. Extractability of nutrients using Mehlich 3 and ammonium bicarbonate-DTPA methods for selected grassland soils of China. Plant Soil Environ. 2018, 64, 448–454. [Google Scholar]
- Wang, L.; Reddy, K.J.; Munn, L.C. Comparison of ammonium bicarbonate-DTPA, ammonium carbonate, and ammonium oxalate to assess the availability of molybdenum in mine spoils and soils. Commun. Soil Sci. Plant Anal. 1994, 25, 523–536. [Google Scholar] [CrossRef]
- Pierzynski, G.M.; Jacobs, L.W. Molybdenum Accumulation by Corn and Soybeans from a Molybdenum-rich Sewage Sludge. J. Environ. Qual. 1986, 15, 394–398. [Google Scholar] [CrossRef]
- Kirby, J.K.; McLaughlin, M.J.; Ma, Y.; Ajiboye, B. Aging effects on molybdate lability in soils. Chemosphere 2012, 89, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Xie, R.; MacKenzie, A. Molybdate sorption-desorption in soils treated with phosphate. Geoderma 1991, 48, 321–333. [Google Scholar] [CrossRef]
- Al-Essa, K. Activation of Jordanian Bentonite by Hydrochloric Acid and Its Potential for Olive Mill Wastewater Enhanced Treatment. J. Chem. 2018, 8385692. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Xu, N.; Braida, W.; Christodoulatos, C.; Chen, J. A Review of Molybdenum Adsorption in Soils/Bed Sediments: Speciation, Mechanism, and Model Applications. Soil Sediment Contam. Int. J. 2013, 22, 912–929. [Google Scholar] [CrossRef]
- Goldberg, S. Influence of Soil Solution Salinity on Molybdenum Adsorption by Soils. Soil Sci. 2009, 174, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Yang, Z.; Yu, T.; Hou, Q.; Zhong, C.; Zheng, G.; Yang, Z.; Li, J. Evaluation of the potential effects of soil properties on molybdenum availability in soil and its risk estimation in paddy rice. J. Soils Sediments 2015, 15, 1520–1530. [Google Scholar] [CrossRef]
- Beata, R.; Wiesław, S.; Ewa, S.-F.; Natalia, P. Prediction of molybdenum availability to plants in differentiated soil conditions. Plant Soil Environ. 2017, 63, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Vistoso, E.; Theng, B.K.G.; Bolan, N.; Parfitt, R.; Mora, M. Competitive sorption of molybdate and phosphate in Andisols. J. Soil Sci. Plant Nutr. 2012, 12, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Davenport, D.; Wilhelm, N.; Doudle, S. Clay Spreading and Delving on Eyre Peninsula; Government of South Australia; SARDI; GRDC: Adelaide, South Australia, 2006.
- Hall, D.J.M.; Jones, H.R.; Crabtree, W.L.; Daniels, T.L. Claying and deep ripping can increase crop yields and profits on water repellent sands with marginal fertility in southern Western Australia. Soil Res. 2010, 48, 178–187. [Google Scholar] [CrossRef]
- Cornacchione, M.V.; Suarez, D.L. Emergence, Forage Production, and Ion Relations of Alfalfa in Response to Saline Waters. Crop. Sci. 2015, 55, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Noaman, M.H.; El-Haddad, E. Effects of irrigation water salinity and leaching fraction on the growth of six halophythe species. J. Agric. Sci. 2000, 135, 279–285. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Hseu, Z.-Y. In-Situ Immobilization of Cadmium and Lead by Different Amendments in Two Contaminated Soils. Water Air Soil Pollut. 2002, 140, 73–84. [Google Scholar] [CrossRef]

| Treatments | Application Rate | pH | EC | ||
|---|---|---|---|---|---|
| % | value | ±SD 3 | value | ±SD | |
| Soil contaminated with 10 mg Mo kg−1 | |||||
| CK 1 | 0 | 7.74 a 2 | 0.05 | 1.89 a | 0.15 |
| RCD | 2.5 | 7.72 a | 0.02 | 1.91 a | 0.05 |
| 5 | 7.74 a | 0.06 | 1.80 a | 0.16 | |
| 10 | 7.66 ab | 0.05 | 1.81 a | 0.08 | |
| AMCD | 2.5 | 7.56 b | 0.00 | 2.34 b | 0.13 |
| 5 | 7.57 b | 0.06 | 2.70 c | 0.16 | |
| 10 | 7.33 c | 0.10 | 3.00 d | 0.08 | |
| F-value | 13.91 | - | 29.93 | - | |
| p-value | 0.0000 | - | 0.0000 | - | |
| Soil contaminated with 50 mg Mo kg−1 | |||||
| CK | 0 | 7.82 a | 0.10 | 1.76 a | 0.10 |
| RCD | 2.5 | 7.78 a | 0.01 | 1.87 a | 0.06 |
| 5 | 7.74 ab | 0.07 | 1.73 a | 0.05 | |
| 10 | 7.62 bc | 0.02 | 1.78 a | 0.13 | |
| AMCD | 2.5 | 7.60 bc | 0.02 | 2.20 b | 0.11 |
| 5 | 7.56 c | 0.02 | 2.63 c | 0.05 | |
| 10 | 7.37 d | 0.13 | 3.15 d | 0.12 | |
| F-value | 9.73 | - | 65.21 | - | |
| p-value | 0.0003 | - | 0.0000 | - | |
| Parameters | In the Soil Contaminated with 10 mg Mo kg−1 | In the Soil Contaminated with 50 mg Mo kg−1 | ||
|---|---|---|---|---|
| Water-Soluble Mo | AB-DTPA-Extractable Mo | Water-Soluble Mo | AB-DTPA-Extractable Mo | |
| pH | 0.7377 *** | 0.3259 | 0.6560 ** | 0.6471 ** |
| Clay | −0.6425 ** | −0.7183 *** | −0.1015 | −0.7632 *** |
| Parameters | In the Soil Contaminated with 10 mg Mo kg−1 | In the Soil Contaminated with 50 mg Mo kg−1 | Combined Data | |||
|---|---|---|---|---|---|---|
| Dry Matter | Shoot Mo | Dry Matter | Shoot Mo | Dry Matter | Shoot Mo | |
| pH | −0.0309 | 0.6697 *** | −0.0902 | 0.7258 *** | −0.1014 | 0.3649 * |
| EC | −0.0643 | −0.7633 *** | −0.2170 | −0.7728 *** | −0.0952 | −0.3618 * |
| Clay | 0.4840 * | −0.6670 *** | 0.4121 | −0.6919 *** | 0.3266 * | −0.2538 |
| Water-soluble Mo | −0.1593 | 0.8519 *** | 0.1396 | 0.4085 | −0.4692 ** | 0.8582 *** |
| AB-DTPA-Mo | −0.2571 | 0.6498 ** | −0.4816 * | 0.6894 *** | −0.6915 *** | 0.9345 *** |
| Shoot Mo | −0.2310 | 1.0000 | −0.1489 | - | −0.6012 *** | 1.0000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashidi, S.H.; Sallam, A.S.; Usman, A.R.A. Acid-Modified and Unmodified Natural Clay Deposits for In Situ Immobilization and Reducing Phytoavailability of Molybdenum in a Sandy Loam Calcareous Soil. Sustainability 2020, 12, 8203. https://doi.org/10.3390/su12198203
Alrashidi SH, Sallam AS, Usman ARA. Acid-Modified and Unmodified Natural Clay Deposits for In Situ Immobilization and Reducing Phytoavailability of Molybdenum in a Sandy Loam Calcareous Soil. Sustainability. 2020; 12(19):8203. https://doi.org/10.3390/su12198203
Chicago/Turabian StyleAlrashidi, Saleh H., Abdelazeem S. Sallam, and Adel R. A. Usman. 2020. "Acid-Modified and Unmodified Natural Clay Deposits for In Situ Immobilization and Reducing Phytoavailability of Molybdenum in a Sandy Loam Calcareous Soil" Sustainability 12, no. 19: 8203. https://doi.org/10.3390/su12198203





