Growth Performance of Jatropha curcas Cultivated on Local Abandoned Bauxite Mine Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Soil Sample
2.3. Test Plant
2.4. Pot Experimental Design
2.5. Soil Characterization
2.6. Plant Analysis
2.7. Seed and Oil Yield
2.8. Statistical Analysis
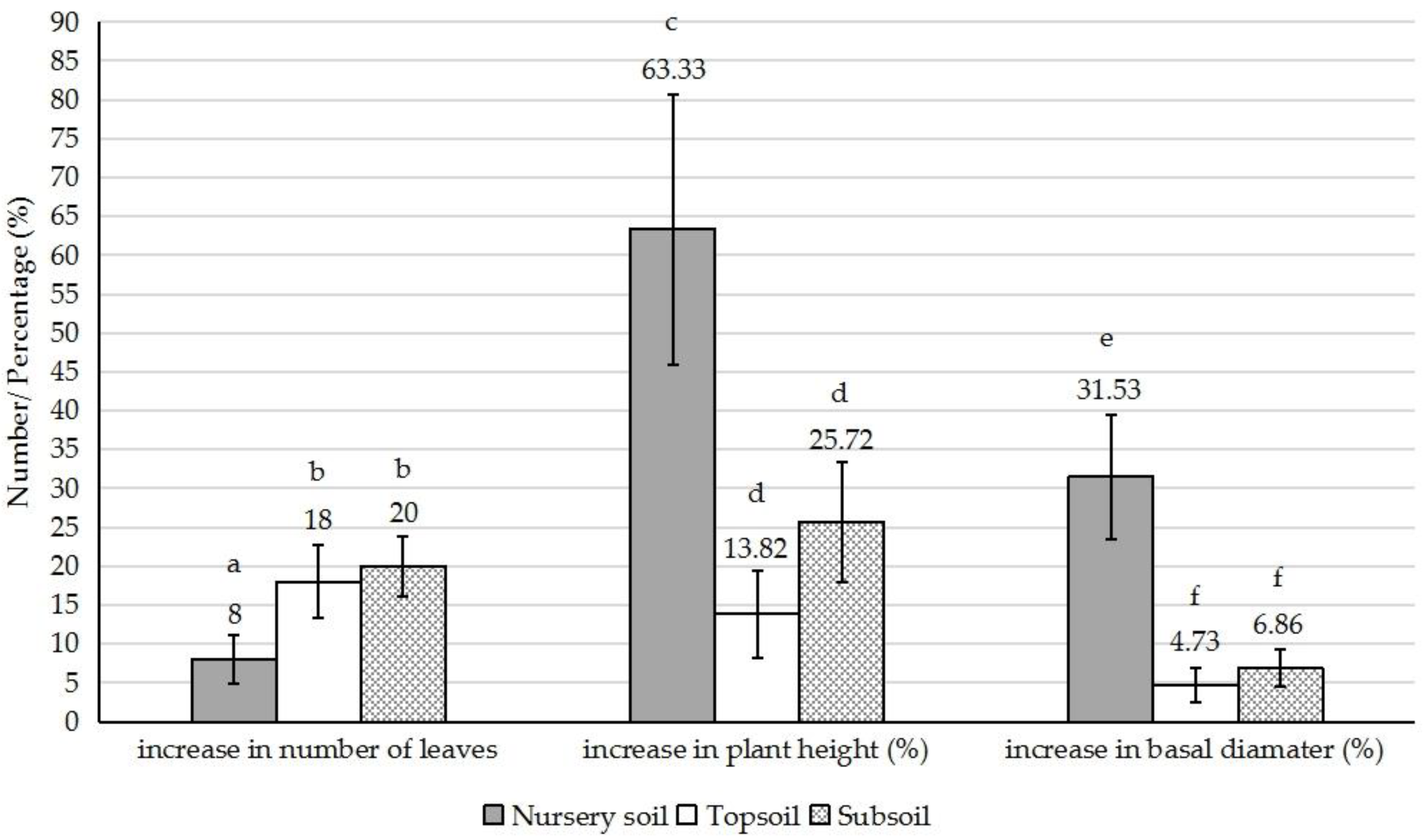
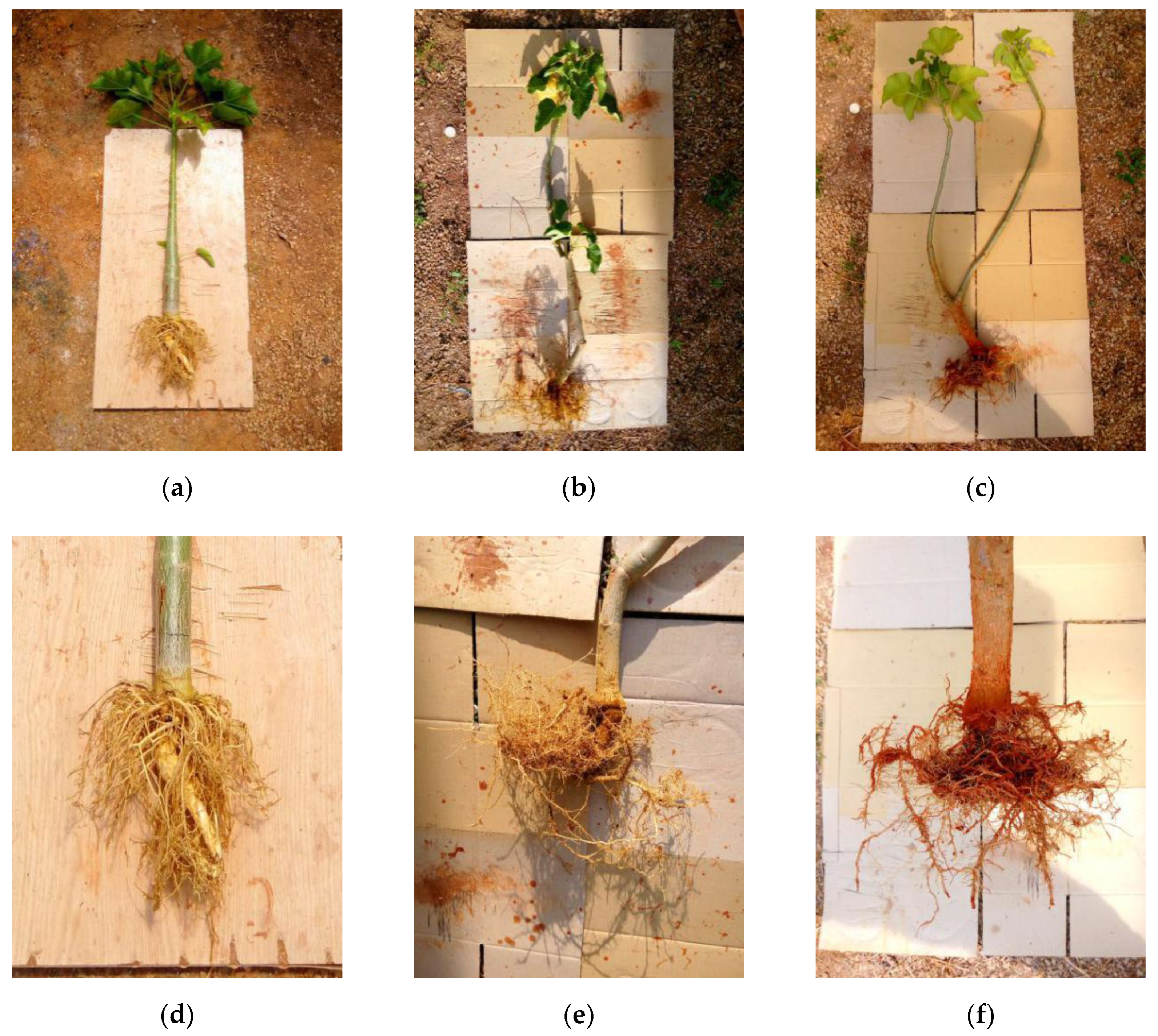
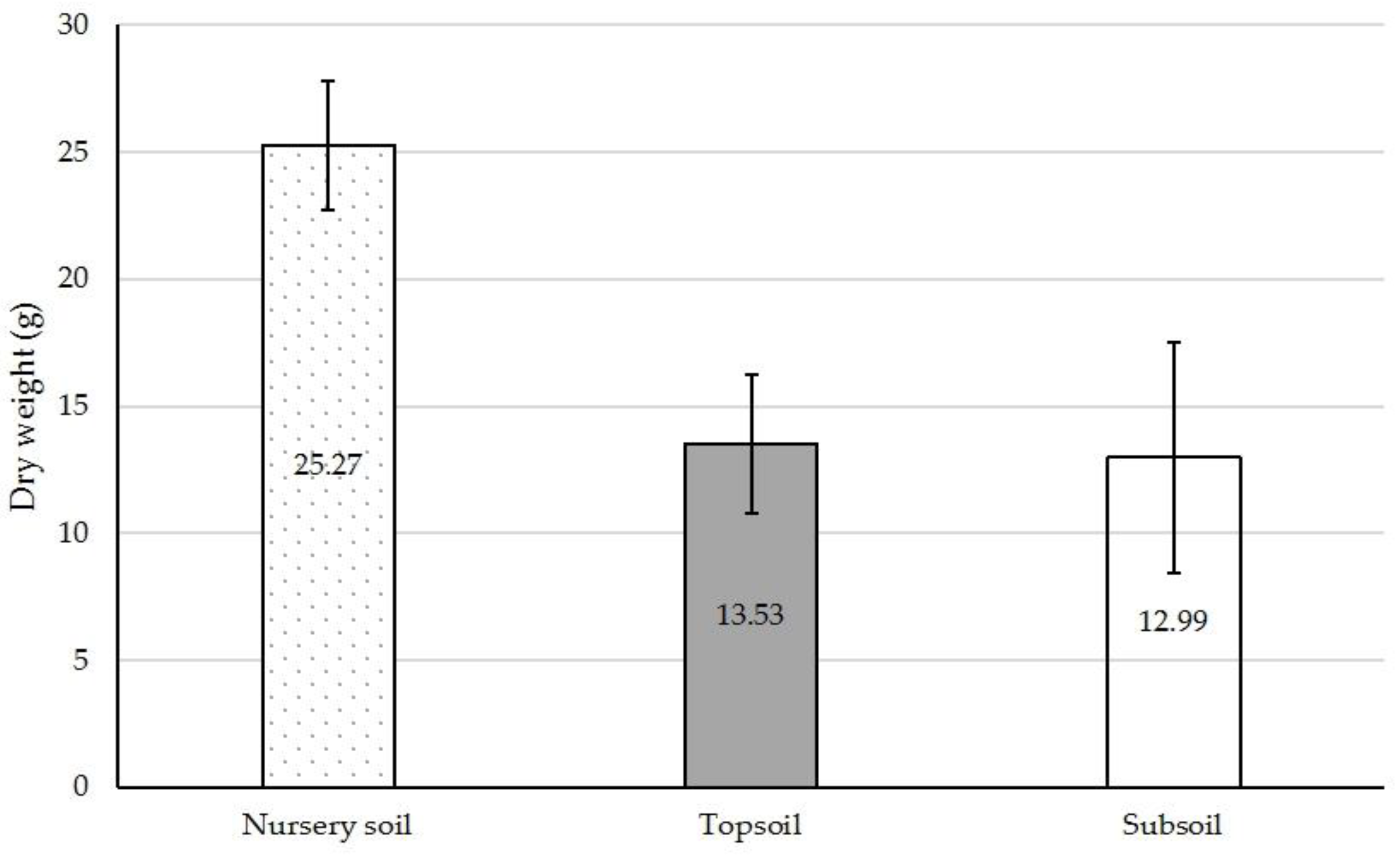
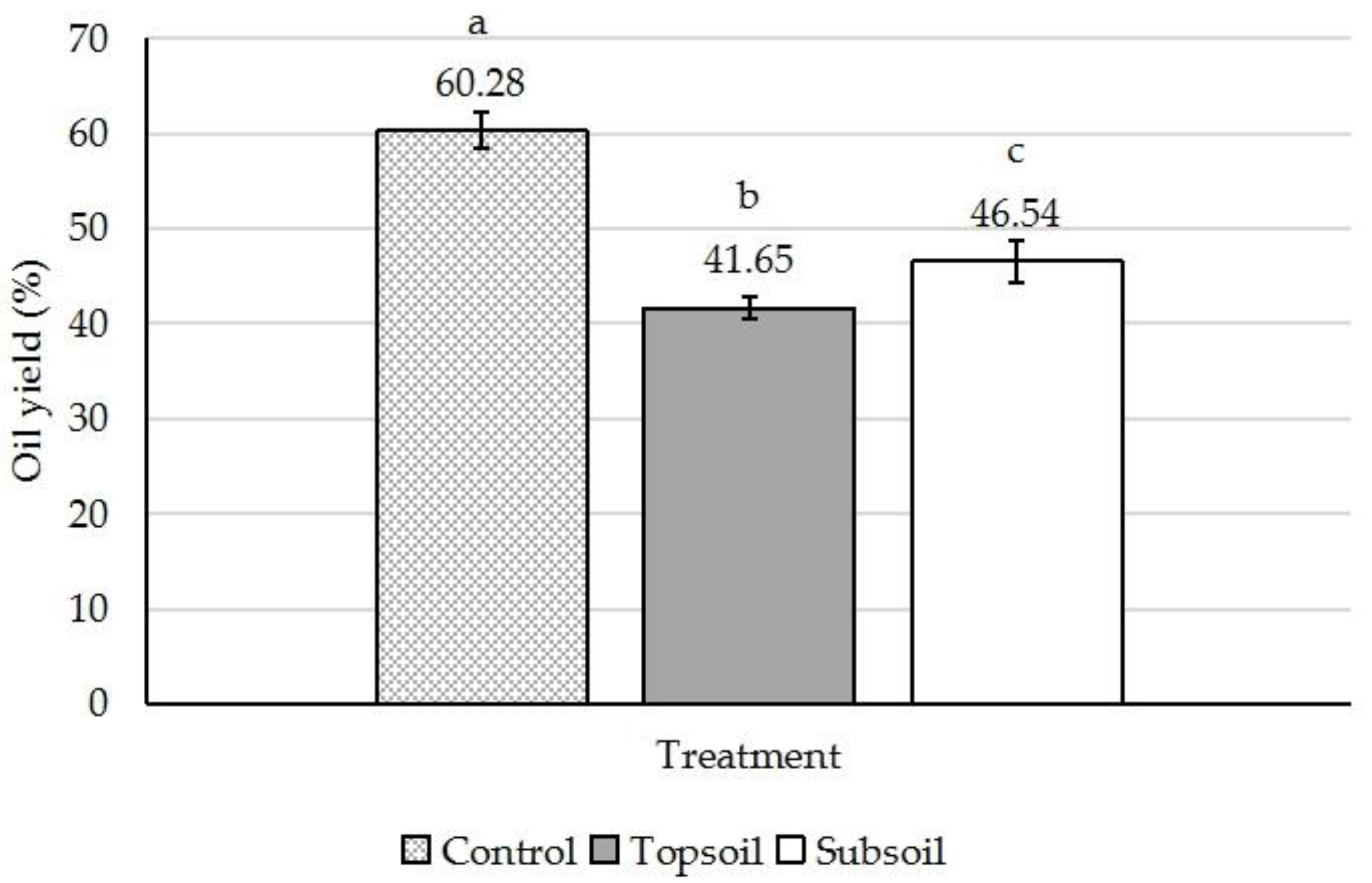
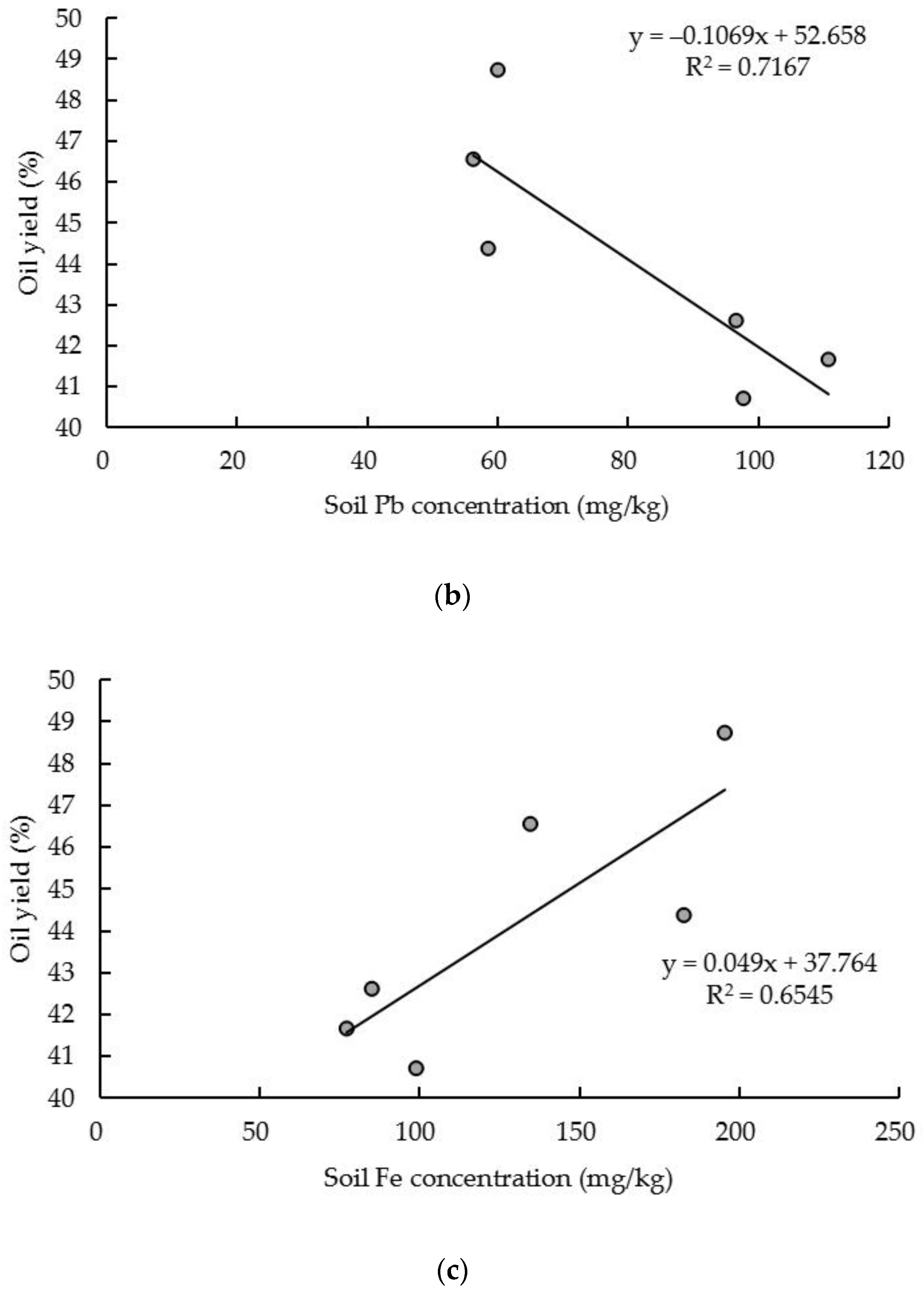
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaheen, M.A.; Rana, S.I.; Tariq, M.I.; Rehman, F.; Karim, A.; Murtaza, N.A.S.; Aziz, M. Evaluation of Bauxite of Khushab (Pakistan) as a Raw Material for Extraction of Aluminum. Pak. J. Sci. 2010, 62, 79–83. [Google Scholar]
- Kusin, F.M.; Rahman, M.S.A.; Madzin, Z.; Jusop, S.; Mohamat-Yusuff, F.; Ariffin, M.; Zahar, M.S.M. The Occurrence and Potential Ecological Risk Assessment of Bauxite Mine-impacted Water and Sediments in Kuantan, Pahang, Malaysia. Environ. Sci. Pollut. Res. 2017, 24, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Omoregie, S.N. Post-mining Deterioration of Bauxite Overburdens in Jamaica: Storage Methods or Subsoil Dilution? Environ. Geol. 2008, 54, 111–115. [Google Scholar] [CrossRef]
- Lewis, D.E.; White, J.R.; Wafula, D.; Athar, R.; Dickerson, T.; Williams, H.N.; Chauhan, A. Soil Functional Diversity Analysis of a Bauxite-Mined Restoration Chronosequence. Soil Microbiol. 2010, 59, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Ghose, M.K. Management of Topsoil for Geo-environmental Reclamation of Coal Mining Areas. Environ. Geol. 2001, 40, 1405–1410. [Google Scholar]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Nat. Biotechnol. 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Gomes, H.I. Phytoremediation for Bioenergy: Challenges and Opportunities. Environ. Technol. Rev. 2012, 1, 59–66. [Google Scholar] [CrossRef]
- Reddy, K.R.; Adams, J.A. Towards Green and Sustainable Remediation of Contaminated Site. In Proceedings of the 6th International Congress on Environmental Geotechnics, New Delhi, India, 8–12 November 2010. [Google Scholar]
- Gautam, M.; Pandey, D.; Agrawal, M. Phytoremediation of Metals Using Lemongrass (Cymbopogon citratus (D.C.) Stapf.) Grown Under Different Levels of Red Mud in Soil Amended with Biowastes. Int. J. Phytoremediation 2017, 19, 555–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Singh, S.P. A Review on Phytoremediation of Heavy Metals and Utilization of Its By-products. Asian J. Energy Environ. 2005, 6, 214–231. [Google Scholar]
- Alshaal, T.; Domokos-Szabolcsy, É.; Márton, L.; Czakó, M.; Kátai, J.; Balogh, P.; Elhawat, N.; El-Ramady, H.; Fári, M. Phytoremediation of Bauxite-derived Red Mud by Giant Reed. Environ. Chem. Lett. 2013, 11, 295–302. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating Heavy-metal Hyperaccumulation using Thlaspi caerulescens as a Model System. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jusop, S. Bauxite Mining in Pengerang; Universiti Putra Malaysia Press: Serdang, Malaysia, 2016; 49p. [Google Scholar]
- Henning, R.K. Using the Indigenous Knowledge of Jatropha: The Use of Jatropha curcas Oil as Raw Material and Fuel. In IK Notes; World Bank: Washington, DC, USA, 2002; No. 47. [Google Scholar]
- Openshaw, K. A Review of Jatropha curcas: An Oil Plant of Unfulfilled Promise. Biomass Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha curcas L.): A Review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Ndong, R.; Montrejaud-Vignoles, M.; Girons, O.S.; Gabrielle, B.; Pirot, R.; Domergue, M.; Sablayrolles, C. Life Cycle Assessment of Biofuels from Jatropha curcas in West Africa: A Field Study. Glob. Chang. Biol. Bioenergy 2009, 1, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Agamuthu, P.; Abioye, O.P.; Abdul Aziz, A. Phytoremediation of Soil Contaminated with Used Lubricating Oil Using Jatropha curcas. J. Hazard. Mater. 2010, 179, 891–894. [Google Scholar] [CrossRef]
- Ahmadpour, P.; Nawi, A.M.; Abdu, A.; Abdul-Hamid, H.; Singh, D.K.; Hassan, A.; Majid, N.M.; Jusop, S. Uptake of Heavy Metals by Jatropha curcas L. Planted in Soils Containing Sewage Sludge. Am. J. Appl. Sci. 2010, 7, 1291–1299. [Google Scholar]
- Jamil, S.; Abhilash, P.C.; Singh, N.; Sharma, P.N. Jatropha curcas: A Potential Crop for Phytoremediation of Coal Fly Ash. J. Hazard. Mater. 2009, 172, 269–275. [Google Scholar] [CrossRef]
- Majid, N.M.; Islam, M.M.; Riasmi, Y. Heavy Metal Uptake and Translocation by Jatropha curcas L. in Sawdust Sludge Contaminated Soils. Aust. J. Crop Sci. 2012, 6, 891–898. [Google Scholar]
- Chang, F.-C.; Ko, C.-H.; Tsai, M.-J.; Wang, Y.-N.; Chung, C.-Y. Phytoremediation of Heavy Metal Contaminated Soil by Jatropha curcas. Ecotoxicology 2014, 23, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.; Saikia, J.; Choudhury, H.K. Economic Benefits and Costs of Jatropha Plantation in North-East India. Agric. Econ. Res. Rev. 2011, 24, 99–108. [Google Scholar]
- Soil and Plant Analysis Council. Soil Analysis: Handbook of Reference Methods; CRC Press: Boca Raton, FL, USA, 2000; 247p. [Google Scholar]
- Jimenez, R.R.; Ladha, J.K. Automated Elemental Analysis: A Rapid and Reliable but Expensive Measurement of Total Carbon and Nitrogen in Plant and Soil Samples. Commun. Soil Sci. Plant Anal. 1993, 24, 1897–1924. [Google Scholar] [CrossRef]
- Sapeta, H.; Costa, J.M.; Lourenço, T.; Maroco, J.; van der Linde, P.; Oliveira, M.M. Drought Stress Response in Jatropha curcas: Growth and Physiology. Environ. Exp. Bot. 2013, 85, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Giwa, S.O.; Yahaya, S.; Ibrahim, M.; Giwa, A. Extraction of Oil from Jatropha Seed Kernels: Optimization and Characterization. Int. J. ChemTech Res. 2016, 9, 758–770. [Google Scholar]
- Fauziah, C.I.; Jamilah, I.; Syed Omar, S.R. An Evaluation of Cation Exchange Capacity Methods for Acid Tropical Soils. Pertanika J. Trop. Agric. Sci. 1997, 20, 113–119. [Google Scholar]
- Sanchez, P.A.; Palm, C.A.; Buol, S.W. Fertility Capability Soil Classification: A Tool to Help Assess Soil Quality in the Tropics. Geoderma 2003, 114, 157–185. [Google Scholar] [CrossRef]
- Cation Exchange Capacity and Base Saturation. Available online: https://extension.uga.edu/publications/detail.html?number=C1040&title=Cation%20Exchange%20Capacity%20and%20Base%20Saturation#:~:text=Depending%20on%20soil%20pH%2C%20the,saturation%20is%20equal%20to%20CEC (accessed on 6 April 2020).
- Saini, J.; Grewal, K.S. Vertical Distribution of Different Forms of Potassium and Their Relationship with Different Soil Properties in Some Haryana Soil under Different Crop Rotation. Adv. Plants Agric. Res. 2014, 1, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.K.; Sidhu, A.S. Dynamics of Potassium and Their Bioavailability for Plant Nutrition. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 187–201. [Google Scholar]
- Afari-Sefa, V.; Kwakye, P.K.; Nyamiah, M.; Okae-Anti, D.; Imoro, A.Z. Potassium Availability in Soils—Forms and Spatial Distribution; International Atomic Energy Agency (IAEA): Vienna, Austria, 2004; p. 20. [Google Scholar]
- Sarkar, G.K.; Chattopadhyay, A.P.; Sanyal, S.K. Release Pattern of Non-exchangeable Potassium Reserves in Alfisols, Inceptisols and Entisols of West Bengal, India. Geoderma 2013, 207–208, 8–14. [Google Scholar] [CrossRef]
- Peck, N.H.; MacDonald, G.E.; Vittum, M.T.; Lathwell, D.J. Effects of Concentrated Superphosphate and Potassium Chloride on Residual Available P, K, and Cl in Three Depths of Soil Derived from Calcareous Glacial Till. Agron. J. 1976, 68, 504–506. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Smith, S.J. Distribution of Potassium Forms in Virgin and Cultivated Soils of the U.S.A. Geoderma 1988, 42, 317–329. [Google Scholar] [CrossRef]
- Zheng, S.J. Crop Production on Acidic Soils: Overcoming Aluminium Toxicity and Phosphorus Deficiency. Ann. Bot. 2010, 106, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Housing, Spatial Planning and Environment Directorate-General for Environmental Protection. Circular on Target Values and Intervention Values for Soil Remediation; Netherlands Government Gazette: Dutch, The Netherlands, 2000; pp. 1–8.
- Mathiyazhagan, N.; Natarajan, D. Phytoremediation Efficiency of Edible and Economical Crops on Waste Dumps of Bauxite Mines, Salem District, Tamil Nadu, India. In On a Sustainable Future of the Earth’s Natural Resources; Ramkumar, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 493–508. [Google Scholar]
- Mathiyazhagan, N.; Natarajan, D. Impact of Mine Waste Dumps on Growth and Biomass of Economically Important Crops. J. Environ. Biol. 2012, 33, 1069–1074. [Google Scholar] [PubMed]
- Rahim, F.A.A.; Hamid, T.H.T.A.; Zainuddin, Z. Jatropha curcas as a Potential Plant for Bauxite Phytoremediation. IOP Conf. Ser. Earth Environ. Sci. 2019, 308, 012006. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, D.; Aarssen, L.W. The Leaf Size/Number Trade-off in Herbaceous Angiosperms. J. Ecol. 2007, 95, 376–382. [Google Scholar] [CrossRef]
- Dombroskie, S.L.; Aarssen, L.W. The Leaf Size/Number Trade-off within Species and Within Plants for Woody Angiosperms. Plant Ecol. Evol. 2012, 145, 38–45. [Google Scholar] [CrossRef]
- Dombroskie, S.L.; Tracey, A.J.; Aarssen, L.W. Leafing Intensity and the Fruit Size/Number Trade-off in Woody Angiosperms. J. Ecol. 2016, 104, 1759–1767. [Google Scholar] [CrossRef] [Green Version]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Kopittke, P.M.; Moore, K.L.; Lombi, E.; Gianoncelli, A.; Ferguson, B.J.; Blamey, F.P.C.; Menzies, N.W.; Nicholson, T.M.; McKenna, B.A.; Wang, P.; et al. Identification of the Primary Lesion of Toxic Aluminum in Plant Roots. Plant Physiol. 2015, 167, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Lad, R.J.; Samant, J.S. Impact of Bauxite Mining on Soil: A Case Study of Bauxite Mines at Udgiri, Dist-Kolhapur, Maharashtra State, India. Int. Res. J. Environ. Sci. 2015, 4, 77–83. [Google Scholar]
- Pramanik, K. Properties and Use of Jatropha curcas Oil and Diesel Fuel Blends in Compression Ignition Engine. Renew. Energy 2003, 28, 239–248. [Google Scholar] [CrossRef]
- Vaknin, Y.; Yermiyahu, U.; Bar-Tal, A.; Samocha, Y. Global Maximization of Jatropha Oil Production Under Semi-arid Conditions by Balancing Vegetative Growth with Reproductive Capacity. Glob. Chang. Biol. Bioenergy 2017, 10, 382–392. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, S.D.; Gautam, P.K.; Kato, S.; Kojima, T. Effect of Climate and Soil Condition on Oil Content of Jatropha Plants Grown in Arid Areas of India. J. Arid Land Stud. 2011, 21, 51–55. [Google Scholar]
- Lama, A.D.; Klemola, T.; Saloniemi, I.; Niemelä, P.; Vuorisalo, T. Factors Affecting Genetic and Seed Yield Variability of Jatropha curcas (L.) Across the Globe: A Review. Energy Sustain. Dev. 2018, 42, 170–182. [Google Scholar] [CrossRef]
- Laviola, B.G.; Alves, A.A.; Rocha, R.B.; Drumond, M.A. The Importance of Jatropha for Brazil. In Jatropha, Challenges for a New Energy Crop; Carels, N., Sujatha, M., Bahadur, B., Eds.; Springer: New York, NY, USA, 2012; Volume 1, pp. 71–94. [Google Scholar]
- Latzel, V.; Allan, E.; Bortolini Silveira, A.; Colot, V.; Fischer, M.; Bossdorf, O. Epigenetic Diversity Increases the Productivity and Stability of Plant Populations. Nat. Commun. 2013, 4, 2875. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Bybordi, A. Effect of Iron Foliar Fertilization on Growth, Seed and Oil Yield of Sunflower Grown under Different Irrigation Regimes. Middle-East J. Sci. Res. 2011, 9, 621–627. [Google Scholar]


| Topsoil | Subsoil | Unmined Soil from Mount Nelson, Jamaica [3] | |||
|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 0 | Day 90 | ||
| CEC (cmol/kg) | 5.31 ± 0.78 | 5.12 ± 0.89 | 3.85 ± 0.20 | 1.65 ± 0.22 | n/a |
| Total C (%) | 0.43 ± 0.02 | 0.45 ± 0.02 | Traces | Traces | 4.2 |
| Total N (%) | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.001 | Traces | 0.242 |
| Total Available P (µg/g) | 4.38 ± 0.34 | 4.34 ± 0.39 | 4.37 ± 0.42 | 4.56 ± 0.70 | 29 |
| Total Extractable K (µg/g) | 24.15 ± 3.25 | 70.13 ± 11.97 | 2.21 ± 0.15 | 47.84 ± 3.85 | 112 |
| pH | 4.94 ± 0.03 | 4.83 ± 0.05 | 5.81 ± 0.06 | 5.84 ± 0.03 | 5.3 |
| EC (µS/cm) | 38 ± 5.29 | 91 ± 11.15 | 30 ± 1.00 | 80 ± 2.65 | n/a |
| Total Extractable K | CEC | Total C | Total N | Total Available P | pH | EC | |
|---|---|---|---|---|---|---|---|
| Total extractable K | 1.000 | 0.924 ** | 0.850 * | 0.967 ** | −0.495 | −0.850 | 0.355 |
| CEC | 0.924 ** | 1.000 | 0.957 ** | 0.964 ** | −0.402 | −0.964 ** | 0.406 |
| Total C | 0.850 * | 0.957 ** | 1.000 | 0.939 ** | −0.238 | −0.999 ** | 0.638 |
| Total N | 0.967 ** | 0.964 ** | 0.939 ** | 1.000 | −0.368 | −0.942 ** | 0.488 |
| Total available P | −0.495 | −0.402 | −0.238 | −0.368 | 1.000 | 0.236 | 0.065 |
| pH | −0.850 * | −0.964 | −0.999 ** | −0.942 ** | 0.236 | 1.000 | −0.609 |
| EC | 0.355 | 0.406 | 0.638 | 0.488 | 0.065 | −0.609 | 1.000 |
| Heavy Metal | Concentration (mg/kg of Soil) | ||||||
|---|---|---|---|---|---|---|---|
| Topsoil | Subsoil | Bauxite Mine Soil in India [41] | Post-Mining Bauxite Mine Topsoil [3] | Post-Mining Bauxite Mine Subsoil [3] | Dutch Target Value [40] | Dutch Intervention Value [40] | |
| Al | 2613.47 ± 396.83 | 1458.00 ± 287.39 | n/a | n/a | n/a | n/a | n/a |
| As | n.d. | n.d. | n/a | n/a | n/a | 29 | 55 |
| Cd | 0.008 ± 0.004 | 0.023 ± 0.020 | 1060 ± 1.31 | n/a | n/a | 0.8 | 12 |
| Cr | 0.604 ± 0.143 | 0.355 ± 0.125 | 533.7 ± 1.73 | n/a | n/a | 100 | 380 |
| Fe | 87.24 ± 11.02 | 171.13 ± 32.08 | 2222 ± 1.01 | 50 | 46 | n/a | n/a |
| Pb | 101.77 ± 7.87 | 58.37 ± 1.89 | 742.6 ± 0.07 | n/a | n/a | 85 | 530 |
| Zn | 1.200 ± 0.391 | 1.493 ± 0.413 | 827.5 ± 2.64 | 2 | 11 | 140 | 720 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingyuan, L.; Samsuri, A.W.; Shukor, M.Y.; Phang, L.Y. Growth Performance of Jatropha curcas Cultivated on Local Abandoned Bauxite Mine Soil. Sustainability 2020, 12, 8263. https://doi.org/10.3390/su12198263
Mingyuan L, Samsuri AW, Shukor MY, Phang LY. Growth Performance of Jatropha curcas Cultivated on Local Abandoned Bauxite Mine Soil. Sustainability. 2020; 12(19):8263. https://doi.org/10.3390/su12198263
Chicago/Turabian StyleMingyuan, Lim, Abd Wahid Samsuri, Mohd Yunus Shukor, and Lai Yee Phang. 2020. "Growth Performance of Jatropha curcas Cultivated on Local Abandoned Bauxite Mine Soil" Sustainability 12, no. 19: 8263. https://doi.org/10.3390/su12198263
APA StyleMingyuan, L., Samsuri, A. W., Shukor, M. Y., & Phang, L. Y. (2020). Growth Performance of Jatropha curcas Cultivated on Local Abandoned Bauxite Mine Soil. Sustainability, 12(19), 8263. https://doi.org/10.3390/su12198263





