Assessing the Impact of Physical and Anthropogenic Environmental Factors in Determining the Habitat Suitability of Seagrass Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Data Collection
2.2.1. Species Data
2.2.2. Environmental Data
2.3. Data Analysis
3. Results
Species Distribution Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lees, F.; Baillie, M.; Gettinby, G.; Revie, C.W. The Efficacy of Emamectin Benzoate against infestations of Lepeoptheirus salmonis on Farmed Atlantic Salmon (Salmo salar L.) in Scotland, 2002–2006. PLoS ONE 2008, 3, e1549. [Google Scholar] [CrossRef]
- Devoy, R.J.N. Coastal Vulnerability and the implications of Sea-Level Rise for Ireland. J. Coast. Res. 2008, 24, 325–341. [Google Scholar] [CrossRef]
- Spencer, M.; Birchenough, S.N.R.; Mieszkowska, N.; Robinson, L.A.; Simpson, S.D.; Burrows, M.T.; Capasso, E.; Cleall-Harding, P.; Crummy, J.; Duck, C.; et al. Temporal change in UK marine communities: Trends or regime shifts? Mar. Ecol. 2011, 32, 10–24. [Google Scholar] [CrossRef]
- Obst, M.; Vicario, S.; Lundin, K.; Berggren, M.; Karlsson, A.; Haines, R.; Williams, A.; Goble, C.; Mathew, C. Marine long-term biodiversity assessment suggests loss of rare species in the Skagerrak and Kattegat region. Mar. Biodivers. 2018, 48, 2165–2176. [Google Scholar] [CrossRef]
- Hillebrand, H.; Brey, T.; Gutt, J.; Hagen, W.; Metflies, K.; Meyer, B.; Lewandowska, A. Climate change: Warming impacts on marine biodiversity. In Handbook on Marine Environment Protection; Salomon, M., Markus, T., Eds.; Springer: Cham, Switzerland, 2018; pp. 353–373. [Google Scholar]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Holloway, P.; Field, R. Can rock-rubble groynes support similar intertidal ecological communities to natural rocky shores? Land 2020, 9, 131. [Google Scholar] [CrossRef]
- Neumann, B.; Vafeidis, A.T.; Zimmermann, J.; Nicholls, R.J. Future coastal population growth and exposure to sea-level rise and coastal flooding-a global assessment. PLoS ONE 2015, 10, e0131375. [Google Scholar] [CrossRef]
- Worm, B. Marine conservation: How to heal an ocean. Nature 2017, 543, 630–631. [Google Scholar] [CrossRef]
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; De Young, C.; Fonseca, L.E.; Grimsdith, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon; Center for Coastal and Ocean Mapping: Durham, NH, USA, 2010; p. 13. [Google Scholar]
- Lovelock, C.E.; Duarte, C.M. Dimensions of blue carbon and emerging perspectives. Biol. Lett. 2019, 15, 20180781. [Google Scholar] [CrossRef]
- Laffoley, D.; Grimsditch, G. The Management of Natural Coastal Carbon Sinks; IUCN: Gland, Switzerland, 2009. [Google Scholar]
- Herr, D.; Landis, E. Coastal Blue Carbon Ecosystems. Opportunities for Nationally Determined Contributions. Policy Brief; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Macreadie, P.I.; Hughes, A.R.; Kimbro, D.L. Loss of ‘blue carbon’ from coastal salt marshes following habitat disturbance. PLoS ONE 2013, 8, e69244. [Google Scholar] [CrossRef]
- Dale, A.L.; McAllen, R.; Whelan, P. Management considerations for subtidal Zostera marina beds in Ireland. In Irish Wildlife Manuals; NPWS: Sydney, Australia, 2007; Volume 28. [Google Scholar]
- Lilley, R.J.; Unsworth, R.K. Atlantic Cod (Gadus morhua) benefits from the availability of seagrass (Zostera marina) nursery habitat. Glob. Ecol. Conserv. 2014, 2, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Jackson, E.L.; Rees, S.E.; Wilding, C.; Attrill, M.J. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 2015, 29, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem services and their variability across genera and geographical regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walls, A.M.; Kennedy, R.; Edwards, M.D.; Johnson, M.P. Impact of kelp cultivation on the Ecological Status of benthic habitats and Zostera marina seagrass biomass. Mar. Pollut. Bull. 2017, 123, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkes, R.; Bennion, M.; McQuaid, N.; Beer, C.; McCullough-Annett, G.; Colhoun, K.; Inger, R.; Morrison, L. Intertidal seagrass in Ireland: Pressures, WFD status and an assessment of trace element contamination in intertidal habitats using Zostera noltei. Ecol. Indic. 2017, 82, 117–130. [Google Scholar] [CrossRef]
- Ruiz-Frau, A.; Gelcich, S.; Hendriks, I.E.; Duarte, C.M.; Marbà, N. Current state of seagrass ecosystem services: Research and policy integration. Ocean Coast. Manag. 2017, 149, 107–115. [Google Scholar] [CrossRef]
- Röhr, M.E.; Holmer, M.; Baum, J.K.; Björk, M.; Boyer, K.; Chin, D.; Chalifour, L.; Cimon, S.; Cusson, M.; Dahl, M.; et al. Blue Carbon Storage Capacity of Temperate Eelgrass (Zostera marina) Meadows. Glob. Biogeochem. Cycles 2018, 32, 1457–1475. [Google Scholar] [CrossRef] [Green Version]
- Green, A.; Chadwick, M.A.; Jones, P.J.S. Variability of UK seagrass sediment carbon: Implications for blue carbon estimates and marine conservation management. PLoS ONE 2018, 19, e0204431. [Google Scholar] [CrossRef] [Green Version]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction, 1st ed.; Cambridge Univeristy Press: Cambridge, UK, 2010. [Google Scholar]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, E.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Miller, J.A.; Holloway, P. Niche theory and models. In International Encyclopedia of Geography: People, the Earth, Environment and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–10. [Google Scholar]
- Van der Heide, T.; Peeters, E.T.H.M.; Hermus, D.C.R.; Van Katwijk, M.M.; Roelofs, J.G.M.; Smolders, A.J.P. Predicting habitat suitability in temperate seagrass ecosystems. Limnol. Oceanogr. 2009, 54, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Downie, A.L.; von Numers, M.; Boström, C. Influence of model selection on the predicted distribution of the segrass Zostera marina. Estuar. Coast. Shelf Sci. 2013, 121–122, 8–19. [Google Scholar] [CrossRef]
- Thomson, M.; Jackson, E.; Kakkonen, J. Seagrass (Zostera) Beds in Orkney; Scottish Natural Heritage Commissioned Report No. 765; Scottish Natural Heritage Commission: Edinburgh, UK, 2014. [Google Scholar]
- Brown, G.D. Modelling the Potential Distribution of Zostera Marina in Wales; Swansea; Swansea University: Swansea, UK, 2015. [Google Scholar]
- Adams, M.P.; Saunders, M.I.; Maxwell, P.S.; Tuazon, D.; Roelfsema, C.M.; Callaghan, D.P.; Leon, J.; Grinham, A.R.; O’Brien, K.R. Prioritizing localized mana gement actions for seagrass conservation and restoration using a species distribution model. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 639–659. [Google Scholar] [CrossRef]
- Boscutti, F.; Marcorin, I.; Sigura, M.; Bressan, E.; Tamberlich, F.; Vianello, A.; Casolo, V. Distribution modeling of seagrasses in brackish waters of Grado-Marano lagoon (Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2015, 164, 183–193. [Google Scholar] [CrossRef]
- Folmer, E.O.; van Beusekom, J.E.; Dolch, T.; Gräwe, U.; van Katwijk, M.M.; Kolbe, K.; Philippart, C.J. Consensus forecasting of intertidal seagrass habitat in the Wadden Sea. J. Appl. Ecol. 2016, 53, 800–1813. [Google Scholar] [CrossRef]
- Jayathilake, D.R.M.; Costello, M.J. A modelled global distribution of the seagrass biome. Biol. Conserv. 2018, 226, 120–126. [Google Scholar] [CrossRef]
- Traganosa, D.; Reinartz, P. Mapping Mediterranean seagrasses with Sentinel-2 imagery. Mar. Pollut. Bull. 2018, 134, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, J.P.; Pratt, L.; Anderson, K.; Land, P.E.; Shutler, J.D. Spatial assessment of intertidal seagrass meadows using optical imaging systems and a lightweight drone. Estuar. Coast. Shelf Sci. 2018, 200, 169–180. [Google Scholar] [CrossRef]
- Zellmer, A.J.; Claisse, J.T.; Williams, C.M.; Schwab, S.; Pondella, D.J. Predicting optimal sites for ecosystem restoration using stacked-species distribution modeling. Front. Mar. Sci. 2019, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Beca-Carreteroa, P.; Varela, S.; Stengel, D.B. A novel method combining species distribution models, remote sensing, and field surveys for detecting and mapping subtidal seagrass meadows. Aquat. Conserv. 2020, 30, 1098–1110. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. J. Exp. Mar. Biol. Ecol. 2005, 320, 57–63. [Google Scholar]
- Duarte, C.M.; Marbà, N.; Krause-Jensen, D.; Sánchez-Camacho, M. Testing the predictive power of seagrass depth limit models. Estuar. Coast. 2007, 30, 652. [Google Scholar] [CrossRef]
- Nejrup, L.B.; Pedersen, M.F. Effects of salinity and water temperature on the ecological performance of Zostera marina. Aquat. Bot. 2008, 88, 239–246. [Google Scholar] [CrossRef]
- Pagès, J.F.; Pérez, M.; Romero, J. Sensitivity of the seagrass Cymodocea nodosa to hypersaline conditions: A microcosm approach. J. Exp. Mar. Biol. Ecol. 2010, 386, 34–38. [Google Scholar] [CrossRef]
- Beca-Carreteroa, P.; Stanschewski, C.S.; Julia-Miralles, M.; Sanchez-Gallego, A.; Stengel, D.B. Temporal and depth-associated changes in the structure, morphometry and production of near-pristine Zostera marina meadows in western Ireland. Aquat. Bot. 2019, 155, 5–17. [Google Scholar] [CrossRef]
- Borum, J.; Duarte, C.M.; Krause-Jensen, D.; Greve, T.M. European Seagrasses: An Introduction to Monitoring and Management; The M&MS Project; European Commission: Brussels, Belgium, 2004. [Google Scholar]
- Maslo, B.; Lockwood, J.L. Coastal Conservation; Cambridge University Press: Cambridge, UK, 2014; Volume 19. [Google Scholar]
- Mangiacotti, M.; Scali, S.; Sacchi, R.; Bassu, L.; Nulchis, V.; Corti, C. Assessing the spatial scale effect of anthropogenic factors on species distribution. PLoS ONE 2013, 8, e67573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloway, P. Simulating movement-related resource dynamics to improve species distribution models: A case study with oilbirds in Northern South America. Prof. Geogr. 2018, 70, 528–540. [Google Scholar] [CrossRef]
- McGovern, J.V.; Nash, S.; Hartnett, M. Interannual improvement in sea lettuce blooms in an agricultural catchment. Front. Mar. Sci. 2019, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jessopp, M.; McAllen, R.; O’Halloran, J.; Kelly, T. Nutrient and Ecosystem Dynamics in Ireland’s Only Marine Nature Reserve (NEIDIN); Final Report for the STRIVE-Funded Project: 2007-FS-B-4-M5, 66; Environmental Protection Agency: Wexford, Ireland, 2011. [Google Scholar]
- McCarthy, G.D.; Gleeson, E.; Walsh, S. The influence of ocean variations on the climate of Ireland. Weather 2015, 70, 242–245. [Google Scholar] [CrossRef] [Green Version]
- Cronin, K.M.; Devoy, R.J.N.; Gault, J. Modelling Estuarine Morphodynamics on the South Coast of Ireland. J. Coast. Res. 2007, 50, 474–479. [Google Scholar]
- Gallagher, S.; Tiron, R.; Dias, F. A long-term nearshore wave hindcast for Ireland: Atlantic and Irish Sea coasts (1979–2012). Present wave climate and energy resource assessment. Ocean Dyn. 2014, 64, 1163–1180. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Schubert, P.R.; Hukriede, W.; Karez, R.; Reusch, T.B.H. Mapping and modeling eelgrass Zostera marina distribution in the western Baltic Sea. Mar. Ecol. Prog. Ser. 2015, 522, 79–95. [Google Scholar] [CrossRef] [Green Version]
- GEBCO Compilation Group. GEBCO 2020 Grid (doi:10.5285/836f016a-33be-6ddc-e053- 6c86abc0788e); GEBCO Compilation Group: White Marsh, MD, USA, 2020. [Google Scholar]
- Amo, A.; Reffray, G.; Sotillo, M.G.; Aznar, R.; Guihou, K. Atlantic-Iberian Biscay Irish-Ocean Physics Analysis and Forecast Product: IBI_Analysis_Forecast_PHYS_005_001. 6.3; VDOKUMENTS: Mexico City, Mexico, 2020. [Google Scholar]
- Marine Institute. Predominant Habitat Type. 2017. Available online: https://data.gov.ie/dataset/predominant-habitat-type (accessed on 1 August 2019).
- Marine Institute. Collated Seabed Structure. 2017. Available online: https://data.gov.ie/dataset/collated-seabed-substrate (accessed on 1 August 2019).
- National Parks and Wildlife Services. Site Specific Conservation Objectives. 2019. Available online: https://www.npws.ie/maps-and-data/habitat-and-species-data (accessed on 1 August 2019).
- EMODnet-Geology. Seabed Substrate 1:250k. Available online: https://www.emodnet-geology.eu/data-products/seabed-substrates/ (accessed on 1 August 2019).
- Environmental Protection Agency (EPA). WFD Transitional Waterbody Status 2010–2015. Available online: https://data.gov.ie/dataset/wfd-transitional-waterbody-status-2010-2015 (accessed on 1 August 2019).
- Environmental Protection Agency (EPA). WFD Coastal Waterbody Status 2010–2015. Available online: https://data.gov.ie/dataset/wfd-coastal-waterbody-status-2010-2015 (accessed on 1 August 2019).
- Environmental Protection Agency (EPA). WFD Coastal Waterbody Approved Risk. Available online: https://data.gov.ie/dataset/wfd-coastal-waterbody-approved-risk (accessed on 1 August 2019).
- Environmental Protection Agency (EPA). WFD Transitional Waterbody Approved Risk. Available online: https://data.gov.ie/dataset/wfd-transitional-waterbody-approved-risk (accessed on 1 August 2019).
- Marine Institute. Inshore Dredge Fishing Activity. Available online: https://data.gov.ie/dataset/inshore-dredge-fishing-activity (accessed on 1 August 2019).
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- EMODnet Human Activities. Dredging. 2017. Available online: https://www.emodnet-humanactivities.eu/search-results.php?dataname=Dredging (accessed on 1 August 2019).
- EMODnet Human Activities. Dredged Dumping Sites. 2005. Available online: https://www.emodnet-humanactivities.eu/search-results.php?dataname=Dredge+Spoil+Dumping+%28Polygons%29 (accessed on 1 August 2019).
- EMODnet Human Activities. Finfish Production. 2019. Available online: https://www.emodnet-humanactivities.eu/search-results.php?dataname=Finfish+Production (accessed on 1 August 2019).
- Ferris, B.E.; Conway-Cranos, L.L.; Sanderson, B.L.; Hoberecht, L. Bivalve aquaculture and seagrass: A global meta-analysis. Aquaculture 2019, 498, 254–262. [Google Scholar] [CrossRef]
- EMODnet Human Activities. Shellfish Production. 2014. Available online: https://www.emodnet-humanactivities.eu/search-results.php?dataname=Shellfish+Production (accessed on 1 August 2019).
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Greiner, J.T.; McGlathery, K.J.; Gunnell, J.; McKee, B.A. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE 2013, 8, e72469. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.H.; Marbá, N.N.; van Katwijk, M.M.; Pickerell, C.; Henriques, M.; Bernard, G.; Ferreira, M.A.; Garcia, S.; Garmendia, J.M.; Manent, P. Changing paradigms in seagrass restoration. Restor. Ecol. 2012, 20, 427–430. [Google Scholar] [CrossRef]
- Paulo, D.; Cunha, A.H.; Boavida, J.; Serrão, E.A.; Gonçalves, E.J.; Fonseca, M. Open coast seagrass restoration. Can we do it? Large scale seagrass transplants. Front. Mar. Sci. 2019, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Van Katwijk, M.M.; Thorhaug, A.; Marbà, N.; Orth, R.J.; Duarte, C.M.; Kendrick, G.A.; Althuizen, I.H.; Balestri, E.; Bernard, G.; Cambridge, M.L.; et al. Global analysis of seagrass restoration: The importance of large-scale planting. J. Appl. Ecol. 2016, 53, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Burden, A.; Garbutt, A.; Evans, C.D. Effect of restoration on saltmarsh carbon accumulation in Eastern England. Biol. Lett. 2019, 15, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, O.; Lavery, P.S.; Rozaimi, M.; Mateo, M.Á. Influence of water depth on the carbon sequestration capacity of seagrasses. Glob. Biogeochem. Cycles 2014, 28, 950–961. [Google Scholar] [CrossRef]
- Postlethwaite, V.R.; McGowan, A.E.; Kohfeld, K.E.; Robinson, C.L.; Pellatt, M.G. Low blue carbon storage in eelgrass (Zostera marina) meadows on the Pacific Coast of Canada. PLoS ONE 2018, 13, e0198348. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Olbert, A.I.; Dabrowski, T.; Nash, S.; Hartnett, M. Regional modelling of the 21st century climate changes in the Irish Sea. Cont. Shelf Res. 2012, 41, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Masson-Delmotte, V. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Ed.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Wan, A.H.L.; Wilkes, R.J.; Heesch, S.; Bermejo, R.; Johnson, M.P.; Morrison, L. Assessment and characterisation of Ireland’s Green Tides (Ulva Species). PLoS ONE 2017, 12, e0169049. [Google Scholar] [CrossRef]
- Bermejo, R.; Heesch, S.; Mac Mongail, M.; O’Donnell, M.; Daly, E.; Wilkes, R.J.; Morrison, L. Spatial and temporal variability of biomass and composition of green tides in Ireland. Harmful Algae 2019, 81, 94–105. [Google Scholar] [CrossRef]



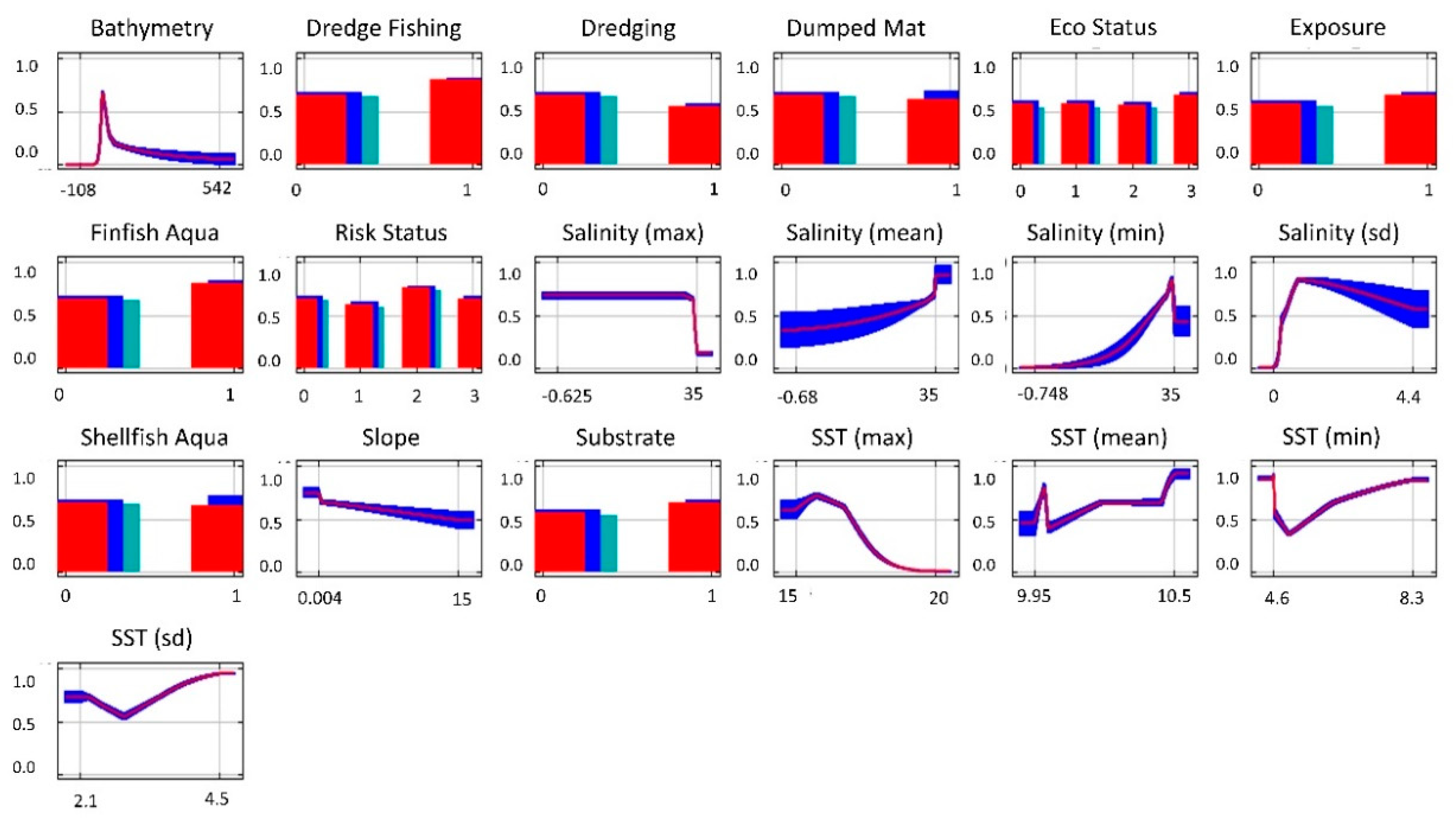
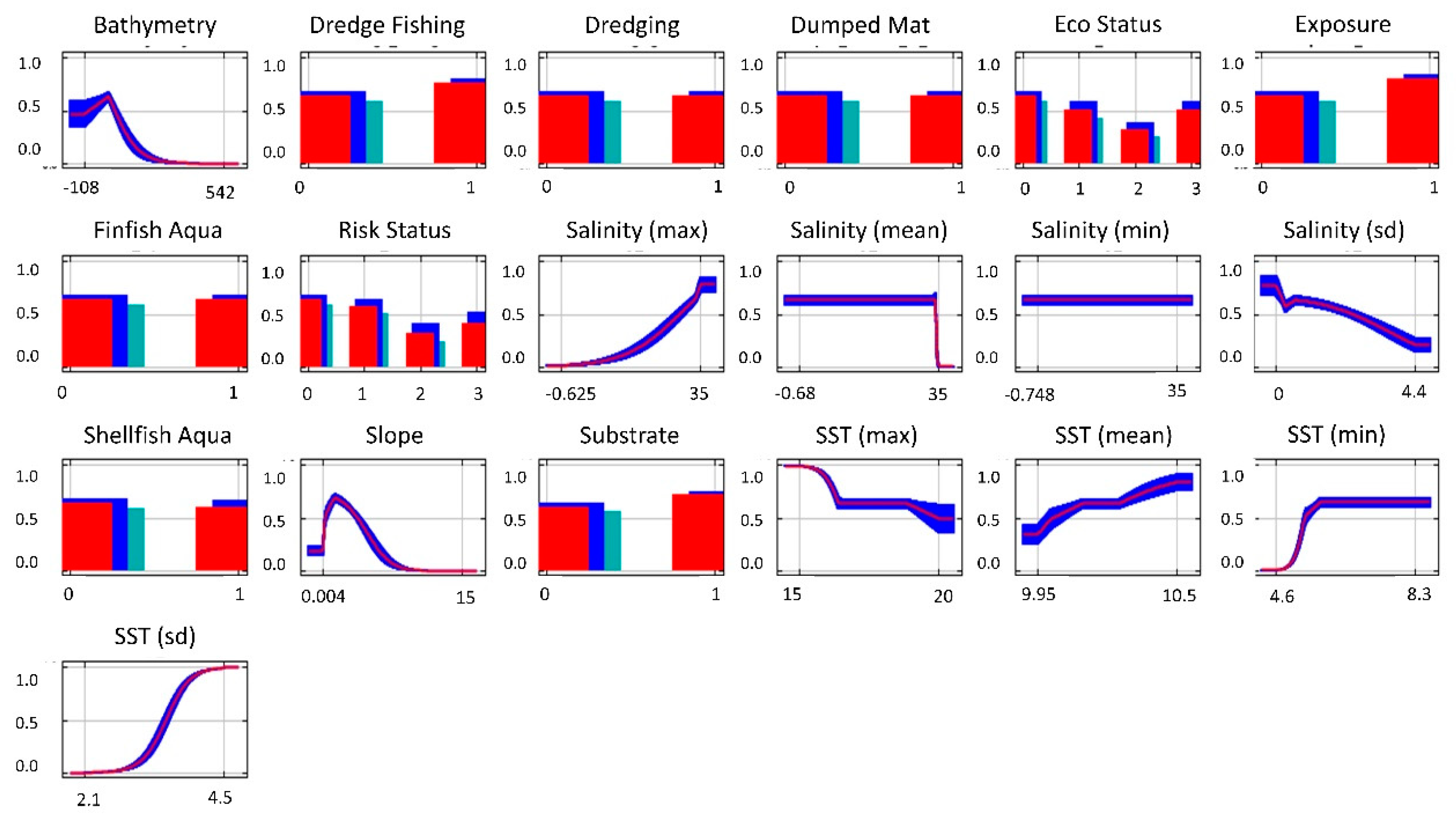
| Layer | Justification | Spatial Data Pre-Processing | Source(s) |
|---|---|---|---|
| Bathymetry (Physical) | Often implemented as a proxy for light availability [57], which impacts photosynthesis | Implemented in downloaded gridded format. Original resolution 15 arc seconds. | [58] |
| SST—Mean, Max, Min, SD (Physical) | SST influences phytoplankton and algae growth, which reduces light availability [35] | Monthly mean data from 2013 to 2018 combined into a single time-series. Summary statistics then generated. Original gridded resolution, 0.028° | [59] |
| Salinity—Mean, Max, Min, SD (physical) | Needed for seagrass, influencing growth and reproduction [46] | Monthly mean data from 2013 to 2018 combined into a single time-series. Summary statistics then generated Original gridded resolution, 0.028° | [59] |
| Slope (physical) | Slope affects both subtidal currents and the degree of beach aspect influencing substrate and wave forces [34,45] | Created from bathymetry layer | [58] |
| Substrate (physical) | Sand and mud are preferred substrates for seagrass attachment [15,45] | Categorical representation of unfavourable (rock), and favourable (mud, sand, estuaries, sandbanks, inlets, bays, and lagoons). | [60,61,62,63] |
| Exposure (physical) | Seagrass requires sheltered locations to prevent disturbance and dislodgement [45] | Sheltered and moderately exposed were recorded as ‘sheltered’ and exposed areas were recorded as ‘exposed’. Converted to raster | [64,65,66,67] |
| Dredge Fishing (anthropogenic) | Negatively affects seagrass meadows through scaring and smothering [20] | Polygons of dredge fishing locations converted into raster | [68] |
| Dredging (anthropogenic) | Negatively affects seagrass meadows through scaring and smothering [69] | Polygons of European dredging activities converted into raster | [70] |
| Dumped Mat (anthropogenic) | Negatively affects seagrass meadows through smothering [69] | Polygons of dumping of dredge spoil material at sea converted into raster | [71] |
| Eco Status (anthropogenic) | Historical condition of water. Eutrophication can negatively affect seagrass distribution [49] | WFD Coastal and Transitional Waterbody Status 2010–2015 for ecological and chemical status of waterbodies. NoData (0), Poor or Bad (1), Moderate (2), Good (3) | [64,65,66,67] |
| Finfish Aqua (anthropogenic) | Negatively affects seagrass through increased nutrients [69] | Point data of European finfish aquaculture locations converted into raster | [72] |
| Risk Status (anthropogenic) | Risk status relating to condition of water. Eutrophication negatively affects seagrass [49] | WFD Coastal and Transitional Waterbody Approved Risk 2016–present. NoData (0), Poor or Bad (1), Moderate (2), Good (3) | [64,65,66,67] |
| Shellfish Aqua (anthropogenic) | Negatively effects seagrass through increased nutrients [73] | Point data of European shellfish aquaculture locations converted into raster | [74] |
| Zostera marina | Zostera noltei | |||
|---|---|---|---|---|
| Variable | Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance |
| Bathymetry (physical) | 39.2 | 57.1 | 1.6 | 1.1 |
| Dredge Fishing (anthropogenic) | 10.3 | 0.6 | 2.2 | 0.7 |
| Dredging (anthropogenic) | 0.0 | 0.0 | 0.0 | 0.0 |
| Dumped Mat (anthropogenic) | 0.0 | 0.0 | 0.0 | 0.0 |
| Eco Status (anthropogenic) | 10.1 | 0.3 | 6.2 | 3.3 |
| Exposure (physical) | 8.0 | 0.0 | 0.9 | 0.9 |
| Finfish Aqua (anthropogenic) | 0.5 | 0.1 | 0.0 | 0.0 |
| Risk Status (anthropogenic) | 0.7 | 0.2 | 3.1 | 2.8 |
| Salinity (Max) (physical) | 0.4 | 3.9 | 0.7 | 4.6 |
| Salinity (Mean) (physical) | 0.3 | 1.7 | 6.8 | 15.8 |
| Salinity (Min) (physical) | 4.7 | 8.4 | 1.8 | 0.0 |
| Salinity (SD) (physical) | 2.5 | 7.9 | 0.8 | 0.7 |
| Shellfish Aqua (anthropogenic) | 0.0 | 0.0 | 0.0 | 0.0 |
| Slope (physical) | 1.5 | 0.0 | 7.1 | 2.3 |
| SST (Max) (physical) | 8.1 | 8.1 | 6.4 | 10.5 |
| SST (Mean) (physical) | 4.1 | 2.8 | 2.1 | 2.0 |
| SST (Min) (physical) | 3.6 | 5.6 | 21.2 | 8.9 |
| SST (SD) (physical) | 3.3 | 3.0 | 38.3 | 46.4 |
| Substrate (physical) | 2.6 | 0.2 | 0.9 | 0.1 |
| Species | 25th Percentile | 50th Percentile | 75th Percentile | Beca-Carretero et al. [39] |
|---|---|---|---|---|
| Zostera marina | 25.54 km2 | 94.34 km2 | 345.33 km2 | 255.21 km2 |
| Zostera noltei | 14.45 km2 | 69.67 km2 | 177.52 km2 | 182.65 km2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hastings, R.; Cummins, V.; Holloway, P. Assessing the Impact of Physical and Anthropogenic Environmental Factors in Determining the Habitat Suitability of Seagrass Ecosystems. Sustainability 2020, 12, 8302. https://doi.org/10.3390/su12208302
Hastings R, Cummins V, Holloway P. Assessing the Impact of Physical and Anthropogenic Environmental Factors in Determining the Habitat Suitability of Seagrass Ecosystems. Sustainability. 2020; 12(20):8302. https://doi.org/10.3390/su12208302
Chicago/Turabian StyleHastings, Ryan, Valerie Cummins, and Paul Holloway. 2020. "Assessing the Impact of Physical and Anthropogenic Environmental Factors in Determining the Habitat Suitability of Seagrass Ecosystems" Sustainability 12, no. 20: 8302. https://doi.org/10.3390/su12208302





