Changing Levels of Myokines after Aerobic Training and Resistance Training in Post-Menopausal Obese Females: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Material and Methods
2.1. Participation
2.2. Measurement of Body Composition
2.3. Physical Fitness Test
2.4. Exercise Program
2.5. One-Repetition Maximum Test
2.6. Blood Collection and Myokine Analysis
2.7. Statistical Analysis
3. Results
3.1. Changes in Body Composition after Training
3.2. Changes in Physical Fitness after Training
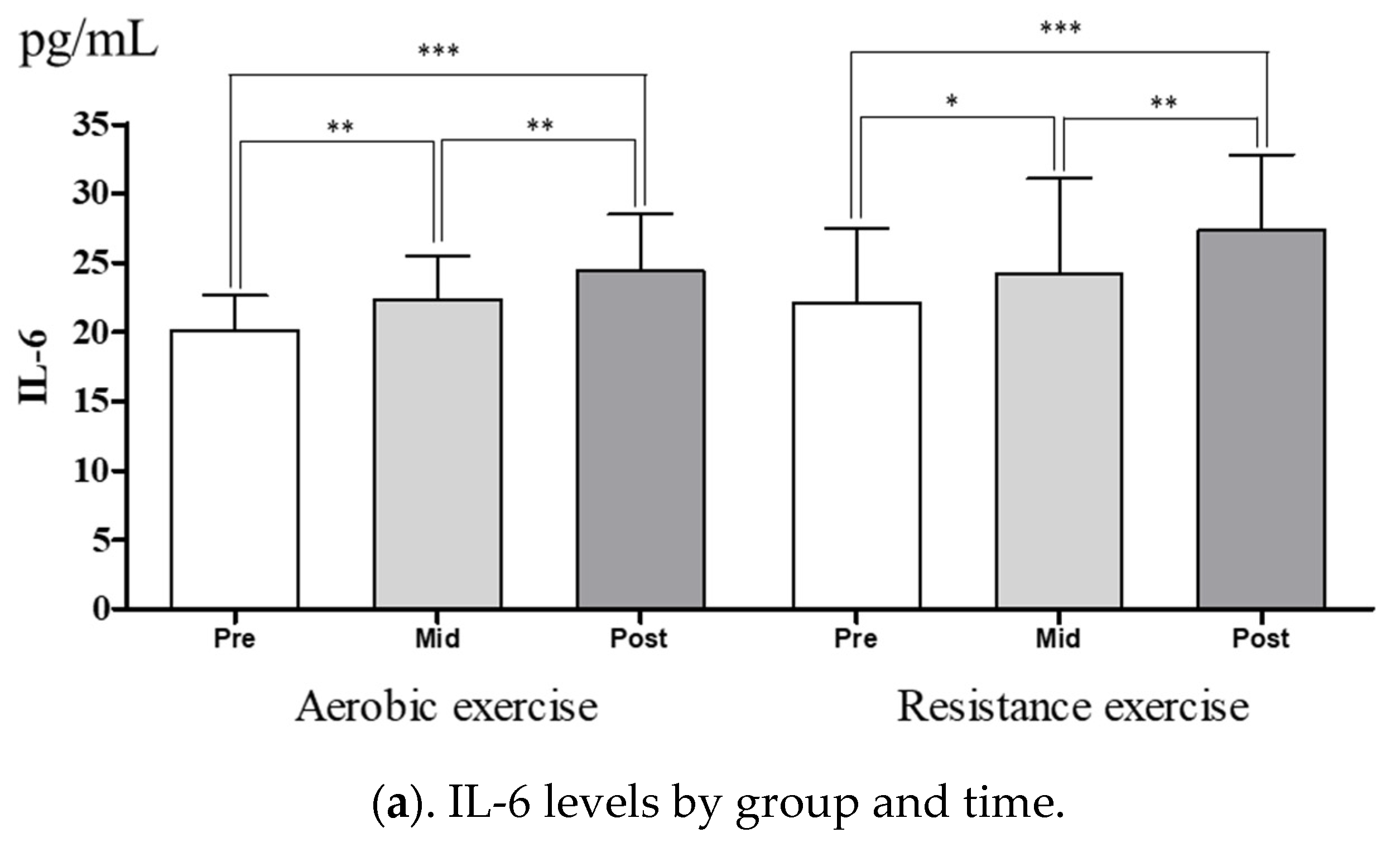
3.3. Change in Myokine Levels after Training
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IL-6 | interleukin-6 |
| IL-15 | interleukin-15 |
| BDNF | brain-derived neurotrophic factor |
| IGF-1 | insulin-like growth factor |
| FGF2 | fibroblast growth factor 2 |
| FGF21 | fibroblast growth factor 21 |
| FSTL1 | follistatin-related protein 1 |
| BMI | body mass index |
| WHR | waist-hip ratio |
| RM | repetition maximum |
| HR | heart rate |
| SPSS | statistical package for social science |
| ANOVA | analysis of variance |
| AMP | adenosine monophosphate |
| TNF-α | tumor-necrosis factor-alpha |
| AMPK | activated protein kinase |
| PMOF | post- menopausal obese females |
References
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Henningsen, J.; Rigbolt, K.T.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol. Cell. Proteom. 2010, 9, 2482–2496. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Pedersen, L.; Hojman, P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 2012, 1, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, B.B.; González, D.A.; Gannar, F.; Pérez, M.C.R.; de León, A.C. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol. Lett. 2018, 203, 1–5. [Google Scholar] [CrossRef]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 3, 51–60. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef]
- Matthews, V.B.; Aström, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Javadivala, Z.; Kousha, A.; Allahverdipour, H.; Jafarabadi, M.A.; Tallebian, H. Modeling the relationship between physical activity and quality of life in menopausal-aged women: A cross-sectional study. J. Res. Health Sci. 2013, 13, 168–175. [Google Scholar]
- Cebula, A.; Tyka, A.K.; Tyka, A.; Pałka, T.; Pilch, W.; Luty, L.; Mucha, D. Physiological response and cardiorespiratory adaptation after a 6-week Nordic Walking training targeted at lipid oxidation in a group of post-menopausal women. PLoS ONE 2020, 15, e0230917. [Google Scholar] [CrossRef] [Green Version]
- Aubertin-Leheudre, M.; Lord, C.; Goulet, E.D.; Khalil, A.; Dionne, I.J. Effect of sarcopenia on cardiovascular disease risk factors in obese postmenopausal women. Obesity 2006, 14, 2277–2283. [Google Scholar] [CrossRef]
- Cauley, J.A.; Gutai, J.P.; Kuller, L.H.; le Donne, D.; Powell, J.G. The epidemiology of serum sex hormones in postmenopausal women. Am. J. Epidemiol. 1989, 129, 1120–1131. [Google Scholar] [CrossRef]
- Helge, J.W.; Stallknecht, B.; Pedersen, B.K.; Galbo, H.; Kiens, B.; Richter, E.A. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J. Physiol. 2003, 546, 299–305. [Google Scholar] [CrossRef]
- Coles, C.A. Adipokines in healthy skeletal muscle and metabolic disease. Adv. Exp. Med. Biol. 2016, 900, 133–160. [Google Scholar]
- Pedersen, B.K.; Fischer, C.P. Beneficial health effects of exercise-the role of IL-6 as a myokine. Trends Pharmacol. Sci. 2007, 28, 152–156. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G. Interleukin-15, IL-15 receptor-α, and obesity: Concordance of laboratory animal and human genetic studies. J. Obes. 2011, 2011, 456347. [Google Scholar] [CrossRef] [Green Version]
- Dozio, E.; Malavazos, A.E.; Vianello, E.; Briganti, S.; Dogliotti, G.; Bandera, F. Interleukin-15 and soluble interleukin-15 receptor α in coronary artery disease patients: Association with epicardial fat and indices of adipose tissue distribution. PLoS ONE 2014, 9, e90960. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Hanatani, A.; Izumi, Y.; Kitada, R.; Iwata, S.; Yoshiyama, M. Serum brain-derived neurotrophic factor level and exercise tolerance complement each other in predicting the prognosis of patients with heart failure. Heart Vessels 2018, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Urzi, F.; Marusic, U.; Ličen, S.; Buzan, E. Effects of elastic resistance training on functional performance and myokines in older women-a randomized controlled trial. J. Am. Med. Dir. Assoc. 2019, 20, 830–834. [Google Scholar] [CrossRef]
- Eaton, M.; Granata, C.; Barry, J.; Safdar, A.; Bishop, D.; Little, J.P. Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J. Sport Health Sci. 2018, 7, 191–196. [Google Scholar] [CrossRef]
- Banitalebi, E.; Kazemi, A.; Faramarzi, M.; Nasiri, S.; Haghighi, M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019, 217, 101–109. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.Y.; Moon, S.; Park, D.H.; Kwak, H.B.; Kang, J.H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflug. Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Kuroda, T.; Saito, M.; Shiraki, M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos Int. 2013, 24, 69–76. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Peake, J.M.; Gatta, P.D.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab. Res. Rev. 2015, 31, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesch, P.A. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med. Sci. Sports Exerc. 1988, 20, 132–134. [Google Scholar] [CrossRef]
- Holloszy, J.P.; Booth, F.W. Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 1976, 38, 273–291. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Patton, J.F.; Gordon, S.E.; Harman, E.A.; Deschenes, M.R.; Reynolds, K.; Newton, R.U.; Triplett, N.T.; Dziados, J.E. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J. Appl. Physiol. 1995, 78, 976–989. [Google Scholar] [CrossRef]
- Mora-Gonzalez, J.; Esteban-Cornejo, I.; Cadenas-Sanchez, C.; Migueles, J.H.; Rodriguez-Ayllon, M.; Molina-García, P.; Hillman, C.H.; Catena, A.; Pontifex, M.B.; Ortega, F.B.; et al. Fitness, physical activity, working memory, and neuroelectric activity in children with overweight/obesity. Scand. J. Med. Sci. Sports 2018, 29, 1352–1363. [Google Scholar] [CrossRef]
- Francois, M.E.; Durrer, C.; Pistawka, K.J.; Halperin, F.A.; Little, J.P. Resistance-based interval exercise acutely improves endothelial function in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, 1258–1267. [Google Scholar] [CrossRef]
- Talebi-Garakani, E.; Safarzade, A. Resistance training decreases serum inflammatory markers in diabetic rats. Endocrine 2013, 43, 564–570. [Google Scholar] [CrossRef]

| Variable | Resistance Exercise (n = 20) | Aerobic Exercise (n = 21) | p-Value |
|---|---|---|---|
| Age (years) | 52.50 ± 7.65 | 56.67 ± 5.43 | 0.051 |
| Height (cm) | 157.89 ± 4.30 | 155.67 ± 5.96 | 0.180 |
| Weight (kg) | 62.82 ± 10.09 | 62.06 ± 9.19 | 0.801 |
| BMI (kg/m2) | 25.16 ± 3.67 | 25.67 ± 3.67 | 0.655 |
| % fat (%) | 36.02 ± 5.87 | 36.53 ± 5.89 | 0.781 |
| Muscle mass (kg) | 21.60 ± 3.00 | 21.26 ± 2.98 | 0.719 |
| SBP (mmHg) | 129.60 ± 18.29 | 129.24 ± 16.38 | 0.947 |
| DBP (mmHg) | 81.00 ± 8.69 | 82.00 ± 9.95 | 0.734 |
| Exercise | Type | Time (min) | Intensity |
|---|---|---|---|
| Resistance Exercise | Warm-up | 10 | 3 times per week, 12 weeks 3 sets 55~65% RM |
| 1. Squat | 40 | ||
| 2. Chest press | |||
| 3. Lat pull-down | |||
| 4. Lunge | |||
| 5. Vertical fly | |||
| 6. Long pull | |||
| 7. Crunch | |||
| Cool-down | 10 | ||
| Aerobic Exercise | Warm-up | 10 | 3 times per week, 12 weeks 50~60% HRR |
| Treadmill running | 30 | ||
| Cool-down | 10 |
| Variable | Time | Type of Exercise | p-Value (Interaction) | |
|---|---|---|---|---|
| Resistance Exercise (n = 20) | Aerobic Exercise (n = 21) | |||
| Weight (kg) | Pre | 62.82 ± 10.09 a* | 62.06 ± 9.19 a** | 0.050 |
| Mid | 62.57 ± 9.70 | 60.90 ± 9.00 b** | ||
| Post | 61.58 ± 8.92 c* | 60.69 ± 9.24 | ||
| BMI (kg/m2) | Pre | 25.16 ± 3.67 a* | 25.67 ± 3.67 a** | 0.024 |
| Mid | 25.12 ± 3.68 | 25.15 ± 3.58 b*** | ||
| Post | 24.69 ± 3.29 c* | 25.07 ± 3.70 | ||
| % fat (%) | Pre | 36.02 ± 5.87 a** | 36.53 ± 5.89 a*** | 0.983 |
| Mid | 34.52 ± 6.09 b* | 34.90 ± 5.55 b*** | ||
| Post | 33.88 ± 5.17 | 34.30 ± 5.28 c* | ||
| Muscle mass (kg) | Pre | 21.60 ± 3.00 a* | 21.26 ± 2.98 | 0.295 |
| Mid | 22.10 ± 3.10 | 21.34 ± 3.01 | ||
| Post | 22.02 ± 3.17 | 21.47 ± 3.15 | ||
| WHR | Pre | 0.91 ± 0.05 a* | 0.91 ± 0.05 a** | 0.519 |
| Mid | 0.89 ± 0.05 | 0.89 ± 0.04 | ||
| Post | 0.89 ± 0.04 c* | 0.89 ± 0.04 | ||
| SBP (mmHg) | Pre | 129.6 ± 18.3 a* | 129.2 ± 16.4 | 0.987 |
| Mid | 130.8 ± 16.1 | 130.3 ± 15.9 | ||
| Post | 122.6 ± 19.3 c** | 122.7 ± 11.7 c* | ||
| DBP (mmHg) | Pre | 81.00 ± 8.69 | 82.00 ± 9.95 | 0.636 |
| Mid | 84.40 ± 10.80 | 85.90 ± 9.43 | ||
| Post | 80.00 ± 11.01 c** | 79.14 ± 10.22 c* | ||
| Variable | Time | Type of Exercise | p-Value (Interaction) | |
|---|---|---|---|---|
| Resistance Exercise (n = 20) | Aerobic Exercise (n = 21) | |||
| Muscle strength (kg) | Pre | 21.18 ± 5.10 | 19.72 ± 6.53 a* | 0.389 |
| Mid | 22.60 ± 5.30 | 22.67 ± 5.17 b* | ||
| Post | 21.64 ± 4.63 | 21.81 ± 4.51 | ||
| Flexibility (cm) | Pre | 14.72 ± 8.02 | 17.68 ± 6.17 a* | 0.293 |
| Mid | 18.20 ± 6.01 b* | 19.78 ± 6.41 b** | ||
| Post | 17.24 ± 6.71 | 19.97 ± 6.82 | ||
| Muscle endurance (rep/30 s) | Pre | 14.05 ± 11.88 a*** | 10.48 ± 8.35 a*** | 0.489 |
| Mid | 19.35 ± 11.86 b** | 13.52 ± 9.51 b** | ||
| Post | 20.80 ± 13.04 | 16.26 ± 9.10 c* | ||
| Power (cm) | Pre | 121.85 ± 24.27 a** | 115.26 ± 22.93 | 0.309 |
| Mid | 129.75 ± 22.64 b** | 121.52 ± 18.39 | ||
| Post | 131.95 ± 24.47 | 115.42 ± 32.31 | ||
| Agility (rep/20 s) | Pre | 28.50 ± 3.68 a*** | 28.04 ± 5.13 a*** | 0.710 |
| Mid | 32.45 ± 4.11 b*** | 31.83 ± 3.10 b*** | ||
| Post | 34.10 ± 4.32 c** | 32.96 ± 3.84 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Park, I.B.; Lim, S.-T. Changing Levels of Myokines after Aerobic Training and Resistance Training in Post-Menopausal Obese Females: A Randomized Controlled Trial. Sustainability 2020, 12, 8413. https://doi.org/10.3390/su12208413
Kang S, Park IB, Lim S-T. Changing Levels of Myokines after Aerobic Training and Resistance Training in Post-Menopausal Obese Females: A Randomized Controlled Trial. Sustainability. 2020; 12(20):8413. https://doi.org/10.3390/su12208413
Chicago/Turabian StyleKang, Sunghwun, Il Bong Park, and Seung-Taek Lim. 2020. "Changing Levels of Myokines after Aerobic Training and Resistance Training in Post-Menopausal Obese Females: A Randomized Controlled Trial" Sustainability 12, no. 20: 8413. https://doi.org/10.3390/su12208413





