Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes
Abstract
:1. Introduction
2. Materials and Methods
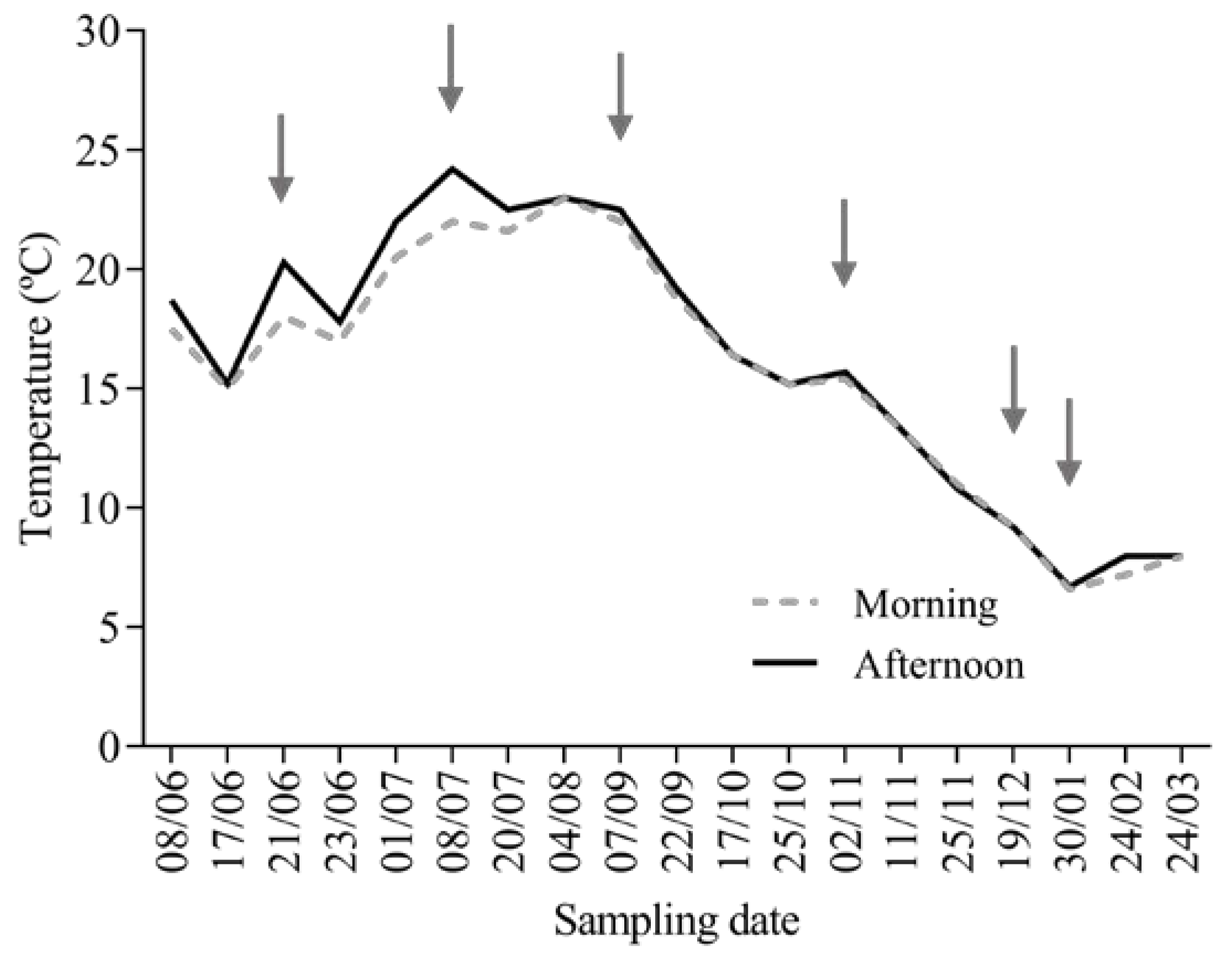
2.1. Fish Rearing Conditions and Sampling
2.2. Hematological Procedures
2.3. Innate Humoral Parameters
2.4. Sample Preparation for Oxidative Stress and Cellular Energy Allocation Analysis
2.4.1. Oxidative Stress-Related Biomarkers
2.4.2. Cellular Energy Allocation
2.5. Statistical Analysis
3. Results
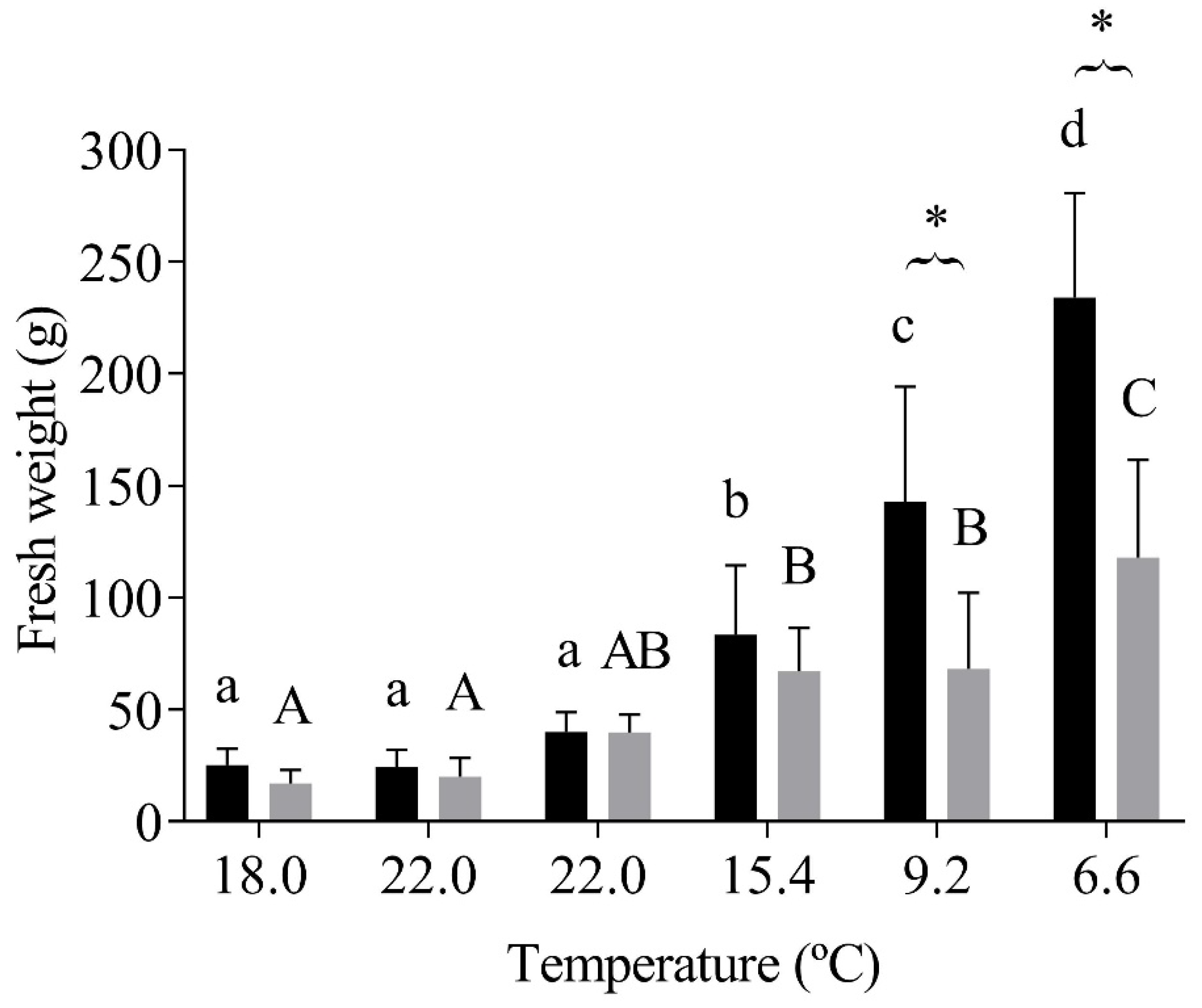
3.1. O. mykiss Weight Gain
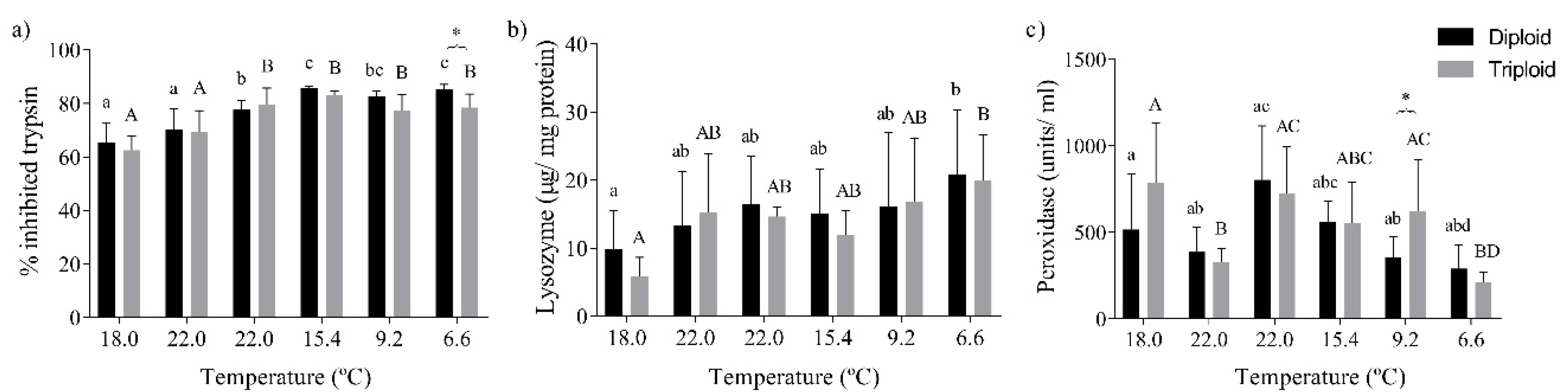
3.2. Hematology and Immunological Parameters
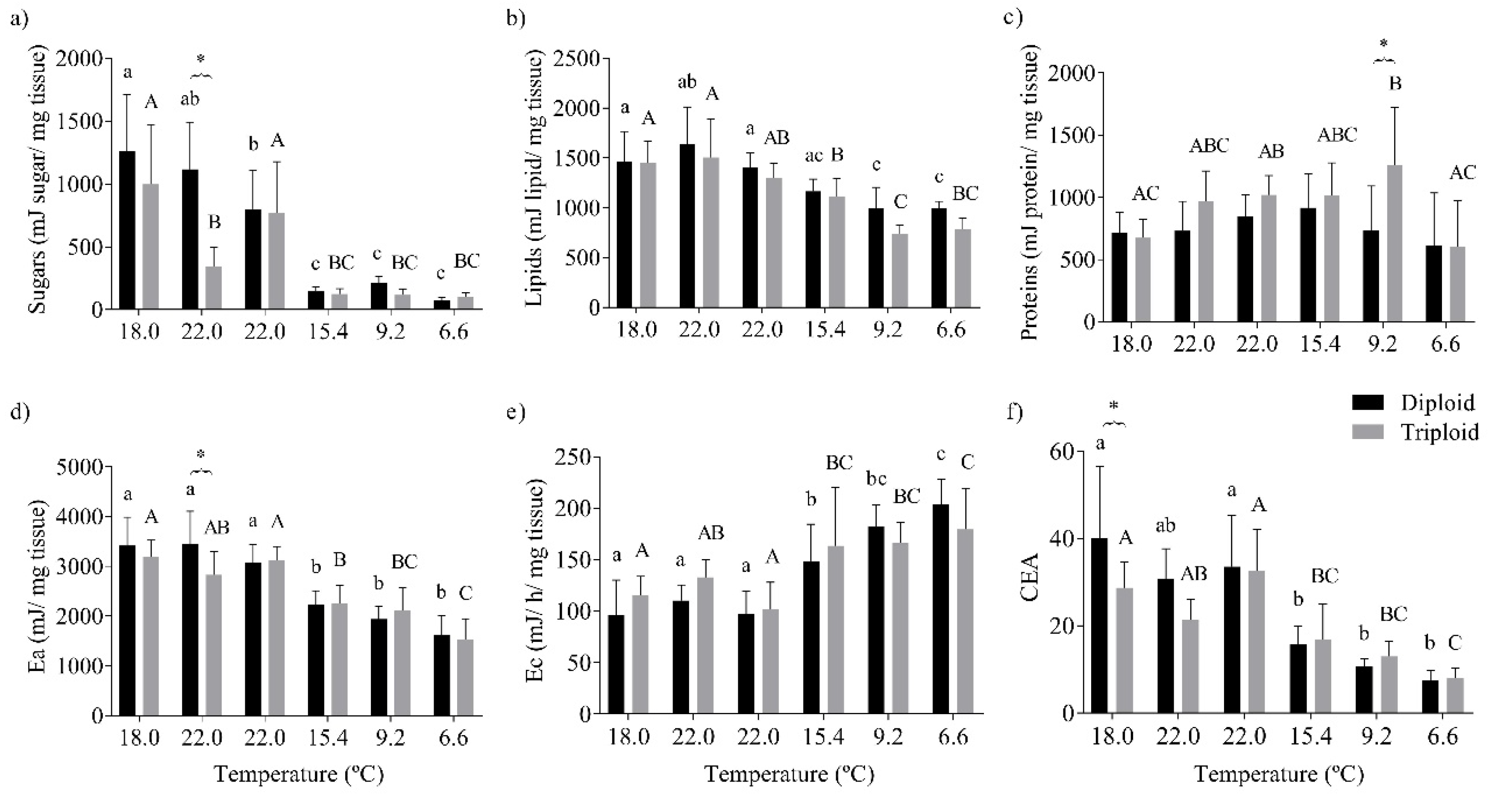
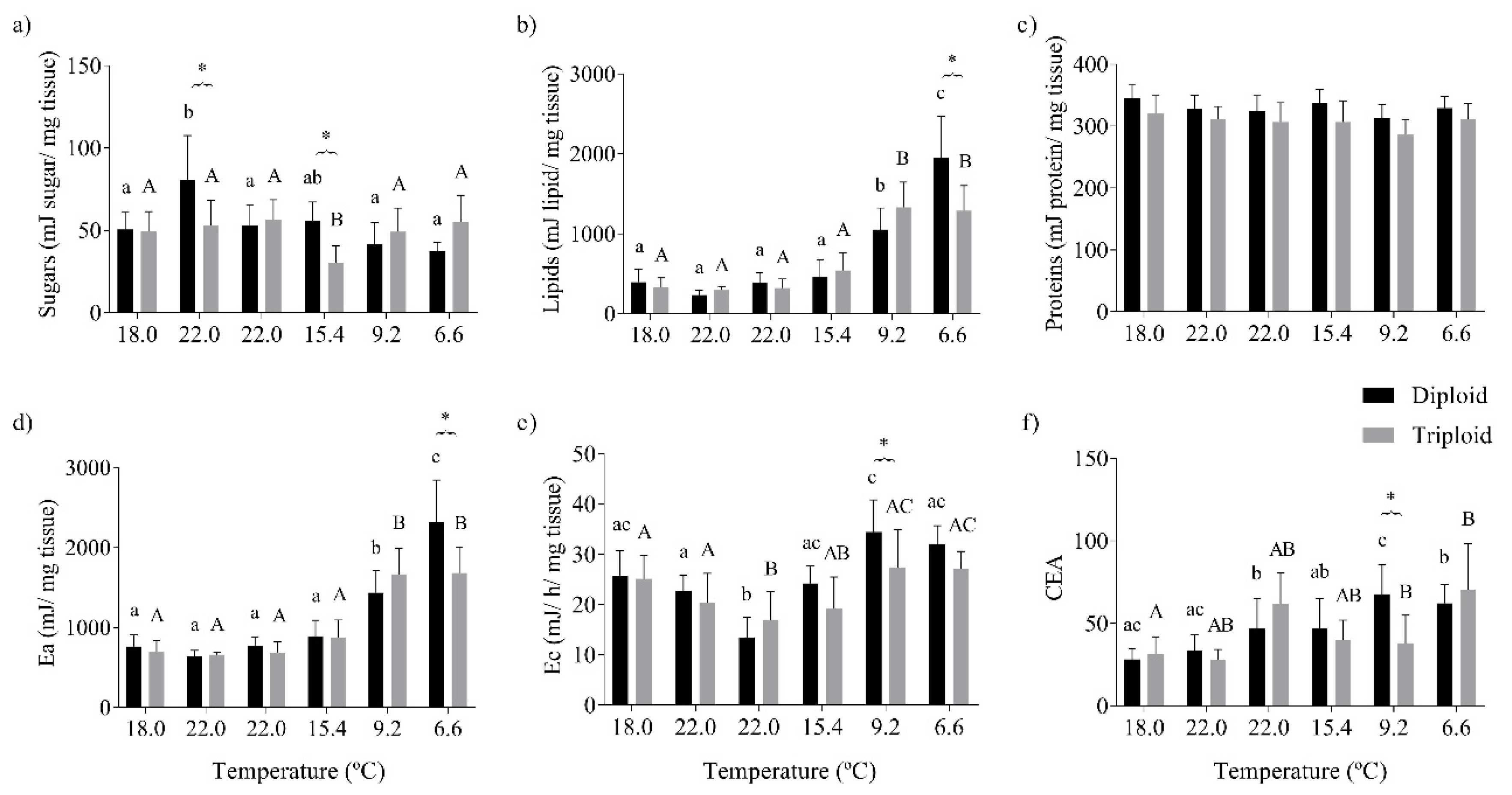
3.3. Oxidative Stress Status and the Energy Budget of O. mykiss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO Fisheries and Aquaculture Department. The State of World Fisheries and Aquaculture-Meeting the Sustainable Development Goals; FAO Fisheries and Aquaculture Department: Rome, Italy, 2018. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2018; ISBN 9789250069753. [Google Scholar]
- Chen, Z.; Snow, M.; Lawrence, C.S.; Church, A.R.; Narum, S.R.; Devlin, R.H.; Farrell, A.P. Selection for upper thermal tolerance in rainbow trout (Oncorhynchus mykiss Walbaum). J. Exp. Biol. 2015, 218, 803–812. [Google Scholar] [CrossRef] [Green Version]
- FAO. Impacts of climate change on fisheries and aquaculture. Synthesis of current knowledge, adaptation and mitigation options. In FAO Fisheries and Aquaculture Technical Paper 627; Barange, M., Bahri, T., Beveridge, M.C.M., Cochrane, K.L., Funge-Smith, S., Poulain, F., Eds.; FAO: Rome, Italy, 2018; pp. 76–82. ISBN 9789604742332. [Google Scholar]
- Lincoln, R.F.; Scott, A.P. Production of all-female triploid rainbow trout. Aquaculture 1983, 30, 375–380. [Google Scholar] [CrossRef]
- Ojolick, E.J.; Cusack, R.; Benfey, T.J.; Kerr, S.R. Survival and growth of all-female diploid and triploid rainbow trout (Oncorhynchus mykiss) reared at chronic high temperature. Aquaculture 1995, 131, 177–187. [Google Scholar] [CrossRef]
- Maxime, V. The physiology of triploid fish: Current knowledge and comparisons with diploid fish. Fish Fish. 2008, 9, 67–78. [Google Scholar] [CrossRef]
- Sambraus, F.; Olsen, R.E.; Remen, M.; Hansen, T.J.; Torgersen, T.; Fjelldal, P.G. Water temperature and oxygen: The effect of triploidy on performance and metabolism in farmed Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture 2017, 473, 1–12. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Fjelldal, P.G.; Hansen, T.; Mayer, I. Welfare considerations of triploid fish. Rev. Fish. Sci. 2012, 20, 192–211. [Google Scholar] [CrossRef]
- Leal, M.J.; Van Eenennaam, J.P.; Schreier, A.D.; Todgham, A.E. Triploidy in white sturgeon (Acipenser transmontanus): Effects of acute stress and warm acclimation on physiological performance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 229, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Verhille, C.; Anttila, K.; Farrell, A.P. A heart to heart on temperature: Impaired temperature tolerance of triploid rainbow trout (Oncorhynchus mykiss) due to early onset of cardiac arrhythmia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 653–657. [Google Scholar] [CrossRef]
- Berrill, I.K.; MacIntyre, C.M.; Noble, C.; Kankainen, M.; Turnbull, J.F. Bio-Economic Costs and Benefits of Using Triploid Rainbow Trout in Aquaculture: Reduced Mortality. Aquac. Econ. Manag. 2012, 16, 365–383. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Lahnsteiner, E.; Kletzl, M. Differences in metabolism of triploid and diploid Salmo trutta f. lacustris under acclimation conditions and after exposure to stress situations. Aquac. Res. 2019, 2444–2459. [Google Scholar] [CrossRef]
- Babaheydari, S.B.; Keyvanshokooh, S.; Dorafshan, S.; Johari, S.A. Modifications in the proteome of rainbow trout (Oncorhynchus mykiss) embryo and fry as an effect of triploidy induction. Fish Physiol. Biochem. 2017, 43, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Papežíková, I.; Mareš, J.; Vojtek, L.; Hyršl, P.; Marková, Z.; Šimková, A.; Bartoňková, J.; Navrátil, S.; Palíková, M. Seasonal changes in immune parameters of rainbow trout (Oncorhynchus mykiss), brook trout (Salvelinus fontinalis) and brook trout × Arctic charr hybrids (Salvelinus fontinalis × Salvelinus alpinus alpinus). Fish Shellfish Immunol. 2016, 57, 400–405. [Google Scholar] [CrossRef]
- Machado, M.; Azeredo, R.; Díaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Kaplow, L.S. Simplified Myeloperoxidase Stain Using Benzidine Dihychloride. J. Haematol. 1965, 26, 215. [Google Scholar]
- Afonso, A.; Lousada, S.; Silva, J.; Ellis, A.E.; Silva, M.T. Neutrophil and macrophage responses to inflammation in the peritoneal cavity of rainbow trout Oncorhynchus mykiss. A light and electron microscopic cytochemical study. Dis. Aquat. Org. 1998, 34, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, A.E. Serum antiproteases in fish. In Techniques in Fish Immunology; SOS: Fair Haven, NJ, USA, 1990. [Google Scholar]
- Costas, B.; Conceição, L.E.C.; Dias, J.; Novoa, B.; Figueras, A.; Afonso, A. Dietary arginine and repeated handling increase disease resistance and modulate innate immune mechanisms of Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol. 2011, 31, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Life history and biochemical effects of chlorantraniliprole on Chironomus riparius. Sci. Total Environ. 2015, 508, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Energetic costs and biochemical biomarkers associated with esfenvalerate exposure in Sericostoma vittatum. Chemosphere 2017, 189, 445–453. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein sutilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In CRC Handbook of Methods in Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.H.; Pabst, M.J.J.; Jacoby, W.B.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and soxidised glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Baker, M.; Cerniglia, G.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Exposure to chlorantraniliprole affects the energy metabolism of the caddisfly Sericostoma vittatum. Environ. Toxicol. Chem. 2017, 36, 1584–1591. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar]
- De Coen, W.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV.Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Verslycke, T.; Ghekiere, A.; Janssen, C.R. Seasonal and spatial patterns in cellular energy allocation in the estuarine mysid Neomysis integer (Crustacea: Mysidacea) of the Scheldt estuary (The Netherlands). J. Exp. Mar. Biol. Ecol. 2004, 306, 245–267. [Google Scholar] [CrossRef]
- Gnaiger, E. Calculation of Energetic and Biochemical Equivalents of Respiratory Oxygen Consumption. In Polarographic Oxygen Sensors. Aquatic and Physiological Applications; Gnaiger, E., Forstner, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 337–345. ISBN 978-3-642-81865-3. [Google Scholar]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Matthews, K.R.; Berg, N.H. Rainbow trout responses to water temperature and dissolved oxygen stress in two southern California stream pools. J. Fish Biol. 1997, 50, 50–67. [Google Scholar] [CrossRef]
- Galbreath, P.F.; Adams, N.D.; Sherrill, L.W.; Martin, T.H. Thermal tolerance of diploid versus triploid rainbow trout and brook trout assessed by time to chronic lethal maximum. Environ. Biol. Fishes 2006, 75, 183–193. [Google Scholar] [CrossRef]
- Johnson, M.A.; Noakes, D.L.G.; Friesen, T.A.; Dittman, A.H.; Couture, R.B.; Schreck, C.B.; Banner, C.; May, D.; Quinn, T.P. Growth, survivorship, and juvenile physiology of triploid steelhead (Oncorhynchus mykiss). Fish. Res. 2019, 220, 105350. [Google Scholar] [CrossRef]
- Bowden, T.J.; Thompson, K.D.; Morgan, A.L.; Gratacap, R.M.L.; Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 2007, 22, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Benfey, T.J.; Biron, M. Acute stress response in triploid rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture 2000, 184, 167–176. [Google Scholar] [CrossRef]
- Morgan, A.L.; Thompson, K.D.; Auchinachie, N.A.; Migaud, H. The effect of seasonality on normal haematological and innate immune parameters of rainbow trout Oncorhynchus mykiss L. Fish Shellfish Immunol. 2008, 25, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Houston, A.H.; Dobric, N.; Kahurananga, R. The nature of hematological response in fish: Studies on rainbow trout Oncorhynchus mykiss exposed to simulated winter, spring and summer conditions. Fish Physiol. Biochem. 1996, 15, 339–347. [Google Scholar] [CrossRef]
- Köllner, B.; Kotterba, G. Temperature dependent activation of leucocyte populations of rainbow trout, Oncorhynchus mykiss, after intraperitoneal immunisation with Aeromonas salmonicida. Fish Shellfish Immunol. 2002, 12, 35–48. [Google Scholar] [CrossRef]
- Weyts, F.A.A.; Flik, G.; Verburg-Van Kemenade, B.M.L. Cortisol inhibits apoptosis in carp neutrophilic granulocytes. Dev. Comp. Immunol. 1998, 22, 563–572. [Google Scholar] [CrossRef]
- Ainsworth, A.J.; Dexiang, C.; Waterstrat, P.R.; Greenway, T. Effect of temperature on the immune system of channel catfish (Ictalurus punctatus)-I. Leucocyte distribution and phagocyte function in the anterior kidney at 10 °C. Comp. Biochem. Physiol. Part A Physiol. 1991, 100, 907–912. [Google Scholar] [CrossRef]
- Chalmers, L.; Thompson, K.D.; Taylor, J.F.; Black, S.; Migaud, H.; North, B.; Adams, A. A comparison of the response of diploid and triploid Atlantic salmon (Salmo salar) siblings to a commercial furunculosis vaccine and subsequent experimental infection with Aeromonas salmonicida. Fish Shellfish Immunol. 2016, 57, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Magnadoóttir, B.; Jónsdóttir, H.; Helgason, S.; Bjornsson, B.; Jorgensen, T.O.; Pilstrom, L. Humoral immune parameters in Atlantic cod (Gadus morhua L.) I. The effects of environmental temperature. Comp. Biochem. Physiol. Part B 1999, 122, 173–180. [Google Scholar] [CrossRef]
- Valero, Y.; García-Alcázar, A.; Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E. Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2014, 39, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, I.; Serra, C.R.; Enes, P.; Couto, A.; Salvador, A.; Costas, B.; Oliva-Teles, A. Effect of short chain fructooligosaccharides (scFOS) on immunological status and gut microbiota of gilthead sea bream (Sparus aurata) reared at two temperatures. Fish Shellfish Immunol. 2016, 49, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langston, A.L.; McGruer, S.; Ellis, A.E.; Johnstone, R. Comparison of some non-specific immune parameters in diploid and triploid atlantic salmon. Dev. Comp. Immunol. 1997, 21, 216. [Google Scholar] [CrossRef]
- Simčič, T.; Jesenšek, D.; Brancelj, A. Effects of increased temperature on metabolic activity and oxidative stress in the first life stages of marble trout (Salmo marmoratus). Fish Physiol. Biochem. 2015, 41, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Leggatt, R.A.; Scheer, K.W.; Afonso, L.O.B.; Iwama, G.K. Triploid and Diploid Rainbow Trout Do Not Differ in Their Stress Response to Transportation. N. Am. J. Aquac. 2006, 68, 1–8. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 36–41. [Google Scholar] [CrossRef]
- Sokolova, I. Mitochondrial Adaptations to Variable Environments and Their Role in Animals’ Stress Tolerance. Integr. Comp. Biol. 2018, 58, 519–531. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Atkins, M.E.; Benfey, T.J. Effect of acclimation temperature on routine metabolic rate in triploid salmonids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 157–161. [Google Scholar] [CrossRef]
- Leal, M.J.; Eenennaam, V.; Schreier, A.D.; Todgham, A.E. Diploid and triploid white sturgeon (Acipenser transmontanus) differ in magnitude but not kinetics of physiological responses to exhaustive exercise at ambient and elevated temperature. Can. J. Fish. Aquat. Sci. 2019. [Google Scholar] [CrossRef]
- Benfey, T.J. The Physiology and Behavior of Triploid Fishes. Rev. Fish. Sci. 1999, 7, 39–67. [Google Scholar] [CrossRef]

| Biomarker | Ploidy | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| 22.0 | 22.0 | 15.4 | 9.2 | 6.6 | ||
| Neutrophils (% WBC) | D | 3.65 ± 2.19 abc | 6.1 ± 6.22 b | 2.1 ± 1.71 c | 3.3 ± 2.72 abc | 4.65 ± 1.63 abc |
| T | 2.85 ± 2.46 | 1.88 ± 0.52 | 1.81 ± 1.13 | 2.95 ± 1.50 | 4.0 ± 1.71 | |
| Monocytes (% WBC) | D | 3.15 ± 2.59 | 0.9 ± 0.57 | 2.2 ± 1.18 | 2.35 ± 2.80 | 2.5 ± 1.29 |
| T | 3.15 ± 2.37 | 1.35 ± 1.13 | 2.6 ± 1.45 | 2.55 ± 1.92 | 3.3 ± 1.95 | |
| Lymphocytes (% WBC) | D | 62.4 ± 8.75 | 61.7 ± 13.12 | 60.9 ± 7.56 | 57.5 ± 7.85 | 59.6 ± 15.6 |
| T | 52.95 ± 11.68 a | 58.8 ± 7.10 a | 49 ± 9.71 a | 49 ± 10.68 a | 72.8 ± 8.94 b | |
| Thrombocytes (% WBC) | D | 30.8 ± 8.11 | 31.3 ± 15.10 | 34.8 ± 6.68 | 36.85 ± 7.70 | 33.25 ± 15.54 |
| T | 41.05 ± 9.58 a | 36.5 ± 7.98 a | 44.9 ± 10.42 a | 45.5 ± 12.42 a | 19.05 ± 8.09 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.C.M.; Gravato, C.; Silva, C.J.M.; Pires, S.F.S.; Costa, A.P.L.; Conceição, L.E.C.; Santos, P.; Costas, B.; Calheiros, J.; Castro-Cunha, M.; et al. Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes. Sustainability 2020, 12, 8785. https://doi.org/10.3390/su12218785
Rodrigues ACM, Gravato C, Silva CJM, Pires SFS, Costa APL, Conceição LEC, Santos P, Costas B, Calheiros J, Castro-Cunha M, et al. Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes. Sustainability. 2020; 12(21):8785. https://doi.org/10.3390/su12218785
Chicago/Turabian StyleRodrigues, Andreia C. M., Carlos Gravato, Carlos J. M. Silva, Sílvia F. S. Pires, Ana P. L. Costa, Luís E. C. Conceição, Paulo Santos, Benjamín Costas, José Calheiros, Manuela Castro-Cunha, and et al. 2020. "Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes" Sustainability 12, no. 21: 8785. https://doi.org/10.3390/su12218785
APA StyleRodrigues, A. C. M., Gravato, C., Silva, C. J. M., Pires, S. F. S., Costa, A. P. L., Conceição, L. E. C., Santos, P., Costas, B., Calheiros, J., Castro-Cunha, M., Soares, A. M. V. M., & Rocha, R. J. M. (2020). Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes. Sustainability, 12(21), 8785. https://doi.org/10.3390/su12218785









