Degradation of Oxytetracycline in Aqueous Solutions: Application of Homogeneous and Heterogeneous Advanced Oxidative Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
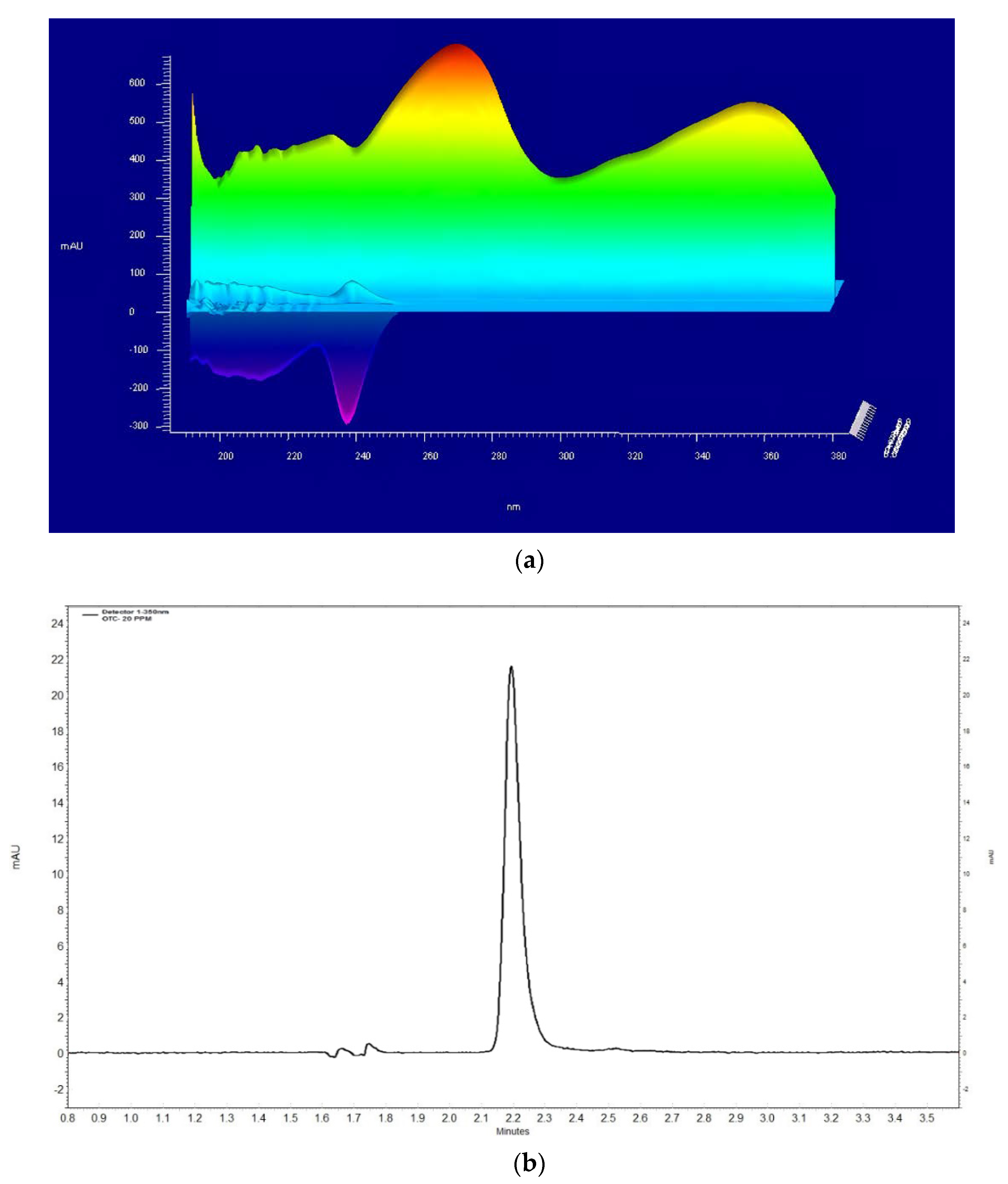
2.2. Sample Determination by HPLC
2.3. Degradation of OTC using Homogeneous Photocatalysis
2.4. Degradation of OTC using Heterogeneous Photocatalysis
2.5. Kinetic Study of OTC Degradation
2.6. Analysis of Operational Costs of the Bench-Top Photolytic Reactor
2.7. Toxicity
3. Results and Discussion
3.1. Determining the OTC and Validating the Method
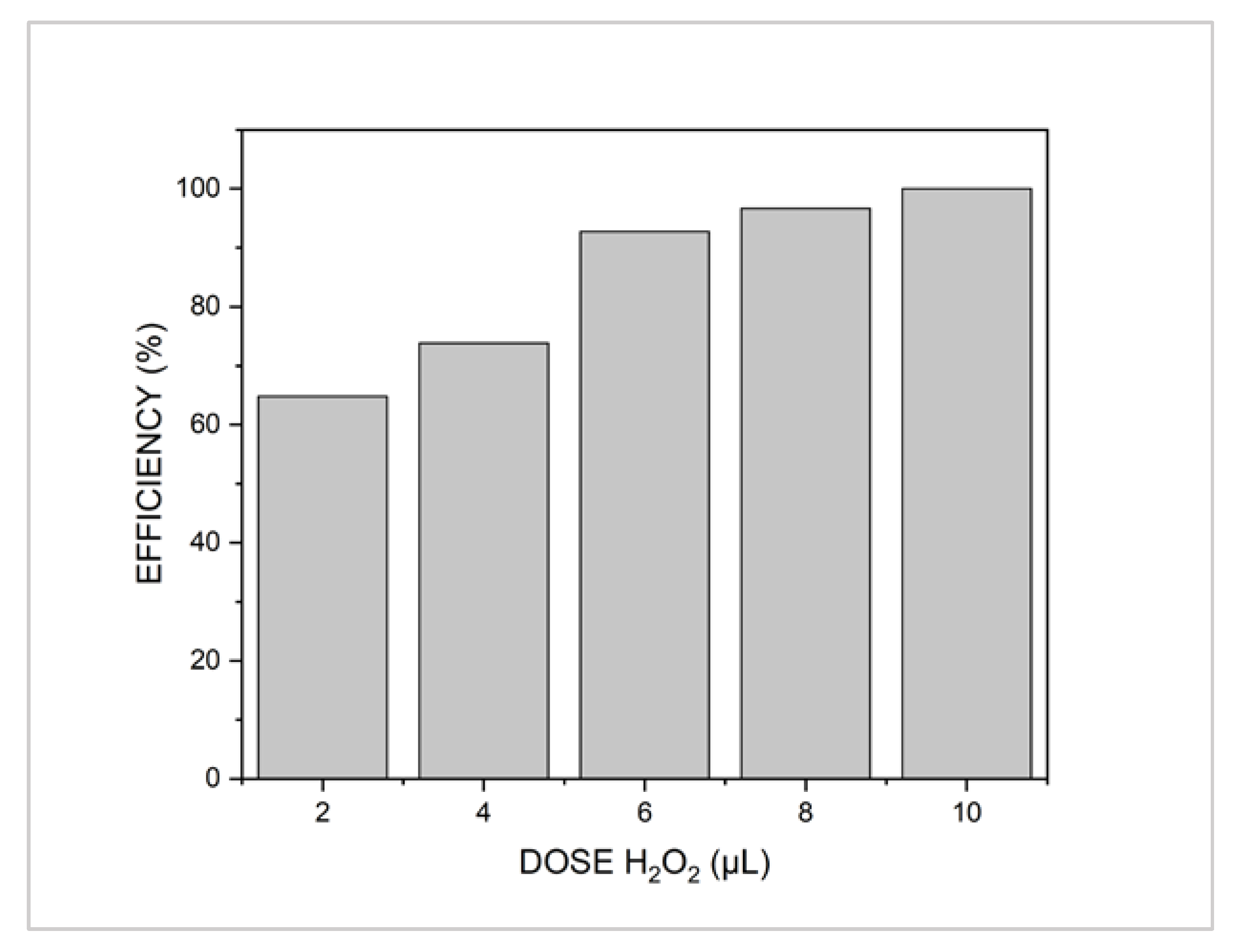
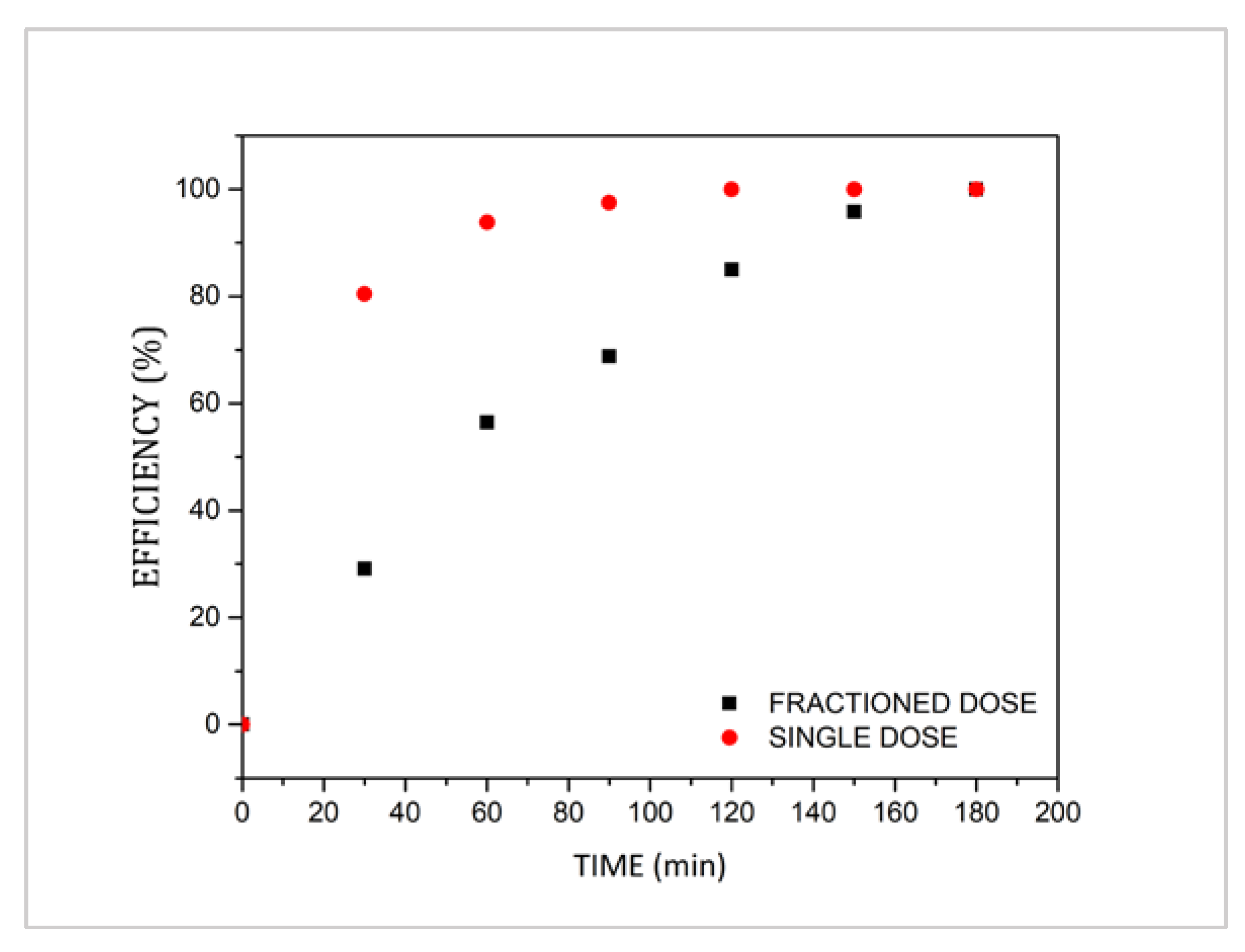
3.2. Preliminary Assessment of the Advanced Oxidation Processes
3.3. The Degradation of OTC
3.4. Kinetic Study
3.5. Bench-top Photolytic Reactor—Associated Costs
3.6. Study of Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total. Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, M.J.; Soto, A.M.; Usma, J.I.; Gutiérrez, O.D. Contaminantes emergentes en aguas, efectos y posibles tratamientos. Prod.+ Limpia 2012, 7, 52–73. [Google Scholar]
- Petrović, M.; Gonzalez, S.; Barceló, D. Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC Trends Anal. Chem. 2003, 22, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Mera, R.; Abalde, J. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J. Hazard. Mater. 2016, 320, 315–325. [Google Scholar] [CrossRef]
- Baguer, A.J.; Jensen, J.; Krogh, P.H. Effects of the antibiotics oxytetracycline and tylosin on soil fauna. Chemosphere 2000, 40, 751–757. [Google Scholar] [CrossRef]
- Barrios, R.L.A.; Sierra, C.A.S.; Morales, J.D.C.J. Bacterias resistentes a antibióticos en ecosistemas acuáticos. Prod.+ Limpia 2015, 10, 160–172. [Google Scholar]
- van der Grinten, E.; Pikkemaat, M.G.; van den Brandhof, E.-J.; Stroomberg, G.J.; Kraak, M.H.S. Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere 2010, 80, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, N.; Reyes, E.; Toro, L. Acumulación/Eliminación de oxitetraciclina en el camarón blanco, Litopenaeus vannamei, y su residualidad en dietas artificiales. El Mundo Acuícola 2002, 8, 34–37. [Google Scholar]
- Santiago, M.L.; Espinosa, A.; del Carmen Bermúdez, M. Uso de antibióticos en la camaronicultura. Rev. Mex. Cienc. Farma. 2009, 40, 22–32. [Google Scholar]
- Pérez Cabeza, S.B. Propuesta de un Modelo para la Degradación Fotocalítica de Oxitetraciclina con Dióxido de Titanio en Soluciones Acuosas. Repositorio Documental Universidad de Valladolid. Master’s Thesis, Universidad de Valladolid, Valladolid, Spain, 2019. [Google Scholar]
- Basile, T.; Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, V.; Colasuonno, S.; Petruzzelli, D. Review of Endocrine-Disrupting-Compound Removal Technologies in Water and Wastewater Treatment Plants: An EU Perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants—Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, M.A.; Mifsut, C.L.; Cavero, E.M.; Alcañiz, L.P.; Muro, J.L.M.; Asensi, J.M.S.; Martínez, M.I.; Hernández, F.J.H. Eliminación de contaminantes emergentes en aguas residuales mediante oxidación avanzada con ozono y ultrasonidos. Tecnoaqua 2013, 4, 22–28. [Google Scholar]
- Pereira, J.H.O.S.; Vilar, V.J.P.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A.R. Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- da Silva Brito, G.F.; Oliveira, R.; Grisolia, C.K.; Guirra, L.S.; Weber, I.T.; de Almeida, F.V. Evaluation of advanced oxidative processes in biodiesel wastewater treatment. J. Photochem. Photobiol. A Chem. 2019, 375, 85–90. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Calderón Peña, J.; Bello Hernández, D.E.; Delgado Niño, P. Remediación fotocatalítica de aguas residuales simuladas contaminadas con tetraciclina. Rev. Av. Investig. Ing. 2019, 16, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, A.R.S.; Andrade de Lucena, A.L.; da Rocha Santana, R.M.; Zaidan, L.E.M.C.; Michelle da Silva, P.; Napoleão, T.H.; Duarte, M.M.M.B.; Napoleão, D.C. Advanced oxidation processes employment for the degradation of lamivudine: Kinetic assessment, toxicity study and mathematical modeling. Water Qual. Res. J. 2020, 55, 249–260. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, D.; Palacios-Antón, M.E.; Santana, R.M.D.R.; Quiroz-Fernández, L.S.; Gómez-Salcedo, Y.; de Lucena, A.L.A.; Napoleão, D.C.; Rodriguez-Diaz, J.M. Comparative Study of the Degradation of the Diclofenac Drug Using Photo-Peroxidation and Heterogeneous Photocatalysis with UV-C and Solar Radiation. Water Air Soil Pollut. 2020, 231, 147. [Google Scholar] [CrossRef]
- Loures, C.C.; Alcântara, M.A.; Izário Filho, H.J.; Teixeira, A.; Silva, F.T.; Paiva, T.C.; Samanamud, G.R. Advanced oxidative degradation processes: Fundamentals and applications. Int. Rev. Chem. Eng. 2013, 5, 102–120. [Google Scholar] [CrossRef]
- Napoleão, D.; Zaidan, L.M.C.; Rodríguez-Díaz, J.; da Rocha Santana, R.; de Mendonça, M.B.D.S.; da Nova Araújo, A.; Benachour, M.; da Silva, V.L. Use of the photo-Fenton process to discover the degradation of drugs present in water from the Wastewater Treatment Plants of the pharmaceutical industry. Afinidad 2018, 75, 581. [Google Scholar]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D.D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Oxidative degradation of the antibiotic oxytetracycline by Cu@Fe3O4 core-shell nanoparticles. Sci. Total. Environ. 2018, 631–632, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Akel, S.; Boughaled, R.; Dillert, R.; El Azzouzi, M.; Bahnemann, D.W. UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles. Molecules 2020, 25, 249. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.-G.; Ko, S.-O. Cu@Fe3O4 core-shell nanoparticle-catalyzed oxidative degradation of the antibiotic oxytetracycline in pre-treated landfill leachate. Chemosphere 2018, 191, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Reis, A.C.; Queirós, D.; Nunes, O.C.; Borges, M.T.; Vilar, V.J.P.; Boaventura, R.A.R. Insights into solar TiO2-assisted photocatalytic oxidation of two antibiotics employed in aquatic animal production, oxolinic acid and oxytetracycline. Sci. Total Environ. 2013, 463–464, 274–283. [Google Scholar] [CrossRef]
- Freire Dávila, M.J.; Gómez López, E.Y. Estudio del Método Fotocatalítico (TiO2) para la Disminución de DQO en Aguas Residuales Domésticas Utilizando Materiales Reciclados. Bachelor’s Thesis, UCE, Quito, Ecuador, 2017. [Google Scholar]
- Chan, K.H.; Chu, W. Modeling the reaction kinetics of Fenton’s process on the removal of atrazine. Chemosphere 2003, 51, 305–311. [Google Scholar] [CrossRef]
- da Rocha Santana, R.M.; Charamba, L.C.V.; do Nascimento, G.E.; de Oliveira, J.G.C.; Sales, D.C.S.; Duarte, M.M.M.B.; Napoleão, D.C. Degradation of Textile Dyes Employing Advanced Oxidative Processes: Kinetic, Equilibrium Modeling, and Toxicity Study of Seeds and Bacteria. Water Air Soil Pollut. 2019, 230, 136. [Google Scholar] [CrossRef]
- Zaidan, L.E.M.C.; Rodriguez-Díaz, J.M.; Napoleão, D.C.; de Silva, M.D.C.B.; da Nova Araújo, A.; Benachour, M.; da Silva, V.L. Heterogeneous photocatalytic degradation of phenol and derivatives by (BiPO4/H2O2/UV and TiO2/H2O2/UV) and the evaluation of plant seed toxicity tests. Korean J. Chem. Eng. 2017, 34, 511–522. [Google Scholar] [CrossRef]
- Napoleão, D. Avaliação e Tratamento de Fármacos Oriundos de Diferentes Estações de Tratamento de Efluentes Empregando Processos Oxidativos Avançado. Ph.D. Thesis, Universidade Federal de Pernambuco, UFPE, Recife, Brazil, 2015. [Google Scholar]
- Napoleão, D.C. Avaliação e Tratamento dos Contaminantes Emergentes (ácido Acetilsalicílico, Diclofenaco e Paracetamol) Utilizando Processos Oxidativos Avançados. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2011. [Google Scholar]
- Lucena, A.L.D. Degradação dos fármacos zidovudina e lamivudina utilizando Fotólise, Foto-Fenton e processo UV/H2O2. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2018. [Google Scholar]
- Oliveira, C.I.S. Estudo da degradação eletroquímica da oxitetraciclina. Ph.D. Thesis, University of Beira Interior, Covilhã, Portugal, 2013. [Google Scholar]
- Leal, J.F. Photo-Degradation of Contaminants as a Way of Remediation of Aquaculture’s Water. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2019. [Google Scholar]
- De Abreu, L.; Jaimes, L. Determinación de residuos de tetraciclinas: Oxitetraciclina, tetraciclina y clortetraciclina en tejido muscular de porcino mediante cromatografía líquida de alta resolución. Rev. Fac. Farm. Vol. 2009, 72, 22. [Google Scholar]
- Horwitz, W.; Albert, R. The Horwitz ratio (HorRat): A useful index of method performance with respect to precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [CrossRef]
- Al-Kdasi, A.; Idris, A.; Saed, K.; Guan, C.T. Treatment of textile wastewater by advanced oxidation processes—A review. Global Nest Int. J. 2004, 6, 222–230. [Google Scholar]
- Castro-Peña, L.; Durán-Herrera, J.E. Degradación y decoloración de agua contaminada con colorantes textiles mediante procesos de oxidación avanzada. Rev. Tecnol. Marcha 2014, 27, ág-40–ág-50. [Google Scholar] [CrossRef]
- Desikan, R.; Hancock, J.; Neill, S. Reactive oxygen species as signalling molecules. Antioxid. React. Oxyg. Species Plants 2005, 169–196. [Google Scholar]
- Giraldo, L.F.G.; Restrepo, A.R. Evaluación de la fotodegradación sensibilizada con TiO2 Y Fe3+ para aguas coloreadas. Rev. Lasallista Investig. 2004, 1, 54–60. [Google Scholar]
- Yuan, F.; Hu, C.; Hu, X.; Wei, D.; Chen, Y.; Qu, J. Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J. Hazard. Mater. 2011, 185, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Paola, A.D.; García-López, E.; Loddo, V.; Marcì, G.; Palmisano, L. Removal of drugs in aqueous systems by photoassisted degradation. J. Appl. Electrochem. 2005, 35, 765–774. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; d’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414–422. [Google Scholar] [CrossRef]
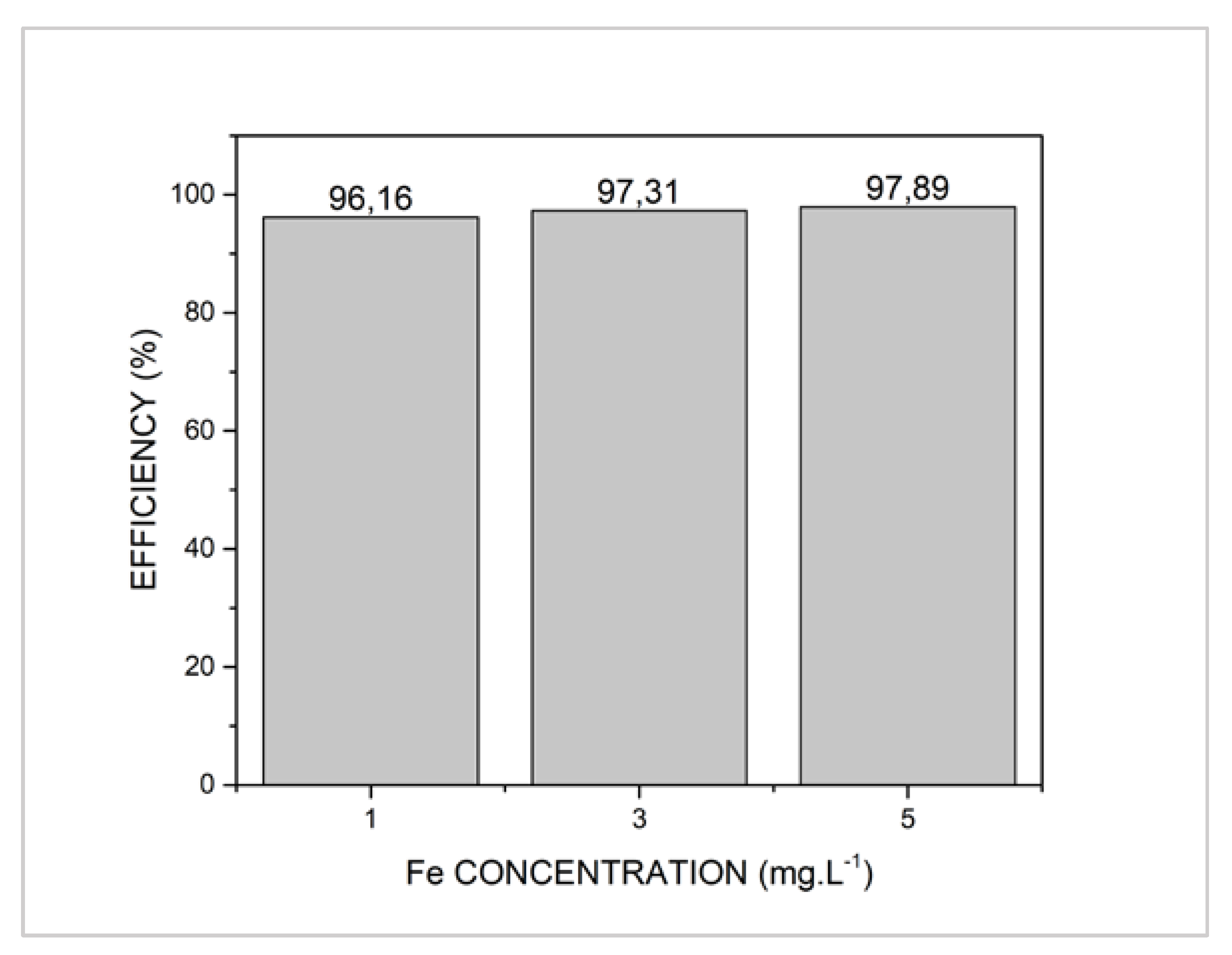


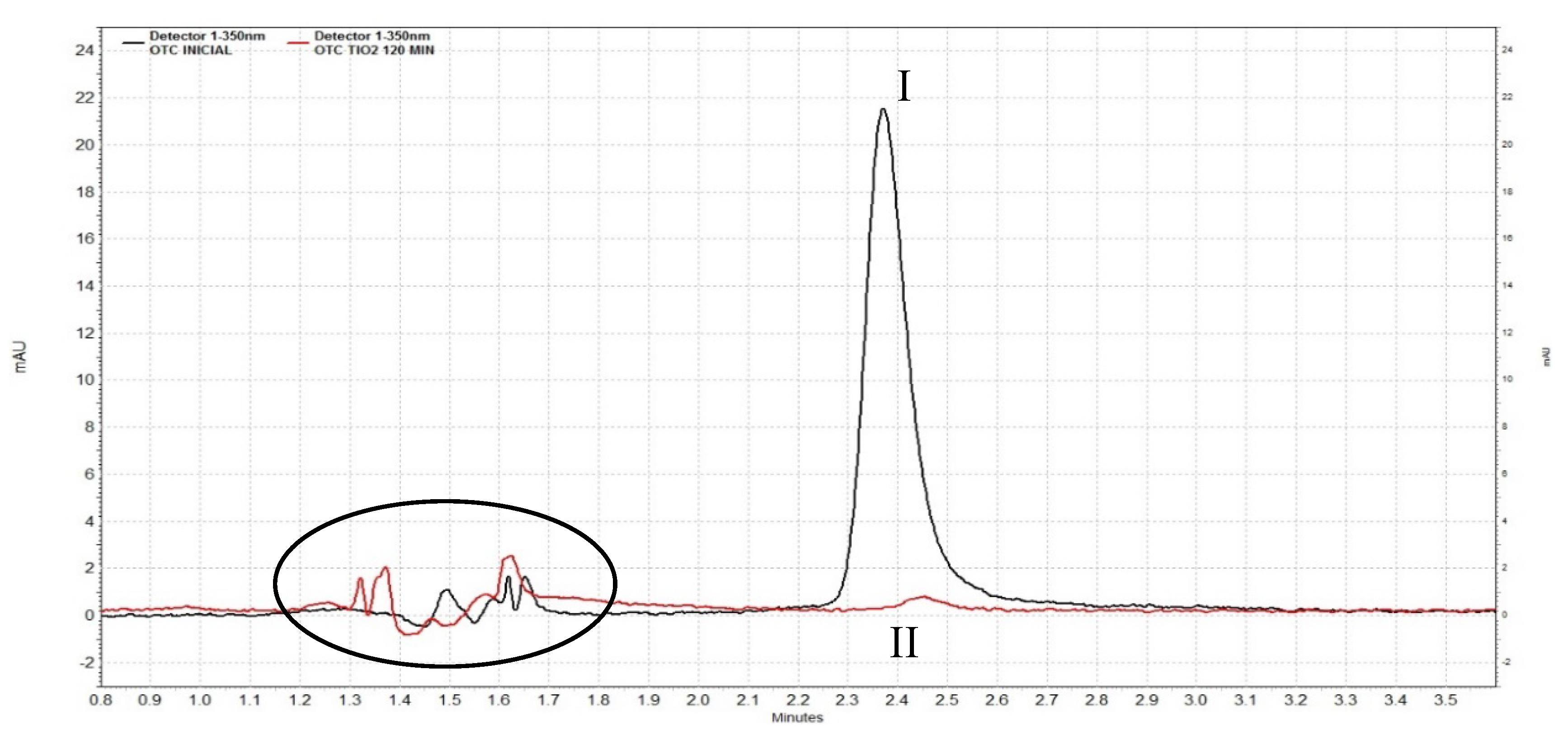
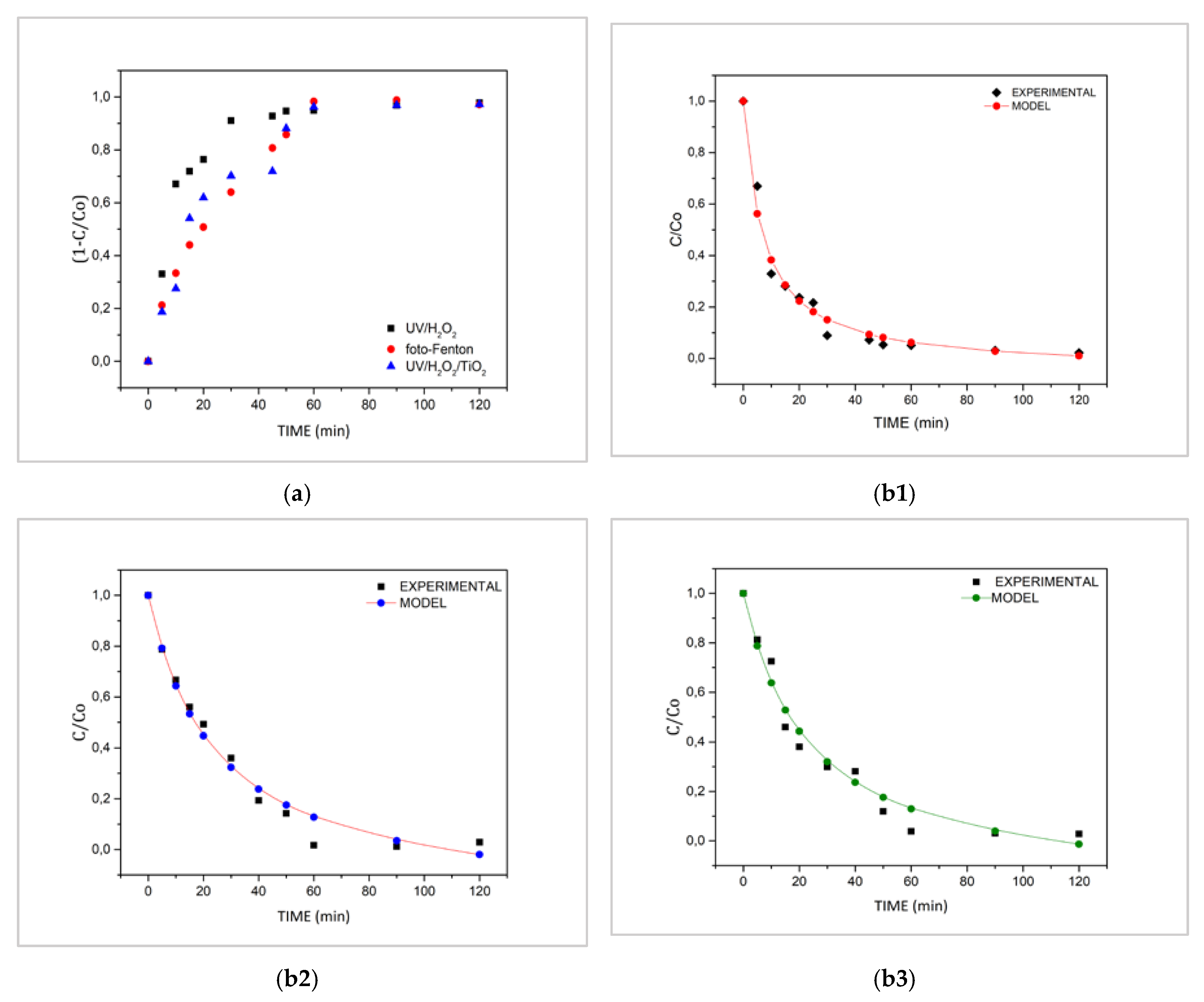

| Treatment | Degradation (%) after 120 min | 1/ρ (min−1) | 1/σ | R2 |
|---|---|---|---|---|
| UV/H2O2 | 97.76 | 0.1504 | 1.0469 | 0.9975 |
| Photo-Fenton | 97.16 | 0.0526 | 1.2277 | 0.9837 |
| Heterogeneous Photocatalysis | 97.21 | 0.0516 | 1.2112 | 0.9757 |
| Material Costs | Unit Price (USD $) | Quantity | Total Price (USD $) |
|---|---|---|---|
| Germicidal Tubular Ultraviolet Lamp (UV-C)—30 W | 15.00 | 3 | 45.00 |
| Wood to build the reactor | 25.00 | 1 | 25.00 |
| Tools | 5.00 | 1 | 5.00 |
| Total material | 75.00 | ||
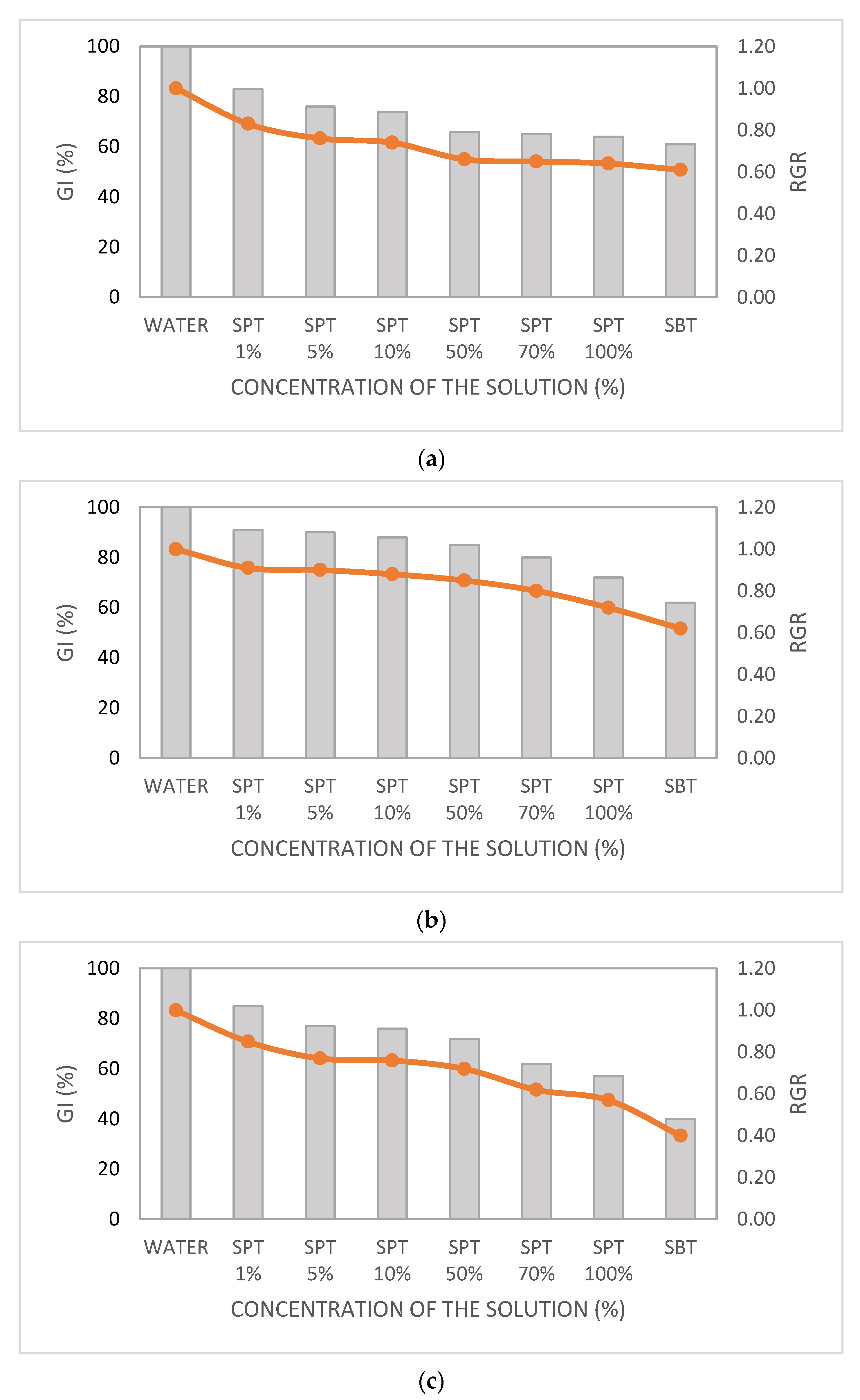
| AOP Treatment | UV/H2O2 | Photo-Fenton | Heterogeneous Photocatalysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Lactuca sativa | Daucus carota | Lactuca sativa | Daucus carota | Lactuca sativa | Daucus carota | ||||||
| Aqueous Solution | * RG ± DP | RGR/GI (%) | * RG ± DP | RGR/GI (%) | * RG ± DP | RGI/GI (%) | * RG ± DP | RGR/GI (%) | * GR ± DP | RGR/GI (%) | * CR ± DP | RGR/GI (%) |
| Water | 4.29 ± 0.33 | 1/100 | 2.44 ± 0.15 | 1/100 | 4.29 ± 0.33 | 1/100 | 2.44 ± 0.15 | 1/100 | 4.29 ± 0.33 | 1/100 | 2.44 ± 0.15 | 1/100 |
| SPT 1% | 3.54 ± 0.27 | 0.83/83 | 2.36 ± 0.20 | 0.97/87 | 3.91 ± 0.26 | 0.91/91 | 1.24 ± 0.18 | 0.55/44 | 3.63 ± 0.27 | 0.85/85 | 2.18 ± 0.34 | 0.89/71 |
| SPT 5% | 3.28 ± 0.38 | 0.76/76 | 2.31 ± 0.20 | 0.95/85 | 3.85 ± 0.57 | 0.9/90 | 1.13 ± 0.21 | 0.46/37 | 3.32 ± 0.38 | 0.77/77 | 1.96 ± 0.25 | 0.8/64 |
| SPT 10% | 3.19 ± 0.58 | 0.74/74 | 2.11 ± 0.20 | 0.87/78 | 3.76 ± 0.33 | 0.88/88 | 1.09 ± 0.19 | 0.44/31 | 3.26 ± 0.58 | 0.76/76 | 1.89 ± 0.29 | 0.77/62 |
| SPT 50% | 2.84 ± 0.46 | 0.66/66 | 2.03 ± 0.41 | 0.83/67 | 3.63 ± 0.22 | 0.85/85 | 1.03 ± 0.35 | 0.42/30 | 3.07 ± 0.46 | 0.72/72 | 1.79 ± 0.36 | 0.73/59 |
| SPT 70% | 2.79 ± 0.31 | 0.65/65 | 1.5 ± 0.30 | 0.61/49 | 3.45 ± 0.19 | 0.8/80 | 0.91 ± 0.16 | 0.41/27 | 2.66 ± 0.31 | 0.62/62 | 1.61 ± 0.47 | 0.66/46 |
| SPT 100% | 2.75 ± 0.22 | 0.64/64 | 1.34 ± 0.18 | 0.55/44 | 3.07 ± 0.41 | 0.72/72 | 0.83 ± 0.23 | 0.37/26 | 2.43 ± 0.22 | 0.57/57 | 1.34 ± 0.33 | 0.55/39 |
| SBT | 2.6 ± 0.24 | 0.61/61 | 1.3 ± 0.42 | 0.53/37 | 2.68 ± 0.37 | 0.62/62 | 0.68 ± 0.22 | 0.36/21 | 1.73 ± 0.24 | 0.4/40 | 0.99 ± 0.28 | 0.4/28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giler-Molina, J.M.; Zambrano-Intriago, L.A.; Quiroz-Fernández, L.S.; Napoleão, D.C.; dos Santos Vieira, J.; Simões Oliveira, N.; Rodríguez-Díaz, J.M. Degradation of Oxytetracycline in Aqueous Solutions: Application of Homogeneous and Heterogeneous Advanced Oxidative Processes. Sustainability 2020, 12, 8807. https://doi.org/10.3390/su12218807
Giler-Molina JM, Zambrano-Intriago LA, Quiroz-Fernández LS, Napoleão DC, dos Santos Vieira J, Simões Oliveira N, Rodríguez-Díaz JM. Degradation of Oxytetracycline in Aqueous Solutions: Application of Homogeneous and Heterogeneous Advanced Oxidative Processes. Sustainability. 2020; 12(21):8807. https://doi.org/10.3390/su12218807
Chicago/Turabian StyleGiler-Molina, José Miguel, Luis Angel Zambrano-Intriago, Luis Santiago Quiroz-Fernández, Daniella Carla Napoleão, Judite dos Santos Vieira, Nelson Simões Oliveira, and Joan Manuel Rodríguez-Díaz. 2020. "Degradation of Oxytetracycline in Aqueous Solutions: Application of Homogeneous and Heterogeneous Advanced Oxidative Processes" Sustainability 12, no. 21: 8807. https://doi.org/10.3390/su12218807