Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles
Abstract
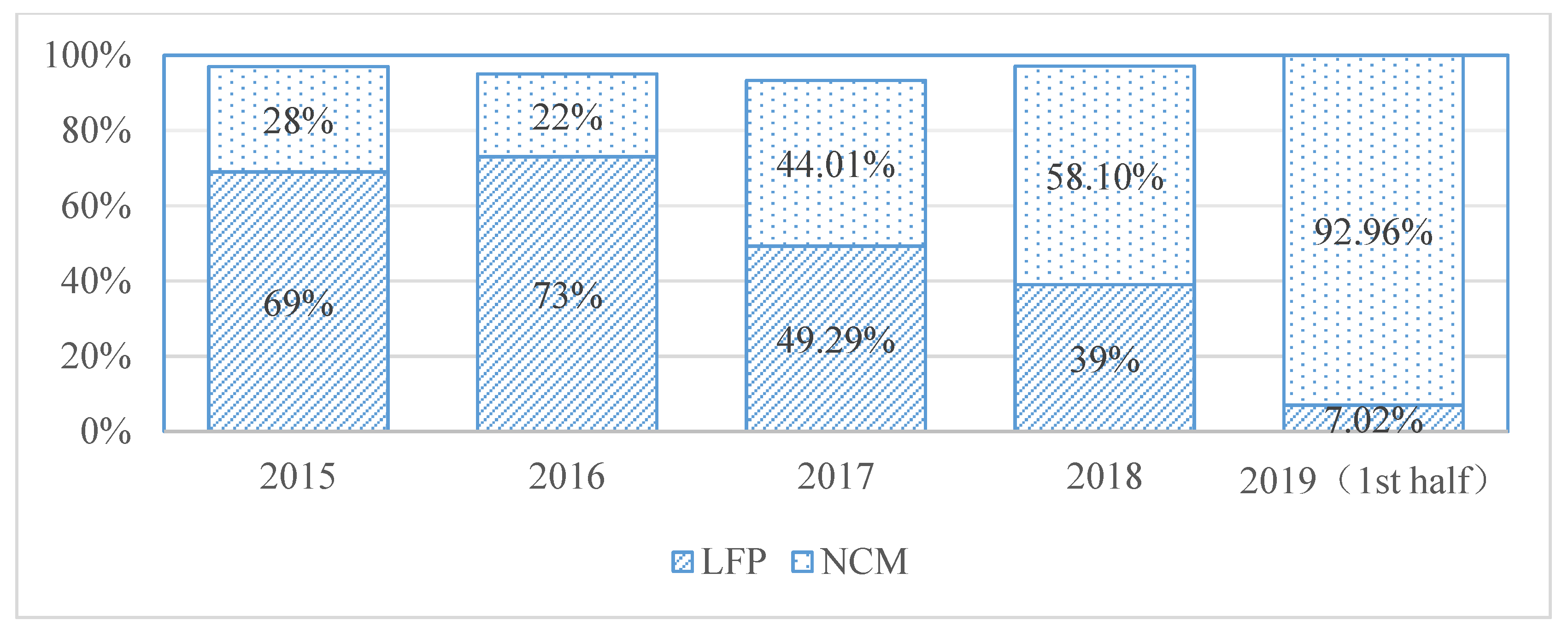
1. Introduction
2. Materials and Methods
2.1. Materials
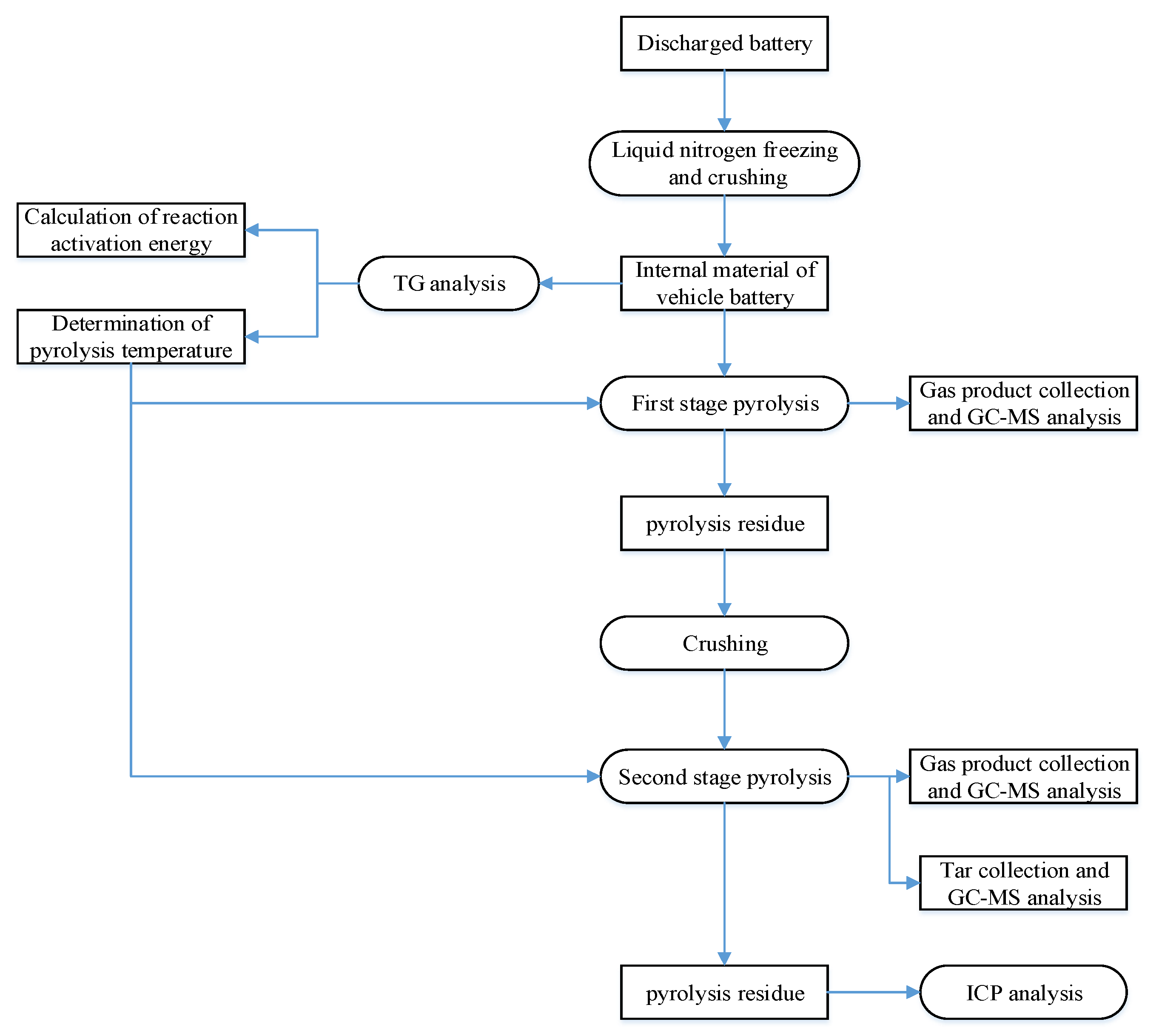
2.2. Experiment Process
3. Results and Discussion
3.1. Pyrolysis Characteristics
3.1.1. Determination of Pyrolysis Temperature
3.1.2. Calculation of Reaction Activation Energy
3.2. Pyrolysis Products and Analysis
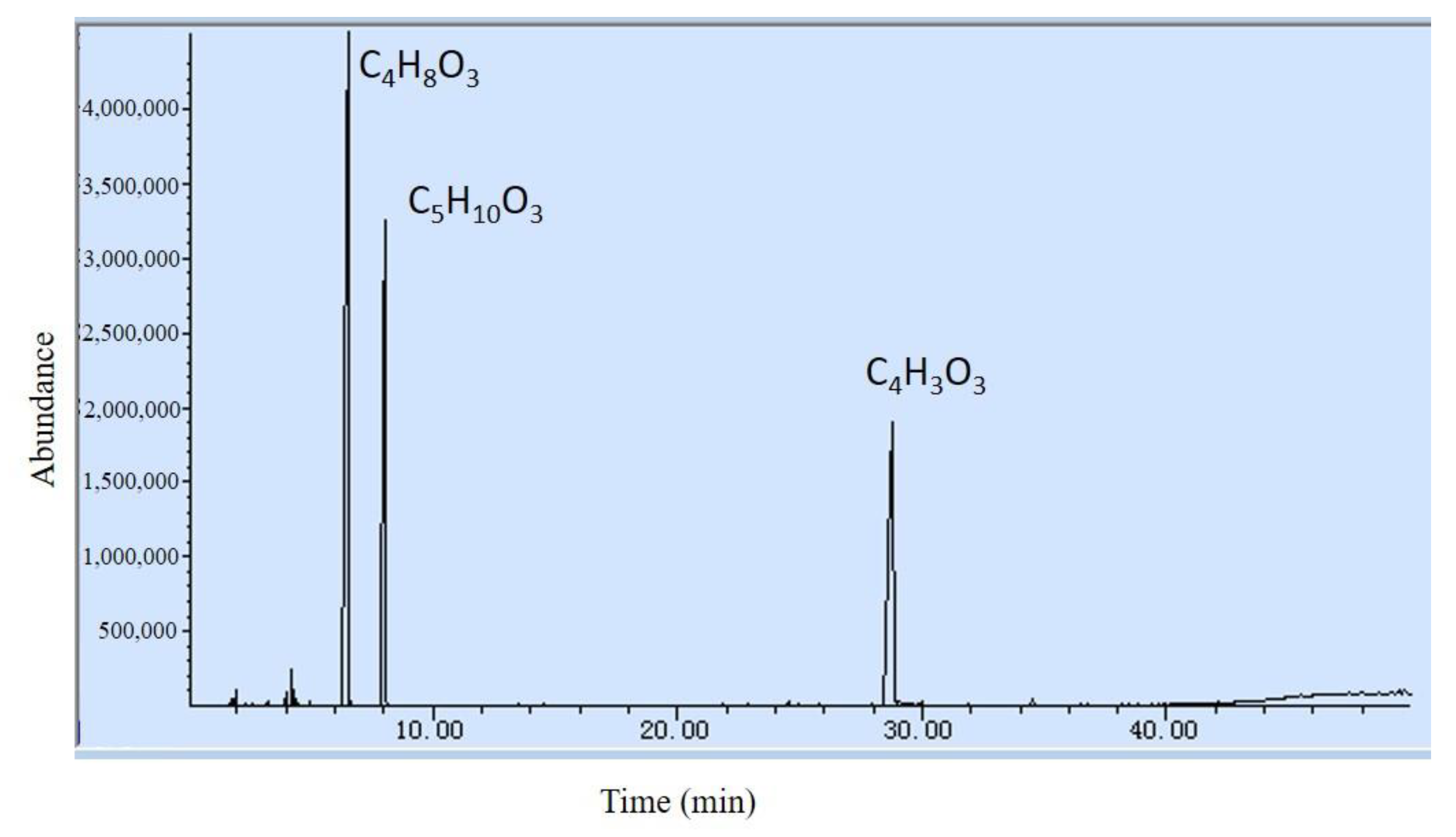
3.2.1. The First Stage of Pyrolysis
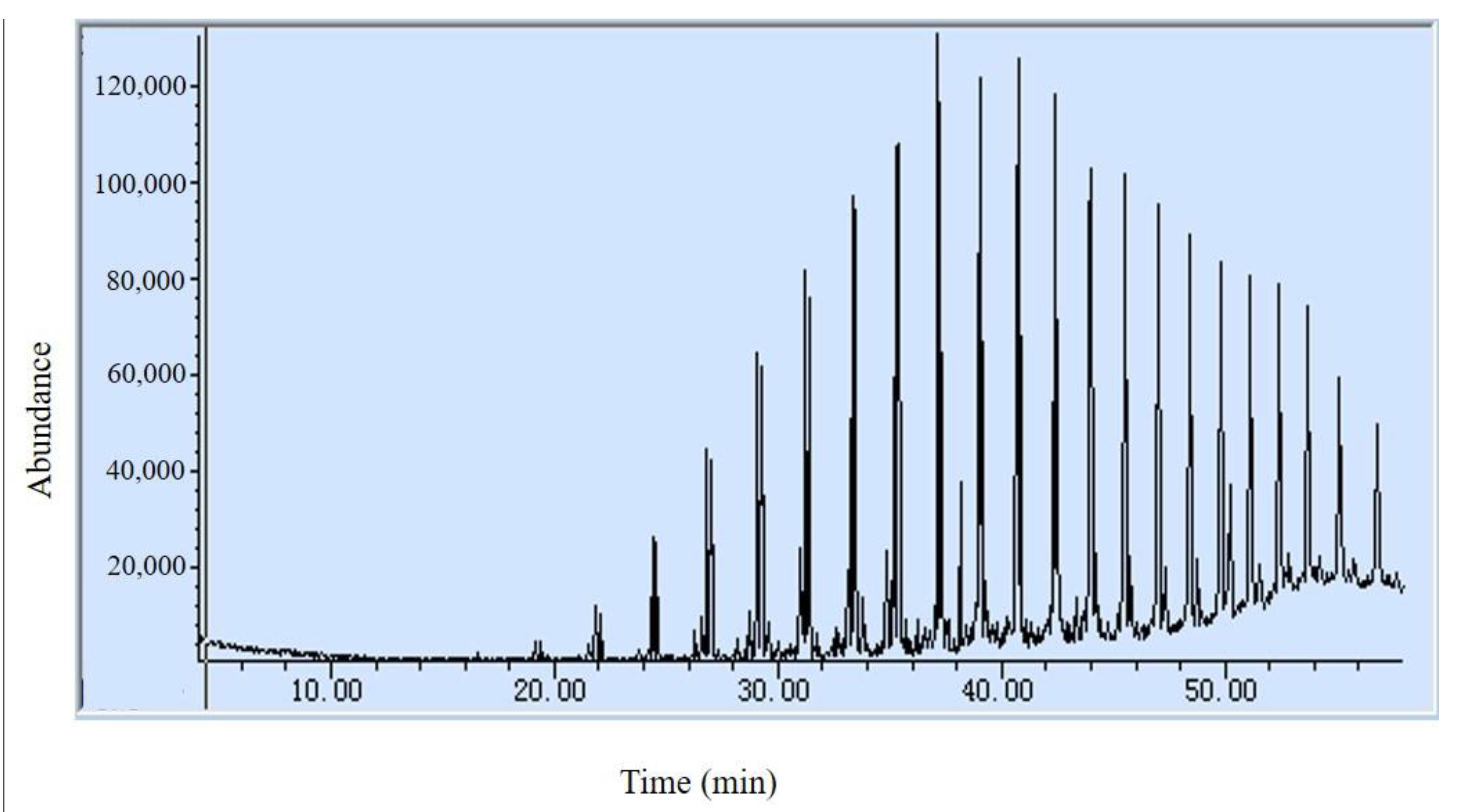
3.2.2. The Second Stage of Pyrolysis
- (1)
- Gas Production Analysis
- (2)
- Solid Production Analysis
3.3. Industrial Process Vision for NCM Vehicle Battery Recycling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, L.; Chen, M. A SWOT and AHP Methodology for the formulation of development strategies for China’s waste EV battery recycling industry. In Cascade Use in Technologies 2018; Springer: Berlin, Germany, 2019; pp. 83–92. [Google Scholar]
- China Industry Information. Available online: http://www.chyxx.com/industry/201905/741529.html (accessed on 23 May 2019).
- China Industry Information. Available online: http://www.chyxx.com/industry/201609/447420.html (accessed on 10 September 2016).
- China D1EV. Available online: https://www.d1ev.com/kol/85871 (accessed on 13 January 2019).
- Industry Information. Available online: http://www.chyxx.com/industry/201705/523691.html (accessed on 18 May 2017).
- D1EV. Available online: https://www.d1ev.com/kol/75830 (accessed on 28 August 2018).
- D1EV. Available online: https://www.d1ev.com/kol/86099 (accessed on 18 January 2019).
- D1EV. Available online: https://www.d1ev.com/kol/108151 (accessed on 16 January 2020).
- Sohu Car. Available online: https://www.sohu.com/a/327364133_99957909 (accessed on 16 January 2020).
- Hu, Y.; Cheng, H.; Tao, S. Retired Electric Vehicle (EV) batteries: Integrated waste management and research needs. Environ. Sci. Technol. 2017, 51, 10927–10929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J. A comparative life cycle assessment on lithium-ion battery: Case study on electric vehicle battery in China considering battery evolution. Waste Manag. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L.; Sullivan, J.; Burnham, A.; Belharouak, I. Life-cycle analysis for lithium-ion battery productionand recycling. In Proceedings of the Transportation Research Board 90th Annual Meeting, Washington, DC, USA, 23–27 January 2011; pp. 23–27. [Google Scholar]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Shanghai Metals Market. Available online: http://www.metal.com/pricing (accessed on 15 January 2020).
- Lupi, C.; Pasquali, M.; Dell’Era, A. Nickel and cobalt recycling from lithium-ion batteries by electrochemical processes. Waste Manag. 2005, 25, 215–220. [Google Scholar] [CrossRef]
- Yun, L.; Linh, D.; Shui, L.; Peng, X.; Garg, A.; LE, M.L.P.; Asghari, S.; Sandoval, J. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles. Resour. Conserv. Recycl. 2018, 136, 198–208. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, W.Y.; Niu, W.Y. Recovery of Co (II) and Ni (II) from hydrochloric acid solution of alloy scrap. Trans. Nonferrous Met. Soc. China 2008, 18, 1262–1268. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, X.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Porvali, A.; Aaltonen, M.; Ojanen, S.; Velázquez-Martínez, O.; Eronen, E.; Liu, F.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour. Conserv. Recycl. 2019, 142, 257–266. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Ravdel, B.; Abraham, K.; Gitzendanner, R.; Dicarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119, 805–810. [Google Scholar] [CrossRef]
- Bankole, O.E.; Lei, L. Silicon exchange effects of glassware on the recovery of LiPF6: Alternative route to preparation of Li2SiF6. J. Solid Waste Technol. Manag. 2014, 39, 254–259. [Google Scholar] [CrossRef]
- Sonoc, A.C.; Jeswiet, J.; Soo, V.K. Opportunities to improve recycling of automotive lithium ion batteries. Procedia CIRP 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Lingyun, Z.; Ming, C. Research on reverse logistics model and network for used electric vehicle batteries. China Mech. Eng. 2019, 30, 1828–1836. [Google Scholar]
- Ministry of Industry and Information Technology of the People’s Republic of China (2020). Available online: http://www.miit.gov.cn/n1146295/n1652858/n1652930/n4509607/c7595282/content.html (accessed on 16 December 2019).
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhang, T.; Xie, W.; Zhu, X. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Ni, F.; Chen, M. Research on ASR in China and its energy recycling with pyrolysis method. J. Mater. Cycles Waste Manag. 2014, 17, 107–117. [Google Scholar] [CrossRef]





| Organic Solvent | DMC (C2H6O2) | DEC (C5H10O2) | EC (C3H4O3) | EMC (C4H8O3) |
|---|---|---|---|---|
| Flash/boiling point | 18 °C/89 °C | 33 °C/126 °C | 157 °C/248 °C | 23 °C/109 °C |
| Policies [30] | Highlights |
| 2006, Automotive product recycling technology policy | Extended producer responsibility for electric vehicle manufacturers |
| 2012, Planning for the development of the energy-saving and new energy vehicle industry (2012–2020) | Cascade use and standards construction of waste battery |
| 2016, Electric vehicle battery recycling technology policy | Recovery rate: Ni, Co, Mn ≥ 98% |
| 2018, New energy vehicle battery recycling interim measures | Reiterate the responsibility of batteries recycling; |
| 2018, New energy vehicle battery recycling on traceability management interim measures | Establishment of traceability platform and coding identification of battery products |
| 2019, New energy vehicle battery recycling on service network construction and operation guide [31] | Recycling network construction |
| 2019, Industry standard conditions of new energy vehicle used battery utilization and interim administrative measures [31] | Recovery rate: Ni, Co, Mn ≥ 98% by hydrometallurgy; Ni and Rare earth elements ≥ 97% by pyrometallurgy |
| Standards | Highlights |
| GBT 33059-2016: Methods for disposal and recycling of lithium-ion battery material wastes | Recovery rate: Cu and Al ≥ 90%, Ni and Co ≥ 99% and Mn ≥ 95%; The removal of binder and separator by 500–600 °C pyrolysis |
| GBT 33598-2017: Recycling of traction battery used in electric vehicle—dismantling specification | Waste battery dismantling specification |
| GBT 34013-2017: Dimension of traction battery for electric vehicles | Battery classification |
| GBT 34014-2017: Coding regulation for automotive traction battery | Code for battery products |
| GBT 34015-2017: Recycling of traction battery used in electric vehicle—test of residual capacity | Residual capacity detection method for battery |
| Composition | Mass Ratio |
|---|---|
| Cathode material | 28.2% |
| Anode material | 18.3% |
| Copper | 11.4% |
| Aluminum | 19.7% |
| Binder | 2.4% |
| Electrolyte | 1.9% |
| Electrolyte solvent | 10.8% |
| Separator | 2.0% |
| Plastics | 1.2% |
| Others | 4.1% |
| Heating Rate (K/min) | Mass Loss (wt%) | Temperature T (°C) Corresponding to Conversion Rate x | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x = 0.1 | x = 0.2 | x = 0.3 | x = 0.4 | x = 0.5 | x = 0.6 | x = 0.7 | x = 0.8 | x = 0.9 | ||
| 5 | 13.31% | 35.64 | 63.18 | 97.94 | 109.09 | 117.61 | 126.4 | 156.43 | 399.8 | 459.48 |
| 10 | 11.85% | 54.21 | 104.75 | 115.92 | 124.62 | 132.31 | 139.52 | 212.21 | 419.19 | 464.87 |
| 20 | 16.19% | 112.52 | 124.36 | 134.62 | 142.16 | 149.63 | 162.57 | 362.27 | 449.08 | 485.32 |
| 30 | 15.01% | 122.92 | 137.61 | 148.36 | 156.12 | 162.85 | 170.98 | 320.82 | 456.29 | 488.64 |
| Conversion Rate x | Slope k | Reaction Activation Energy E (kJ/mol) |
|---|---|---|
| 0.1 | −961.19 | 17.49 |
| 0.2 | −1402.81 | 25.54 |
| 0.3 | −2431.81 | 44.27 |
| 0.4 | −2753.01 | 50.12 |
| 0.5 | −2942.72 | 53.57 |
| 0.6 | −2944.79 | 53.61 |
| 0.7 | −1210.91 | 22.04 |
| 0.8 | −6348.81 | 115.58 |
| 0.9 | −12,740.46 | 231.93 |
| Composition (Formula) | Peak Area Ratio (%) | Boiling Point (°C) | Calorific Value (kJ/mol) |
|---|---|---|---|
| EC(C4H3O3) | 28.94 | 39 | 834.0 |
| DEC(C5H10O3) | 27.17 | 126 | 2708.2 |
| EMC(C4H8O3) | 43.14 | 109 | N/A |
| Products | N2 | CO2 | H2O | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | C4H10 | C5H10 | C5H12 | C6H12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak area ratio (%) | 89.27 | 3.87 | 1.73 | 0.39 | 0.26 | 1.11 | 0.41 | 0.66 | 0.50 | 0.66 | 0.39 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Chen, M. Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles. Sustainability 2020, 12, 9164. https://doi.org/10.3390/su12219164
Zhu L, Chen M. Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles. Sustainability. 2020; 12(21):9164. https://doi.org/10.3390/su12219164
Chicago/Turabian StyleZhu, Lingyun, and Ming Chen. 2020. "Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles" Sustainability 12, no. 21: 9164. https://doi.org/10.3390/su12219164
APA StyleZhu, L., & Chen, M. (2020). Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles. Sustainability, 12(21), 9164. https://doi.org/10.3390/su12219164





