3.1. Nutrient Content of V. karroo, M. oleifera and C. tagasaste Vermicomposts
Table 1 shows the nutrient content of
V. karroo,
M. oleifera and
C. tagasaste vermicomposts at the final week of sampling compared with soil critical limits adopted by various researchers [
33,
34,
35]. The results show that nutrient contents in vermicomposts depended on the feedstock i.e., the material being vermicomposted. These results were observed for all the nutrients determined except for Cu, Mn and C, which did not vary among the vermicomposts. Of the three feedstock materials,
M. oleifera had the highest content of most nutrients compared to
V. karroo, and
C. tagasaste. For instance, Ca content in
M. oleifera vermicompost was 154 mg kg
−1, which was more than 170% higher than the other two vermicomposts. Similarly, such large differences in contents among
M. oleifera and the other vermicomposts were also observed with other macronutrients (K, Mg, and P). Primary macronutrients (N, P and K) differed between the vermicomposts and followed the order
M. oleifera >
V. karroo >
C. tagasaste for N. For P and K the trend was
M. oleifera >
C. tagasate >
V. Karroo. Micronutrients such as Fe and Zn were lower in
C. tagasaste compared to the other two vermicomposts. The pH of the vermicomposts were slightly alkaline.
When compared over time, the macronutrients (Ca, Mg, K, P), with the exception of N, were higher at the later stage of vermicomposting during week 6. (
Table 1) while no differences were observed after zero-, two- and four-weeks. The general increase in nutrients particularly basic cations at the later stages of vermicomposting could be a result of earthworm digestion. Several studies have also reported high levels of exchangeable bases in vermicompost, agreeing with the findings of this study [
36,
37]. Higher quantities of basic cations explain the slightly alkaline pH observed at week six.
Nitrogen content was higher at the initial vermicomposting stage and lower at the later stage, a similar trend observed also for some micronutrients such as Mn, Zn, and B. The low N content at later stages of vermicomposting could be attributed to assimilation by microorganisms as a source of energy and growth [
38]. The decrease in the micronutrient as the vermicompost matures are contrary to the findings of some studies [
39]. The contradiction observed in micronutrients may be attributed to the differences in the vermicomposted material where Sharma and Garg [
39] used rice straw. The feedstocks used in this study are of relatively low C:N ratio compared to the rice straw.
M. oleifera,
V. karroo and
C. tagasaste are all leguminous trees and hence have lower C:N ratio than wheat straw. The observed results may partially be attributed to the slightly higher pH in
M. oleifera and
C. tagasaste. In soils, for example, it is well established that micronutrients become less available under alkaline conditions.
After six weeks of vermicomposting,
M. oleifera generally had higher nutrient content compared to the other vermicomposts (
Table 1). However, the pH remained relatively similar. These results suggest
M. oleifera provides high quality vermicompost when mature compared to the other two feedstocks. The low N content for all vermicomposts at week six (
Table 1) may be due to changes in conditions such as the duration of the process which is known to drastically affect the N content [
40]. Other researchers stated that phenolic compounds such as tannins and flavonoids found in plant tissue stimulate microbial activity which subsequently reduces plant available N through assimilation by microorganisms as a source of energy and growth [
38]. In this study,
V. karroo did not contain any tannins nor flavonoids. These phenolic compounds were only consistently present in
M. oleifera (See
Section 3.5). However, the stimulation of microbial activity was not evident as the microbial numbers (CFUss) were rather constant throughout the vermicomposting period (See
Section 3.4). Therefore, a plausible reason for the decrease in N with time can be attributed to the utilisation of N by earthworms themselves for growth. This is indeed supported by the five-fold increase in earthworm numbers as discussed under
Section 3.4.
Phosphorus content in all vermicomposts were above the critical soil values, particularly of in
M. oleifera with a content of 107 mg kg
−1 of P which was seven times higher than the critical soil limit value of 15 mg kg
−1 [
35]. Norman, Arancon [
41] found that P was 64% higher in vermicomposts compared to other organic materials such as animal manure or human wastes.
Molybdenum and B contents are normally very low in most soils [
42]. In this study, Mo and B were relatively high therefore suggesting that vermicomposts could also be used as a source of micronutrients and can act as slow release fertiliser, in agreement with Atiyeh, Domínguez [
43] who reported that plants gown on a vermicompost medium outgrew plants that were grown in other media due to the slow release of the nutrients. The presence of humic and fulvic acids in vermicompost could be one of the reasons for the relatively high content of the, Mo and B which were above critical soil test values. Khaled and Fawy [
44], reported that humic and fulvic acids form chelates with micronutrients increasing their bio-availability for plant use.
Basic cations such as K, Ca, Mg and some micronutrients such Mn, Cu and Zn were below the soil critical values in all vermicomposts. It is unclear why basic cations were so low because according to Elvira, Sampedro [
45] and Parthasarathi, Ranganathan [
46], earthworm cast have enhanced content of such nutrients due to the mineralisation process taking place in their guts. A possible reason for this could be that the earthworms are normally found in soils and they ingest soil material which generally have higher Ca, Mg, and K. In this study the earthworms mineralised nutrients that were in the feedstocks. Therefore, the quantities of these nutrients should be a reflection of the amount of nutrients in the feedstocks. However, the contents of basic cations were still rather too low than expected. A study by Mabapa, Ayisi [
17] showed that
M. oleifera dry biomass contained significantly higher quantities of basic cations than those found in the present study. The lower contents of micronutrients in some of the vermicomposts could be attributed to the high pH which is known to reduce their availability [
47].
The pH value of all the three vermicompost was found to be alkaline at 7.35. A good soil pH is essential in order to ensure the availability of nutrients because some nutrients are found to become unavailable at pH that is extremely alkaline or acidic [
48]. The pH also controls enzyme activity as well as biotic decomposition [
49], hence the need for optimal soil pH. High pH levels in vermicompost may result from the release of excess organic N released as ammonia that quickly dissolved in water producing ammonium and consequently increasing the pH [
50]. It has already been observed that vermicompost tend to have higher N levels [
51]. So, considering the leguminous nature of the feedstocks and the relatively high N content associated with them, ammonification could have occurred. Thus, vermicomposts have the potential to ameliorate soil acidity. However, some studies have shown that in some instances pH may decrease during vermicomposting especially towards maturity [
52,
53].
3.2. Correlation Relationships and Principal Components Analysis
Bivariate correlation analyses for the different vermicomposts shown in
Table 2,
Table 3 and
Table 4 for
V. karroo,
M. oleifera, and
C. tagasaste, respectively, show that
M. oleifera, and
C. tagasaste had positive correlations between pH and basic cations (Ca, Mg, K, and Na) and negative relationship with micronutrients (Cu, Fe, Mn, Zn, B). However, Mo was the only micronutrient positively related to the vermicompost pH. Such relationships confirm earlier discussions where pH had positive and negative relationships with bases and micronutrients, respectively. In contrast to the correlations shown by
M. oleifera, and
C. tagasaste,
V. karroo showed a significant negative relationship between pH and Ca while the other basic cations had non-significant positive relationships with pH.
Principal components analysis for
M. oleifera vermicompost showed that PC1 and PC2 exhibited a variance of >10% and were therefore retained (
Figure 1 and
Table S1). The cumulative variance for the two PCs was 84%. In the first PC, all the bases, P and HR showed a strong positive loading. In contrast, micronutrients Mn, Fe and B showed a strong negative loading. In PC2, only HI and N loaded significantly.
Meanwhile, the principal components analysis for
C. tagasaste vermicompost produced three significant PCs which accounted for 92% of variance (
Figure 2 and
Table S2). In PC1, Na, K, P, Mg, and pH exhibited significant positive loadings while micronutrients, Mn, Fe and Zn showed significant negative loadings. Humification indices, N and HA showed positive loadings in PC2 with carbon and FA showing significant loadings. Bases, P, and HR showed a strong positive loading. Parameters Cu, Ca and Mo loaded significantly in PC3. The strong loading of the above nutrients serves to confirm their dominant occurrence in
C. tagasaste. The negative loading of most micronutrients also confirms the negative interactive effects between micronutrients and the PCA. In the PCA analysis negative values of loadings of variable in the components of the PCA means the existence of an inverse correlation between the factor PCA and the variables.
Also, three PCs were retained for
V. karroo accounting for 95% variance (
Figure 3 and
Table S3). The high loading parameters in PC1 with loading values >0.90 were P, K, Na and Mg. Zn, B and Fe showed a strong negative loading of >−0.88. In PC 2 carbon exhibited a strong negative loading (−0.829). Meanwhile, the humification parameters loaded showed a significant positive loaded with soil pH loading negatively. The strong loading shows the importance of nutrients such as P, K and micronutrients in
V. karroo vermicompost while the negative loading of C indicates the depletion of C sources with vermicomposting. The P loaded strongly in all the three species showing its strong influence in the vermicomposts.
3.3. Humic and Fulvic Acids of V. karroo, C. tagasaste and M. oleifera Vermicomposts
The C content in the FA fraction (FA) was highest in
M. oleifera (1.30%) followed by
V. karroo with 0.63% and
C. tagasaste had the lowest with 0.23% (
Table 5). The carbon in HA was highest in
C. tagasaste (1.72%) but did not differ between
M. oleifera (1.25%) and
V. karroo (1.27%). The TEC which is the sum of FA and HA was higher in
M. oleifera compared to the other two vermicomposts. The indices calculated, showed that the polymerisation index (PI) was highest in
C. tagasaste with an index value of 786.93 while the humification ratio (HR) was highest in
M. oleifera with an index value of 4.25. On the other hand, the humification index (HI) did not differ between the vermicomposts.
Both PI and HI can be used to determine maturity of a compost. According to Raj and Antil [
54], a mature compost should have a PI ratio of >1.9 and an HI of >30%. The mean values of PI ratio observed in this study were found to be higher than 1.9 thus indicating the maturity of the compost [
54,
55]. However, the HI index of the vermicomposts were too low at week 6 (1.92 to 2.53), well below the 30% proposed by Raj and Antil [
54] which suggest that the vermicomposts were probably not fully mature according to this index. Although the HI of the vermicomposts were very low, the PI ratios suggests that the vermicomposts were mature. The low HI could be the result of slow vermicomposting which consequently reflects low humification rate as reported by [
56]. It is worth noting that no individual parameter can be used to determine maturity of the vermicompost [
54]. The mean values for the parameters differ based on the type of feedstock used, which explains the low or high mean values recorded for the week 6 parameters in this study.
Table 5 also shows the mean variation of FA, HA and maturity indices (PI, HR and HI) with time (combined for all vermicomposts) while
Figure 1 shows the relationship of FA and HA at different times. The results in
Table 5 show that the mean FA generally decreased with time with higher values being observed at the beginning of the vermicompost while mean HA generally increased with time. Humification index (HI) behaved similarly to HA with a lower value at the beginning (0.91) which significantly increased at week two and then remained relatively constant throughout the vermicomposting period. As vermicomposting progresses, the percentage of humic substances is expected to increase [
57]. This is because during the initial stage of vermicomposting, the organic matter is progressively mineralized. However, during the later (maturing) stage of vermicomposting, humic substances particularly humic acids increases and most of the organic matter stabilises [
58].The other indices (PI and HR) fluctuated throughout the vermicomposting period.
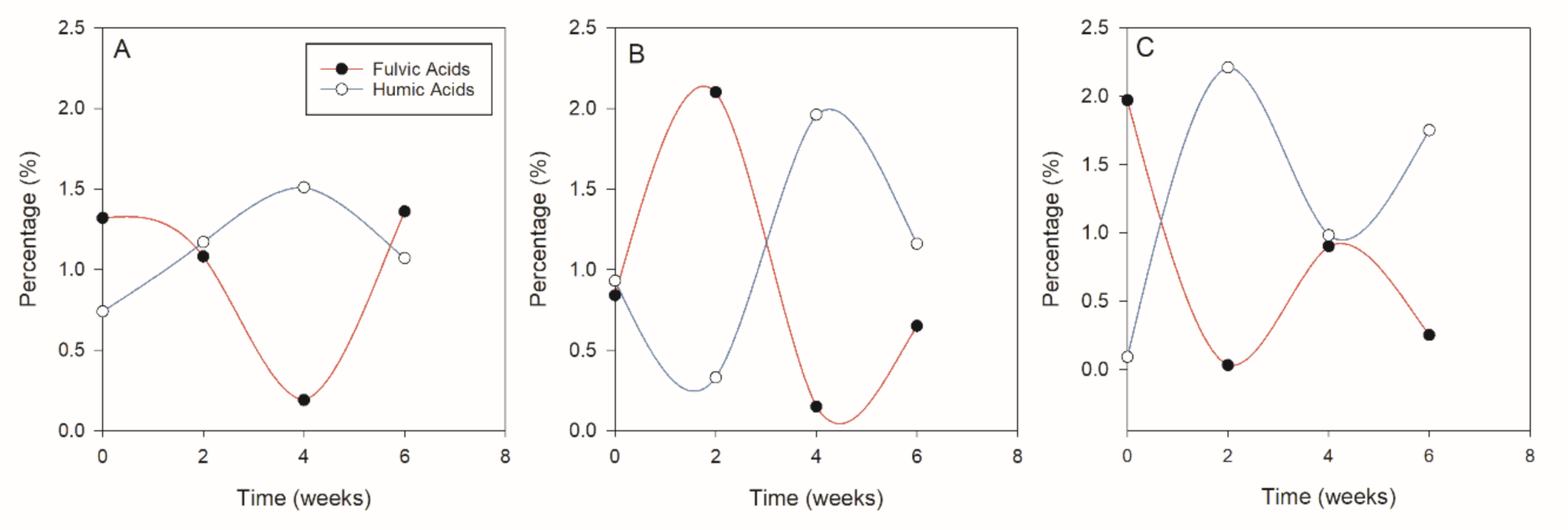
It was observed that
M. oleifera and
C. tagasas all started with relatively high contents of FA compared to HA (
Figure 4A) while
V. karroo had similar levels of HA and FA (
Figure 4B). Notable was the mirror reflection of the contents of HA and FA whereby when HA was high FA was lower. There was also a significant negative relationship (−0.90;
p < 0.001) between FA and HA. The alternating decrease and increase of the humic and fulvic acids may result from the transformation of humic substances through degradation [
59] to the more soluble fulvic acid form at any given time. Hence when the fulvic acids in a certain period increase, the humic acids decrease.
The increase in fulvic acids in this study could have resulted from continued mineralization during the vermicomposting process. The increase in fulvic acids, results in low humic acid content because of the unstable organic matter. Labile forms of fulvic acids are associated with the part of organic carbon referred to as dissolved organic carbon while the less labile forms are humic acids bound to cations and humins [
60,
61]. Degradation of humic acids results in the accumulation of fulvic acids which contributes to their increase while decreasing the humic acids. The presence of humic acids in the vermicompost can enhance the nutrient uptake by plants and thus act as a soil conditioner [
62,
63]. By increasing the permeability of the root cell membrane and also the proliferation of the root hairs, the humic acids improve the nutrient uptake of plants [
64]. They also form chelates with micronutrients thus increasing the bio-availability for plant use [
44].
3.4. Microbial and Earthworm Populations in Vermicomposts
The bacterial populations were found to be lower in
V. karroo compared to other two vermicompost (
Table 6).
V. karroo had 9.00 colony-forming units (cfu) compared to 9.34 in both
M. oleifera and
C. tagasaste. Phosphate Solubilising Bacteria differed between all vermicompost at week six. Phosphate solubilising bacteria followed the order
M. oleifera >
C. tagasaste >
V. karroo. There were no
E coli in
V. karroo and
C. tagasaste at week six. Only
M. oleifera had
E coli at week six with a cfu of 5.33. Fungi on the hand was higher in
M. oleifera (5.75 cfu) compared to
V. karroo and
C. tagasaste, which both had 5.49 CFU. Vermicompost are known to enhance soil biodiversity by promoting the beneficial microbes which in turn enhances plant growth directly by production of plant growth-regulating hormones and enzymes and indirectly by controlling plant pathogens, nematodes and other pests, thereby enhancing plant health and minimizing the yield loss. In addition, earthworms also enhance the beneficial microflora and suppress harmful pathogenic microbes [
65]. However, the existence of
E. coli in
M. oleifera was unexpected because the type of earthworms used in this study (
E. fetida) are known to completely destroy
E. coli in their guts [
65,
66]. Therefore, the non-existence of any
E. coli in
V. karroo and
C. tagasaste is in agreement with findings of Pathma and Sakthivel [
65] and Emperor and Kumar [
66].
Bacterial population were higher at the beginning, decreased at week two and four before increasing again at week six (
Table 6) while PSB fluctuated with time. The mean cfu of
E. coli and fungi did not vary with time.
The results also show that the vermicomposts increased earthworm population by more than 500% in all the composts (
Table 7). The earthworm population count ranged from 584-761 at week six, with
M. oleifera being the highest and
C. tagasaste as the lowest. The increase in earthworm population for each treatment has resulted in improved microbial activity and nutrient availability as shown by the results in this study. Most of the nutrient contents especially for
M. oleifera were found to be at their optimum levels when compared to critical soil values, which is attributed to the increase in earthworm populations.
3.5. Phytochemical Constituents of C. tagasaste, V. karroo and M. oleifera Vermicompost
Qualitative analysis of phytochemicals showed that at any given time during the vermicomposting process; saponins were not detected in the three vermicomposts (
Table 8).
Moringa oleifera contained tannins during the entire period of vermicomposting while no tannins were detected
C. tagasaste and
V. karroo during the period (
Table 8).
Moringa oleifera is known to be rich in different forms of phytochemicals [
67], which could have been the reason for the presence of tannins as well as flavonoids observed in this study. This is also supported by Rengarajan, Melanathuru [
68] who indicated that methanol extracts of
M. oleifera petals contained flavonoids, tannins, saponins and alkaloids. Kraus, Yu [
69] reported that tannins may limit litter decomposition in a number of different ways such as by themselves being resistant to decomposition or by sequestering proteins in protein-tannin complexes that are resistant to decomposition. In this study, the presence of tannins in
M. oleifera vermicompost may benefit growing plants by slowing down decomposition and supplying plants with nutrients for longer as opposed to when vermicomposting is faster and quickly depleting nutrients.
Alkaloids were not detected in
M. oleifera and
V. karroo but only in
C. tagasaste. These results are in contrast with Rengarajan, Melanathuru [
68] who found that
M. oleifera contained alkaloids among other phytochemicals. It is not immediately clear why none of the analysed phytochemicals were not detected in
V. karroo vermicompost in this study. However, previous studies have shown that
M. oleifera,
V. karroo and
C. tagasaste contain secondary metabolites [
67,
70,
71]. The absence could be due to the fact that phytochemicals do not automatically accumulate at their site of synthesis and as a result of genetic variability or leaf age development [
72] may have been translocated to other parts of the plant that were not analysed. Reports also indicate that external stimuli can modulate the synthesis and change the composition or quantities of the phytochemicals in plants [
73,
74]. The quantities are also affected by environmental factors, such as soil composition, temperature, rainfall and ultraviolet radiation incidence [
75,
76]. Research has certified that flavonoid contents in some leaf exudates can be enhanced by ultra-violet radiation induction or by drought [
77]. These findings indicate that the phytochemical contents of the tree species in this study may have possibly been affected by the above-mentioned factors resulting in the absence of saponins and alkaloids.
Flavonoids were detected in
M. oleifera and
C. tagasaste vermicompost throughout the vermicomposting period (
Table 8). These results are also in agreement with Rengarajan, Melanathuru [
68] who noted that
M. oleifera contains flavonoids among other phytochemicals. Legumes exude specific flavonoids that act as signalling molecules to attract N-fixing bacteria [
78], which is essential in making N available in the soil for plant use. Flavonoids are produced when plants are infected or injured [
79] or when there are low nutrients [
80]. This implies that their presence in the vermicompost is essential to plant health. This is supported by the fact that among others, phytochemicals are a source of carbon or energy for microorganisms which are consequently beneficial for plant health and they also release hormone effectors of cell differentiation in plants [
81]. Flavonoids give ultra violet protection to plant tissues, and their accumulation due to ultra-violet exposure is also well documented [
82].
The absence of some phytochemicals such as saponins and tannins in some tree species indicates that they are naturally unavailable [
83], or present in undetectable amounts. This supports the results obtained in this study, since the phytochemicals were not detected in some of the vermicompost treatments. The presence of phytochemicals in the vermicompost indicate possible defence against harmful bacteria and pests [
29]. Therefore, this shows that the vermicomposts have potential to be used for soil fertility enhancement. Seed germination (phytotoxicity) was also above 70%, which indicates that the compost could not have been toxic to plants [
29].