A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings
Abstract
:1. Introduction
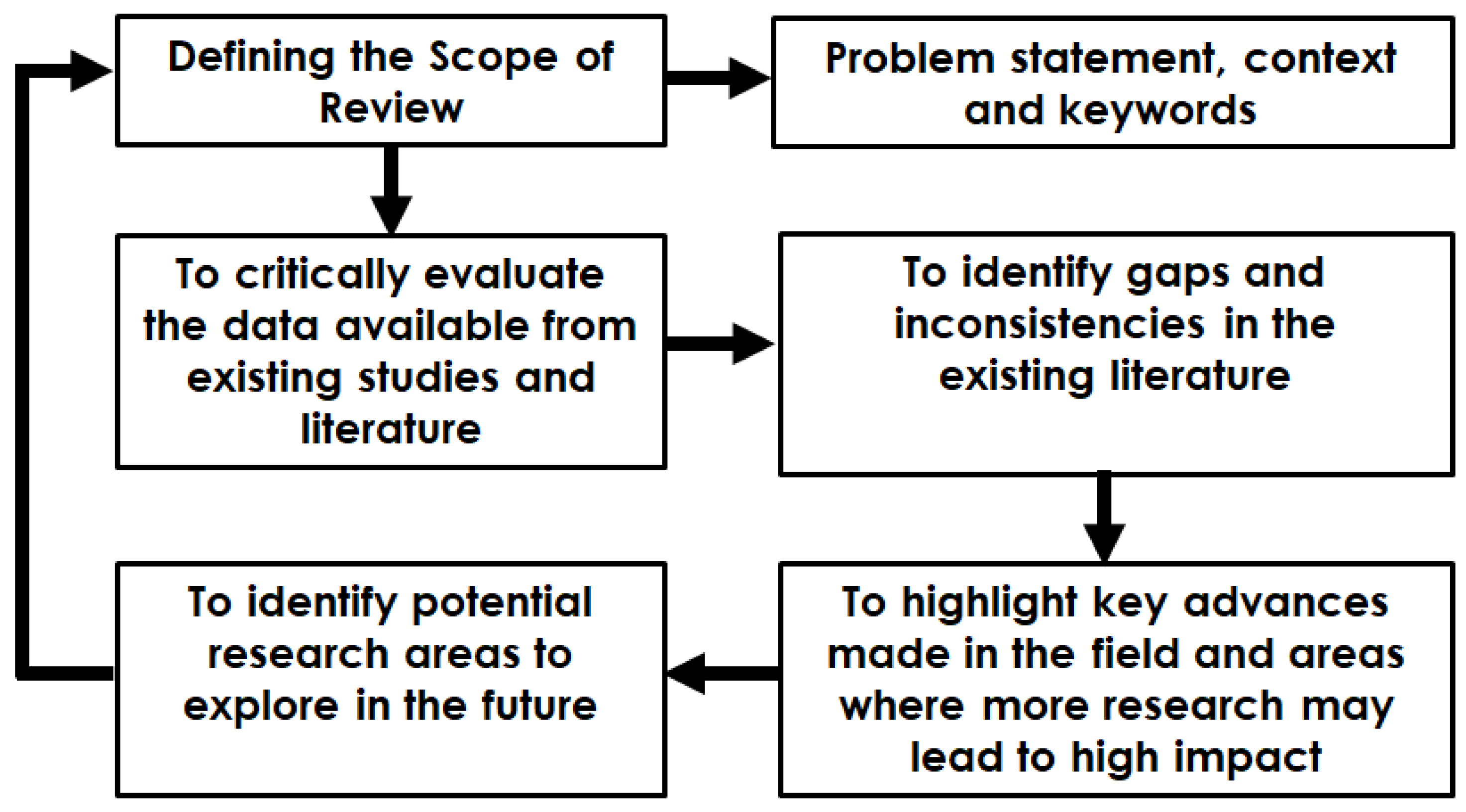
2. Review Methodology
3. Shape-Stabilized Phase Change Materials (SSPCMs)
4. Preparation Methods of Inorganic FSCPCMs
5. Supporting Materials Types
5.1. Expanded Vermiculite (EV) based SSPCM
5.2. Expanded Perlite (EP) Based FSCPCM
5.3. Diatomite based FSCPCM
5.4. Expanded Graphite (EG) based FSCPCM
6. SSPCMs for Building Envelope Applications
6.1. Walls
6.2. Floor
6.3. Roof
6.4. Windows
7. Conclusions and Future Outlook
Summary of the Research Carried Out in the Area of SSPCMs
8. Further Research
- Existing studies have focused either on heating or cooling applications of PCMs. Therefore, PCMs are highly required for both heating as well as cooling applications in buildings. Further research on the performance of PCM in maintaining thermal comfort for a whole year is being carried out by the authors.
- Most of the studies in the literature have focused on walls, and less research reported on incorporating PCMs with roof, floor, and windows. Thus, further research on these areas is needed and authors are researching on it.
- Most of the studies have been conducted at laboratory level and not in a full-scale room. Therefore, real-time, full-scale, experimental studies are essential to analyse the practicability and reliability of SSPCMs under real weather conditions. Hence, the authors are exploring a full-scale prototype in a composite climate where both cooling and heating requirements exist.
- Similarly, further studies with a focus on environmental and economic analysis as a result of incorporating PCMs in building materials are required, as currently these topics are least researched. Further studies are needed to improve the long-term thermal stability and chemical reliability of SSPCMs in buildings, under real weather conditions. The authors are researching it.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Guo, C. Influence of intumescent flame retardant on thermal and flame retardancy of eutectic mixed paraffin/polypropylene form-stable phase change materials. Appl. Energy 2016, 162, 428–434. [Google Scholar] [CrossRef]
- Shen, Q.; Ouyang, J.; Zhang, Y.; Yang, H. Lauric acid/modified sepiolite composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2017, 146, 14–22. [Google Scholar] [CrossRef]
- Alameda, M.; Lacasta, A.M.; Paloma, J.G.; Chimenos, J.M.; Haurie, L.; Formosa, J. Magnesium phosphate cements formulated with low grade magnesium oxide incorporating phase change materials for thermal energy storage. Constr. Build. Mater. 2017, 155, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Thermal performance assessment of phase change material integrated cementitious composites in buildings: Experimental and numerical approach. Appl. Energy 2017, 207, 654–664. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Nian, H. Polyethylene glycol-enwrapped silicon carbide nanowires network/expanded vermiculite composite phase change materials: Form stabilization, thermal energy storage behavior and thermal conductivity enhancement. Sol. Energy Mater. Sol. Cells 2018, 174, 283–291. [Google Scholar] [CrossRef]
- Li, S.; Zhu, N.; Hu, P.; Lei, F.; Deng, R. Numerical study on thermal performance of PCM trombe wall. Energy Procedia. 2018, 158, 2441–2447. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on shell materials used in the encapsulation of phase change materials for high temperature thermal energy storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Li, M.; Shi, J. Review on micropore grade inorganic porous medium based form stable Composite phase change materials: Preparation, performance improvement and effects on the properties of cement mortar. Constr. Build. Mater. 2018, 194, 287–310. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Chi, B.; Yao, Y.; Cui, S.; Jin, X. Prepration of grapheme oxide coated tetradecanol/expanded graphite composite phase change material for thermal energy storage. Mater. Lett. 2020, 282, 128666. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; He, X. Research on the preparation and properties of lauric acid/expanded perlite phase change materials. Energy Build. 2016, 110, 108–111. [Google Scholar]
- Chung, O.; Jeong, S.G.; Kim, S. Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings. Sol. Energy Mater. Sol. Cells 2015, 137, 107–112. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, B.; Zhang, J.; Zhu, Y.; Sun, G.; Li, Z. Preparation and characterization of expanded perlite/paraffin composite as form-stable phase change material. Sol. Energy 2014, 108, 460–466. [Google Scholar] [CrossRef]
- Zhu, N.; Li, S.; Hu, P.; Wei, S.; Deng, R.; Lei, F. A review on applications of shape-stabilized phase change materials embedded in building enclosure in recent ten years. Sustain. Cities Soc. 2018. [Google Scholar] [CrossRef]
- Golnoosh, A.; Ahmad, R.B.; Mahdi, A. Review on nanostructure supporting material strategies in shape-stabilized phase change materials. J. Energy Storage 2020, 29, 1–31. [Google Scholar]
- Anthony, J.R.; Werner, K.; Tina, G.; Florian, K. Selection of compatible metallic phase change materials and containers for thermal storage applications. J. Energy Storage 2020, 32, 1–16. [Google Scholar]
- Meng, G.; Meichen, L.; Yubo, J.; Wei, Z.; Yongxia, D.; Haiqing, L. A review of phase change materials in asphalt binder and asphalt mixture. Constr. Build. Mater. 2020, 258, 1–9. [Google Scholar]
- Zhou, G.; He, J. Thermal performance of a radiant floor heating system with different heat storage materials and heating pipes. Appl. Energy 2015, 138, 648–660. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Li, T.; Cao, X. Lauric–palmitic–stearic acid/expanded perlite composite as form-stable phase change material: Preparation and thermal properties. Energy Build. 2014, 82, 505–511. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, S.; Xu, X.; Ma, Z. A simplified dynamic model of building structures integrated with shaped-stabilized phase change materials. Int. J. Therm. Sci. 2010, 49, 1722–1731. [Google Scholar] [CrossRef]
- Meng, E.; Yu, H.; Zhan, G.; He, Y. Experimental and numerical study of the thermal performance of a new type of phase change material room. Energy Convers. Manag. 2013, 74, 386–394. [Google Scholar] [CrossRef]
- Karim, L.; Barbeon, F.; Gegout, P.; Bontemps, A.; Royon, L. New phase-change material components for thermal management of the light weight envelope of buildings. Energy Build. 2014, 68, 703–706. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; Luo, L.; Wang, J.; Zhang, K.; Fan, J.; Wu, J.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Tengteng, S.; Xiaoguang, Z.; Jiaxin, Q.; Xiaowen, W.; Guo, C.; Guoqin, L.; Fankai, L.; Xin, M.; Zhaohui, H. Preparation and characterization of composite phase change materials based on paraffin and carbon foams derived from starch. Polymer 2020. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Preparation and thermal properties of polyethylene glycol/expanded graphite blends for energy storage. Appl. Energy 2009, 86, 1479–1483. [Google Scholar] [CrossRef]
- Lv, Y.; Zhou, W.; Jin, W. Experimental and numerical study on thermal energy storage of polyethylene glycol/expanded graphite composite phase change material. Energy Build. 2016, 111, 242–252. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Lu, Z.; Li, Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Jia, Y.; Fang, G. Improved thermal properties of stearyl alcohol/ high density polyethylene/expanded graphite composite phase change materials for building thermal energy storage. Energy Build. 2017, 153, 41–49. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, R.; Tang, C.; Wu, B.; Huang, Z. Thermal conductivity enhancement of polyethylene glycol/expanded perlite with carbon layer for heat storage application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement- based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Kalnas, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-air review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Zhou, G. Thermal performance of a window-based cooling unit phase change materials combined with night ventilation. Energy Build. 2015, 108, 267–278. [Google Scholar] [CrossRef]
- Sarı, A. Fabrication and thermal characterization of kaolin-based composite phase change materials for latent heat storage in buildings. Energy Build. 2015, 96, 193–200. [Google Scholar] [CrossRef]
- Xiaofeng, W.; Siyuan, Y.; Jincheng, W. Hyperbranched polyester-modified montmorillonite: A novel phase change material for energy storage. Polym. Int. 2017, 66, 1284–1294. [Google Scholar] [CrossRef]
- Wei, H.; Xie, X.; Li, X.; Lin, X. Preparation and characterization of capric-myristicstearic acid eutectic mixture/modified expanded vermiculite composite as a form-stable phase change material. Appl. Energy 2016, 178, 616–623. [Google Scholar] [CrossRef]
- Li, R.; Zhu, J.; Zhou, W.; Cheng, X.; Li, Y. Thermal properties of sodium nitrate expanded vermiculite form-stable composite phase change materials. Mater. Des. 2016, 104, 190–196. [Google Scholar] [CrossRef]
- Li, X.; Wei, H.; Lin, X. Preparation of stearic acid/modified expanded vermiculite composite phase change material with simultaneously enhanced thermal conductivity and latent heat. Sol. Energy Mater. Sol. Cells 2016, 155, 9–13. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew. Energy 2008, 33, 2599–2605. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Petinakis, E.; Wilson, J. Development of thermal energy storage cementitious composites (TESC) containing a novel paraffin/hydrophobic expanded perlite composite phase change material. Sol. Energy 2017, 158, 626–635. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Huang, Z.; Yin, Z.; Wen, R.; Huang, Y.; Wu, X.; Min, X. Preparation and characterization of capric-palmitic-stearic acid ternary eutectic mixture/expanded vermiculite composites as form-stabilized thermal energy storage materials. J. Mater. Sci. Technol. 2018, 34, 379–386. [Google Scholar] [CrossRef]
- Shadnia, R.; Zhang, L.; Li, P. Experimental study of geopolymer mortar with incorporated PCM. Constr. Build. Mater. 2015, 84, 95–102. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Jafferji, H.; Reynolds, S.E. Application of lightweight aggregate and rice husk ash to incorporate phase change materials into cementitious materials. J. Sustain. Cem. Mater. 2016, 5, 349–369. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Pasupathy, A.; Athanasius, L.; Velraj, R.; Seeniraj, R.V. Experimental investigation and numerical simulation analysis on the thermal performance of a building roof incorporating phase change material (PCM) for thermal management. Appl. Therm. Eng. 2008, 28, 556–565. [Google Scholar] [CrossRef]
- Koksal, F.; Gencel, O.; Kaya, M. Combined effect of silica fume and expanded vermiculite on properties of lightweight mortars at ambient and elevated temperatures. Constr. Build. Mater. 2015, 88, 175–187. [Google Scholar] [CrossRef]
- Schacko, W.A.; Effting, C.; Folgueras, M.V.; Güths, S.; Mendes, G.A. Mechanical and thermal properties of lightweight concretes with vermiculite and EPS using air-entraining agent. Constr. Build. Mater. 2014, 57, 190–197. [Google Scholar] [CrossRef]
- Wen, R.; Huang, Z.; Huang, Y.; Zhang, X.; Min, X.; Fang, M. Synthesis and characterization of lauric acid/expanded vermiculite as form-stabilized thermal energy storage materials. Energy Build. 2016, 116, 677–683. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, X. Thermal analysis of a double layer phase change material floor. Appl. Therm. Eng. 2011, 31, 1576–1581. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Meng, D.; Huang, Z.; Wen, R.; Huang, Y. Shape-stabilized composite phase change materials with high thermal conductivity based on stearic acid and modified expanded vermiculite. Renew. Energy 2017, 112, 113–123. [Google Scholar] [CrossRef]
- Guan, W.M.; Li, J.H.; Qian, T.T.; Wang, X.; Deng, Y. Preparation of paraffin/expanded vermiculite with enhanced thermal conductivity by implanting network carbon in vermiculite layers. Chem. Eng. J. 2015, 277, 56–63. [Google Scholar] [CrossRef]
- Jiao, C.; Ji, B.; Fang, D. Preparation and properties of lauric acid – stearic acid/expanded perlite composite as phase change materials for thermal energy storage. Mater. Lett. 2012, 67, 352–354. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Song, X.; Hou, H.; Yang, Z.; Zhu, J. Preparation and properties of gypsum based energy storage materials with capric acid–palmitic acid/expanded perlite composite PCM. Energy Build. 2015, 92, 155–160. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Impregnation of porous material with phase change material for thermal energy storage. Mater. Chem. Phys. 2009, 115, 846–850. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, W.; Alam, M.; Wilson, J.A. Novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L.; Li, C. Preparation and thermal properties of paraffin/expanded perlite composite as form-stable phase change material. Mater. Lett. 2013, 108, 247–249. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Xiao, Y.; Wang, J.; Lei, J. Preparation and properties of lauric acid/diatomite composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2015, 104, 244–249. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H. Study on preparation and thermal properties of binary fatty acid/diatomite shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 2412–2416. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Li, J.; Qian, T. Enhanced thermal conductivity of peg/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. 2015, 3, 8526–8536. [Google Scholar]
- Kim, D.; Jung, J.; Kim, Y.; Lee, M.; Seo, J.; Khan, S.B. Structure and thermal properties of octadecane/expanded graphite composites as shape-stabilized phase change materials. Int. J. Heat Mass Transf. 2016, 95, 735–741. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Fang, G.Y. Synthesis and characteristics of form-stable n-octadecane/expanded graphite composite phase change materials. Appl. Phys. 2010, 100, 1143–1148. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Acta Energy Sol. Sin. 2006, 47, 303–310. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, L.; Zhao, M. Experimental assessment on the use of phase change materials (PCMs)-bricks in the exterior wall of a full-scale room. Energy Convers. Manag. 2016, 120, 81–89. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Liu, R. Research on composite-phase change materials (PCMs)-bricks in the west wall of room-scale cubicle: Mid-season and summer day cases. Build. Environ. 2017, 123, 494–503. [Google Scholar] [CrossRef]
- Barreneche, C.; Navarro, L.; Gracia, A.; Fernandez, A.I. In situ thermal and acoustic performance and environmental impact of the introduction of a shape-stabilized PCM layer for building. Renew. Energy 2016, 85, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Lu, S.; Huang, J.; Cai, Z.; Wei, S. Experimental research on the use of phase change materials in perforated brick rooms for cooling storage. Energy Build. 2013, 62, 597–604. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Li, Y.; Huang, J.; Liu, S. Numerical study on the thermal performance of building wall and roof incorporating phase change material panel for passive cooling application. Energy Build. 2014, 81, 404–415. [Google Scholar] [CrossRef]
- Jin, X.; Medina, M.A.; Zhang, X. Numerical analysis for the optimal location of a thin PCM layer in frame walls. Appl. Therm. Eng. 2016, 103, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Medina, M.A.; Zhang, X. On the importance of the location of PCMs in building walls for enhanced thermal performance. Appl. Energy 2013, 106, 72–78. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, Y.; Wang, X.; Zhou, S. Numerical analysis of effect of shape-stabilized phase change material plates in a building combined with night ventilation. Appl. Energy 2009, 86, 52–59. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Lin, K.; Xiao, W. Thermal analysis of a direct-gain room with shape-stabilized PCM plates. Renew. Energy 2018, 33, 1228–1236. [Google Scholar] [CrossRef]
- Lei, J.; Yang, J.; Yang, E. Energy performance of building envelopes integrated with phase change materials for cooling load reduction in tropical Singapore. Appl. Energy 2016, 162, 207–217. [Google Scholar] [CrossRef]
- Auzeby, M.; Wei, S.; Underwood, C.; Tindall, J.; Chen, C.; Ling, H.; Buswell, R. Effectiveness of Using Phase Change Materials on Reducing Summer Overheating Issues in UK Residential Buildings with Identification of Influential Factors. Energies 2016, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Diaconu, B.M.; Cruceru, M. Novel concept of composite phase change material wall system for year-round thermal energy savings. Energy Build. 2010, 42, 1759–1772. [Google Scholar] [CrossRef]
- Yao, C.; Kong, X.; Li, Y.; Du, Y.; Qi, C. Numerical and experimental research of cold storage for a novel expanded perlite-based shape-stabilized phase change material wallboard used in building. Energy Convers. Manag. 2018, 155, 20–31. [Google Scholar] [CrossRef]
- Kim, H.B.; Mae, M.; Choi, Y. Application of shape-stabilized phase-change material sheets as thermal energy storage to reduce heating load in Japanese climate. Build. Environ. 2017, 125, 1–14. [Google Scholar] [CrossRef]
- Kim, H.B.; Mae, M.; Choi, Y.; Kiyota, T. Experimental analysis of thermal performance in buildings with shape-stabilized phase change materials. Energy Build. 2017, 152, 524–533. [Google Scholar] [CrossRef]
- Biswas, K.; Lu, J.; Soroushian, P.; Shrestha, S. Combined experimental and numerical evaluation of a prototype nano-PCM enhanced wallboard. Appl. Energy 2014, 131, 517–529. [Google Scholar] [CrossRef]
- Xuetong, S.; Maryam, R.Y.; Rubina, A.; Orlando, J.R. Leakage-proof microencapsulation of phase change material by emulsification with acetylated cellulose nanofibrils. Carbohydr. Polym. 2020. [Google Scholar] [CrossRef]
- Ye, H.; Long, L.; Zhang, H.; Zou, R. The performance evaluation of shape-stabilized phase change materials in building applications using energy saving index. Appl. Energy 2014, 113, 1118–1126. [Google Scholar] [CrossRef]
- Royon, L.; Karim, L.; Bontemps, A. Optimization of PCM embedded in a floor panel developed for thermal management of the lightweight envelope of buildings. Energy Build. 2014, 82, 385–390. [Google Scholar] [CrossRef]
- Belmonte, J.F.; Eguia, P.; Molina, A.E.; Ibanez, J.A. Thermal simulation and system optimization of a chilled ceiling coupled with a floor containing a phase change material (PCM). Sustain. Cities Soc. 2015, 14, 154–170. [Google Scholar] [CrossRef]
- Saffari, M.; Piselli, C.; Gracia, A.; Pisello, A.L.; Cotana, F.; Cabeza, L.F. Thermal stress reduction in cool roof membranes using phase change materials (PCM). Energy Build. 2018, 158, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Roman, K.K.; Brien, T.O.; Alvey, J.B.; Woo, O. Simulating the effects of cool roof and PCM (phase change materials) based roof to mitigate UHI (urban heat island) in prominent US cites. Energy 2016, 96, 103–117. [Google Scholar] [CrossRef]
- Jaworski, M. Thermal performance of building element containing phase change material (PCM) integrated with ventilation system—An experimental study. Appl. Therm. Eng. 2014, 70, 665–674. [Google Scholar] [CrossRef]
- Pasupathy, A.; Velraj, R. Effect of double layer phase change material in building roof for year round thermal management. Energy Build. 2008, 40, 191–203. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Rodrigues, F.; Samagaio, A.; Cardoso, C. Development of a window shutter with phase change materials: Full scale outdoor experimental approach. Energy Build. 2015, 88, 110–121. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Rodrigues, F.; Samagaio, A.; Cardoso, C. Performance of a window shutter with phase change material under summer Mediterranean climate conditions. Appl. Therm. Eng. 2015, 84, 246–256. [Google Scholar] [CrossRef]
- Jeong, S.G.; Chang, S.J.; Wi, S.; Kang, Y.; Lim, J.H.; Chang, J.D.; Kim, S. Energy efficient concrete with n-octadecane/xGnP SSPCM for energy conservation in infrastructure. Constr. Build. Mater. 2016, 106, 543–549. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xu, X.; Lin, X.; Liu, L. A review on the effect of external fields on solidification, melting and heat transfer enhancement of phase change materials. J. Energy Storage 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Jun, O.K.; Lizhong, Y.; Bakytzhan, A.; Alfredo, B.L.; Alessandro, R. Application of granular materials for void space reduction within packed bed thermal energy storage system filled with macro-encapsulated phase change materials. Energy Convers. Manag. 2020, 222, 1–19. [Google Scholar]
- Kapsalis, V.; Karamanis, D. Solar thermal energy storage and heat pumps with phase change materials. Appl. Therm. Eng. 2016, 99, 1212–1224. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Kim, K.H.; Dong, W.; Kim, S. A novel enhancement of shape/thermal stability and energy-storage capacity of phase change materials through the formation of composites with 3D porous (3,6)-connected metal–organic framework. Chem. Eng. J. 2020, 389, 124430. [Google Scholar] [CrossRef]
- Jia, X.; Li, Q.; Ao, C.; Hu, R.; Xia, T.; Xue, Z.; Wang, Q.; Deng, X.; Zhang, W.; Lu, C. High thermal conductive shape-stabilized phase change materials of polyethylene glycol/boron nitride@chitosan composites for thermal energy storage. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105710. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H.-e. Enhanced thermal conductivity of form-stable phase change composite with single-walled carbon nanotubes for thermal energy storage. Sci. Rep. 2017, 7, 44710. [Google Scholar] [CrossRef]
- Yang, H.; Feng, L.; Wang, C.; Zhao, W.; Li, X. Confinement effect of SiO2 framework on phase change of PEG in shape-stabilized PEG/SiO2 composites. Eur. Polym. J. 2012, 48, 803–810. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Ma, B.; Wen, R.; Min, X.; Huang, Y.; Yin, Z.; Liu, Y.; Fang, M.; Wu, X. Preparation and performance of novel form-stable composite phase change materials based on polyethylene glycol/White Carbon Black assisted by super-ultrasound-assisted. Thermochim. Acta 2016, 638, 35–43. [Google Scholar] [CrossRef]

| Reference | PCM | Results | Advantages | Limitations | ||
|---|---|---|---|---|---|---|
| Type | Key Innovation | Melting Temperature/Enthalpy | Freezing Temperature/Enthalpy | |||
| Lv et al. [28] | Eutectic PCM Na2SO4.10H2O/Na2HPO4.12H2O impregnated into expanded graphite. | Coated the composite PCM by paraffin. | High melting enthalpy of 172.3 kJ/kg was observed at 32.05 °C. | Freezing enthalpy of 140.8 kJ/kg was observed at 17.1 °C. | No phase separation and leakage issues. Good thermal reliability. | Poor thermal insulation due to enhanced thermal conductivity. |
| Wang et al. [27] | A composite was prepared by incorporating fatty acid eutectic PCM (capric acid-palmitic acid- stearic acid) into EV via vacuum impregnation. | Added 5% (by weight) Cu powder to improve the thermal conductivity. | Enthalpy and melting temperature were 117.6 kJ/kg and 19.3 °C respectively. | Freezing temperature and enthalpy were 17.1 °C & 118.3 kJ/kg respectively | Thermal conductivity increased by 49.58% by adding Cu powder. | Low thermal insulation property observed due to increased thermal conductivity. |
| Xu et al. [29] | Paraffin/expanded vermiculite composite | Composite PCM was used as aggregate to develop light-weight cement-based composite. | The latent heat and melting temperature of the composite PCM were 27 °C. and 77.6 kJ/kg respectively. | - | Good thermal insulation property of PCM favours its capability to reduce room temperature. | Relatively low latent heat is observed. |
| Tang et al. [30] | Prepared SSPCM by embedding eutectic hydrated salt (Na2SO4.10H2O-Na2HPO4.12H2O) into modified SiO2. | Surface coated with polyvinylpyrrolidone (PVP) to improve thermal cycling performance | Melting temperature: 30.1 °C and melting enthalpy: 106.2 kJ/kg observed. | - | No phase separation issue. No leakage issues. Significant improvement in thermal cycling performance after PVP coating. | Relatively low latent heat storage capacity observed. |
| Zhang et al. [31] | Prepared eutectic acid/EV based SSPCM by incorporating Na2SO4.10H2O-Na2CO3.10H2O in EV by vacuum impregnation. | The resulting composite was shape-stabilized by EV through vacuum impregnation. | Melting temperature: 23.98 °C. Latent heat: 110.3 kJ/kg observed. | - | Shape-stabilization resolves the leakage issue of PCM. Good thermal stability and reliability. Low thermal conductivity. Large latent heat. Non-flammable. | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, M.; Kumar, A.; Elangovan, R.; Meena, C.S.; Kulkarni, K.S.; Kumar, A.; Bhanot, G.; Kapoor, N.R. A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings. Sustainability 2020, 12, 9481. https://doi.org/10.3390/su12229481
Gandhi M, Kumar A, Elangovan R, Meena CS, Kulkarni KS, Kumar A, Bhanot G, Kapoor NR. A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings. Sustainability. 2020; 12(22):9481. https://doi.org/10.3390/su12229481
Chicago/Turabian StyleGandhi, Monika, Ashok Kumar, Rajasekar Elangovan, Chandan Swaroop Meena, Kishor S. Kulkarni, Anuj Kumar, Garima Bhanot, and Nishant R. Kapoor. 2020. "A Review on Shape-Stabilized Phase Change Materials for Latent Energy Storage in Buildings" Sustainability 12, no. 22: 9481. https://doi.org/10.3390/su12229481







