Abstract
D. magna can affect the composition of planktonic bacteria, and can also significantly inhibit the growth of Cyanobacteria at high density. In this study, the inhibitory effects of low-density D. magna groups were stronger than high-density groups due to increases in Acidobacteria abundance in water. Meanwhile, D. magna can inhibit the growth of Planktothrix and Microcystis, but especially the growth of the latter. Alternatively, M. aquaticum and C. demersum can change the community structure of planktonic bacteria. Among them, the inhibitory effect of M. aquaticum on Microcystis and Planktothrix is strong, but it also increases the relative abundance of Chlamydia in water and the risk of pathogenic bacteria. In contrast, the inhibitory effect of C. demersum was more significant on Microcystis than on Planktothrix. Therefore, the combination of “submerged plants-Daphnia”, especially the combination of low density D. magna with M. aquaticum, had a significant inhibitory effect on Planktothrix and Microcystis.
Keywords:
submerged macrophytes; D. magna; plankton; eutrophic water; purification effect; microbial 1. Introduction
Human activities such as agricultural production and rural life have accelerated the discharge of nitrogen, phosphorus, and other nutrients into the water environment [1], resulting in a rapid increase in algae and plankton and a significant decline in transparency and dissolved oxygen in the water column [2]. Some algae metabolites rapidly deteriorate water quality, causing serious damage to the ecological balance of the water environment [3]. In particular, closed water bodies such as ponds and lakes have weak self-purification ability, which aggravates eutrophication [4]. Eutrophication of ponds and lakes will lead to overgrowth of algae and inhibit the growth and reproductive ability of other aquatic organisms, and eventually lead to their high mortality [5].
Although several methods have been investigated to mitigate eutrophication [6,7], biomanipulation is considered one of the most effective ecological methods to control algal growth, mainly through reducing algae abundance by plankton-feeding fish [8,9]. An increase in plankton can promote the growth of highly efficient filter-feeding phytophagous macrozooplankton, and consequently, the efficiency of other feeders such as phytoplankton or zooplankton [10,11,12]. This implies that, ultimately, phytoplankton biomass can be reduced. As a common macrozooplankton in lakes and reservoirs, Daphnia magna has long been a dominant species, with a fast growth and reproduction rate as well as strong feeding potential [13]. At present, there have been many successful cases of purifying eutrophic water by controlling algae growth using cladoceran zooplankton, both in these regions and abroad [14,15]. For example, Gremberghe et al. [16] designed two co-culture experiments: (1) a D. magna culture medium filtrate, and (2) a commercially available Daphnia infochemical consisting of the addition of sodium octyl sulfate to study the effects on strain growth and microcystin production when they were co-cultured with eight Microcystis strains. The results show that both the D. magna culture filtrate and sodium octyl sulfate had a negative effect on the growth of half of the strains. Chislock et al. [17] conducted an enclosure test using Daphnia pulicaria and found that it could tolerate microcystins and significantly reduce Cyanobacteria biomass in Cyanobacteria-dominated waters. Additionally, other studies showed that filter-feeders could not withstand microcystins, giving rise to poor adaptability and a eutrophic environment with Cyanobacteria-dominated waters [18].
Concurrently, the remediation of hot-spot areas through macrophytes, algae bloom inhibition, and the recovery of the aquatic system has been witnessed in recent years [19]. Basically, macrophytes (i) absorb nutrients such as nitrogen and phosphorus that occur in the water during its growth [20]; (ii) boost huge root systems that can provide a living environment and attachment sites for the growth and reproduction of microorganisms, which would entail an increase in the diversity of microorganisms in water [21]; and (iii) are susceptible to some higher plants such as Vallisneria natans, Hydrilla verticillata, Solidago canadensi, Ceratophyllum demersum, or Myriophyllum verticillatum that secrete organic acids, organic esters, ketones, and other allelochemicals during their growth that inhibit algae growth by destroying their cell membrane, and also affecting cell division and photosynthesis [22].
Over recent years, there have been some achievements made in combined treatments for the remediation of eutrophic waters. However, to our knowledge, research on the use of biological treatments for the remediation of eutrophic water bodies by combining D. magna and macrophytes is limited, and its effectiveness has not yet been evaluated. Therefore, we assessed the potential remediation effect of a single organism and its combination with eutrophication water to provide new insights for better remediation of eutrophic waters.
2. Materials and Methods
2.1. Source of Original Materials for Testing
In this study, raw water was taken from a pond nearby farmland in Ninghe County, Tianjin (N 39°33′, E 117°82′). Water characteristics were determined as follows: pH 8.77, total nitrogen (TN) 7.03 mg L−1, ammonia-nitrogen (NH4+-N) 0.21 mg L−1, nitrate nitrogen (NO3−-N) 6.59 mg L−1, total phosphorus (TP) 0.28 mg L−1, dissolved oxygen (DO) 9.28 mg L−1, water transparency (WT) 17.20 cm, and chlorophyll a (chla) content was 272 μg L−1.
The original macrozooplankton of the sampled water were filtered (no. 13 zooplankton net), diluted with a sterilized BG-11 (4%) culture solution in a 1:1 ratio, and an appropriate living space for phytoplankton and bacterial communities was left. This water sample was used to investigate the effect of the test setup on the native phytoplankton and bacterial community in the eutrophic pond.
Given the economic value of aquatic plants, Myriophyllum aquaticum and Ceratophyllum demersum were selected as the study test plants. These were purchased from a flower market in Tianjin and cultured in 4% BG-11 culture solution for 15 days to adapt to the test environment. Well-grown plants of similar size were then selected for the test.
D. magna was purchased from the Institute of Applied Ecology, Chinese Academy of Sciences in Shenyang and its cultivation enlarged in the laboratory before the test. The flask containing D. magna was put into an artificial climate box for more than 60 days before the test at a temperature of 25 °C, light intensity of 1200 lux, and light-to-dark ratio of 12:12 h. Scenedesmus obliquus (20 mL) was fed to the flask daily. Before the test, the filial generation made up of individuals alive, healthy, and of similar size was selected through a filter screen with a 2 mm pore size. Then, they were cleaned in pure water for 24 h so that their intestines were emptied as much as possible for the test.
2.2. Experiment Design
Test barrels were constructed of polyvinylchloride (PVC) with a 30 cm diameter and a 30 cm height. Before the experiment, the barrels were sterilized with ethanol (75%) and repeatedly rinsed with pure water. Fluorescent lamps were used to simulate the sunlight in spring and summer. The light intensity was 1200 ± 100 lux and the light-to-dark ratio was 12:12 h. The test water was mixed thoroughly and then packed into each test barrel. The initial water content was 20 L. The remaining water was used for measurement, which was used at the initial value of 0 d. The heating rod in each test barrel was set to a constant temperature of 25 °C. Thermometers were set to monitor water temperature to ensure constant water temperature during the test. The test time lasted for 20 d. The test setups were all based on the results of the pre-test, and there was a total of nine groups with three replicates (Table 1) (i.e., Time 0: original water samples; K2, K10, and K20 refer to the 2-, 10-, and 20-day water samples in the control treatment; A2, A10, and A20 refer to the 2-, 10-, and 20-day water samples in low-density of the Daphnia magna treatment; B2, B10, and B20 refer to the 2-, 10-, and 20-day water samples in high-density Daphnia magna treatment; C2, C10, and C20 refer to the 2-, 10-, and 20-day water samples in the Ceratophyllum demersum treatment; D2, D10, and D20 refer to the 2-, 10-, and 20-day water samples in the Myriophyllum spicatum treatment; E2, E10, and E20 refer to the 2-, 10-, and 20-day water samples in the low-density combination of Daphnia magna and Ceratophyllum demersum treatment; F2, F10, and F20 refer to the 2-, 10-, and 20-day water samples in the high-density combination of Daphnia magna and Ceratophyllum demersum treatment; G2, G10, and G20 refer to the 2-, 10-, and 20-day water samples in the low-density combination of the Daphnia magna and Myriophyllum spicatum treatment; and H2, H10, and H20 refer to the 2-, 10-, and 20-day water samples in the high-density combination of the Daphnia magna and Myriophyllum spicatum treatment.

Table 1.
Experimental design of different treatments.
2.3. Collection and Determination of Samples
2.3.1. Collection of Samples
This study was conducted in June 2020, when the temperature varied from around 20 to 28 °C, which is why we put heating rods (20 cm height) in the barrels to keep the water at a constant temperature. Sampling was conducted between 8:00–9:00 am, and before sampling, we slightly stirred the water in each barrel to avoid uneven heating. The water level was recorded after each sampling to reduce errors. Distilled water was used to make up the evaporation before the next sampling. The diversity of planktonic bacteria was determined on days 2, 10, and 20 of the test.
2.3.2. Community Structure of Planktonic Bacteria
On day 0 of the test, a fully mixed 300 mL of the test water sample was taken. On days 2, 10, and 20 of the test, a 100 mL water sample was taken from barrels in each test group and mixed well. The 300 mL mixed water sample was then filtered with a sterile filter membrane with a pore size of 0.22 μm and a diameter of 4.5 cm. DNA of the sample on the filter membrane was extracted by a Powerwater DNA Isolation kit (MO BIO, Jefferson City, MO, USA) and purified by a MObio power clean®DNA clean-up kit (MO BIO, Jefferson City, MO, USA). The DNA concentration was detected by Qubit 2.0. Its integrity was detected by 1% of agarose gel.
PCR in the V3–V4 region of the 16S rRNA gene was amplified by the specific primers (338F:5′-ACTCCTACGGGAGGCAGCAG-3′, 806R:5′-GGACTACHVGGGTWTCTAAT-3′). The PCR 20 μL reaction system included a 4 μL 1 × FastPfu buffer, 2.5 mM dNTPs, 5 μM forward and backward primers, 1 unit FastPfu enzyme (2.5 unit μL−1), and a 10 ng DNA template. Initially, it was pre-denatured at 95 °C for three minutes, then went through 27 cycles. Each cycle included denaturation at 95 °C for 30 s and annealing at 55 °C for 30 s, and extended at 72 °C for 45 s, then to 72 °C for 10 min. The reaction product was detected with 2% of agarose gel, and the target band was cut off and then recycled by an AxyPrepGel Kit (Axygen, CA, USA). The products were subjected to double-end sequencing on the Illumina MiSeq platform (Illumina Miseq, Illumina, CA, USA, Shanghai Major Biomedical Technology Co. Ltd., Shanghai, China), according to standard methods.
2.4. Data Analysis and Processing
2.4.1. Chlorophyll a (Chla) and Microcystin (MC) Measurement
Chla was determined through the fluorometric technique using a Turner Designs Model 10–005 fluorometer. The chl-a was extracted at 20 °C in darkness over 24 h with acetone. Microcystin concentrations were measured using enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocol (Abraxis®, Warminster, PA, USA).
2.4.2. Operational Taxonomy Units (OTUs) Taxonomic and Microbial Diversity Analysis
The measured data were screened by QIIME (Version 1.17) and matched after it had its low-quality sequence removed. After removing chimeras using UCHIME, at a similarity level of 97%, operational taxonomy units (OTUs) were generated using UCLUST to cluster. Comparative analysis and species of OTU representative sequences were performed and annotated by SILVA libraries. The high-quality sequences were compared with the SILVA (128) database [23], and reference sequences with a confidence level of more than 70% were identified [24]. OTU groups were identified according to the reference sequences, and the information was annotated to phylum, class, order, family, and genus. In addition, OUT numbers, Chao, and Shannon and Simpson indices were used to indicate the microbial richness and diversity. Calculation methods for these indices can be found at http://www.mothur.org/wiki.
2.4.3. Statistical Analysis
Reshape2, ggplot2, and other R language packages (Version 3.6.3, http://www.r-project.org/)) were used to draw the histogram of relative abundance at the phylum level, and the pheatmap package was used to draw the heat map of relative abundance at the genus level.
According to the plankton bacteria relative abundance data, when entering the formation and stable phases of the microflora structure (days 10 and 20), hypothesis testing was performed on the species groups within the bacterial communities in different groups. The significance level of the abundance difference of the species groups was evaluated using one-way analysis of variance (ANOVA), a Tukey’s honestly significant difference (HSD) test, and redundancy analysis (RDA), with a significance level of 0.05 to obtain the groups with significant differences among them. The RDA analyses between planktonic bacteria in the formation and stable phases and water environmental factors were carried out using Canoco (Version 5.0) software.
3. Results
3.1. Influence of “Submerged Macrophytes—D. magna” Combination on Planktonic Bacteria Diversity
The 415.0 ± 2.9 reads representing mean length amplicons were retained for further analysis (Table S1). Alpha diversity index of each sample was evaluated, and the results are shown in Table 2. Each sample coverage index was above 98%, indicating that the sequencing results in the test water best reflect the composition of planktonic bacterial communities. Values of the richness index (Chao1 index) and diversity indices (Shannon and Simpson index) of each sample are shown in Table 2. The Shannon index of the high-density D. magna treatments (F, H) decreased over the first ten days and increased at the end of the experiment, but the overall trend was downward. In contrast, the Shannon index of the low-density D. magna treatments (E, G) showed the opposite trend. In general, our experimental treatments failed to improve water bacterial diversity.

Table 2.
Microbial richness and community diversity indexes of “submerged macrophytes—Daphnia magna” combination in different treatment group.
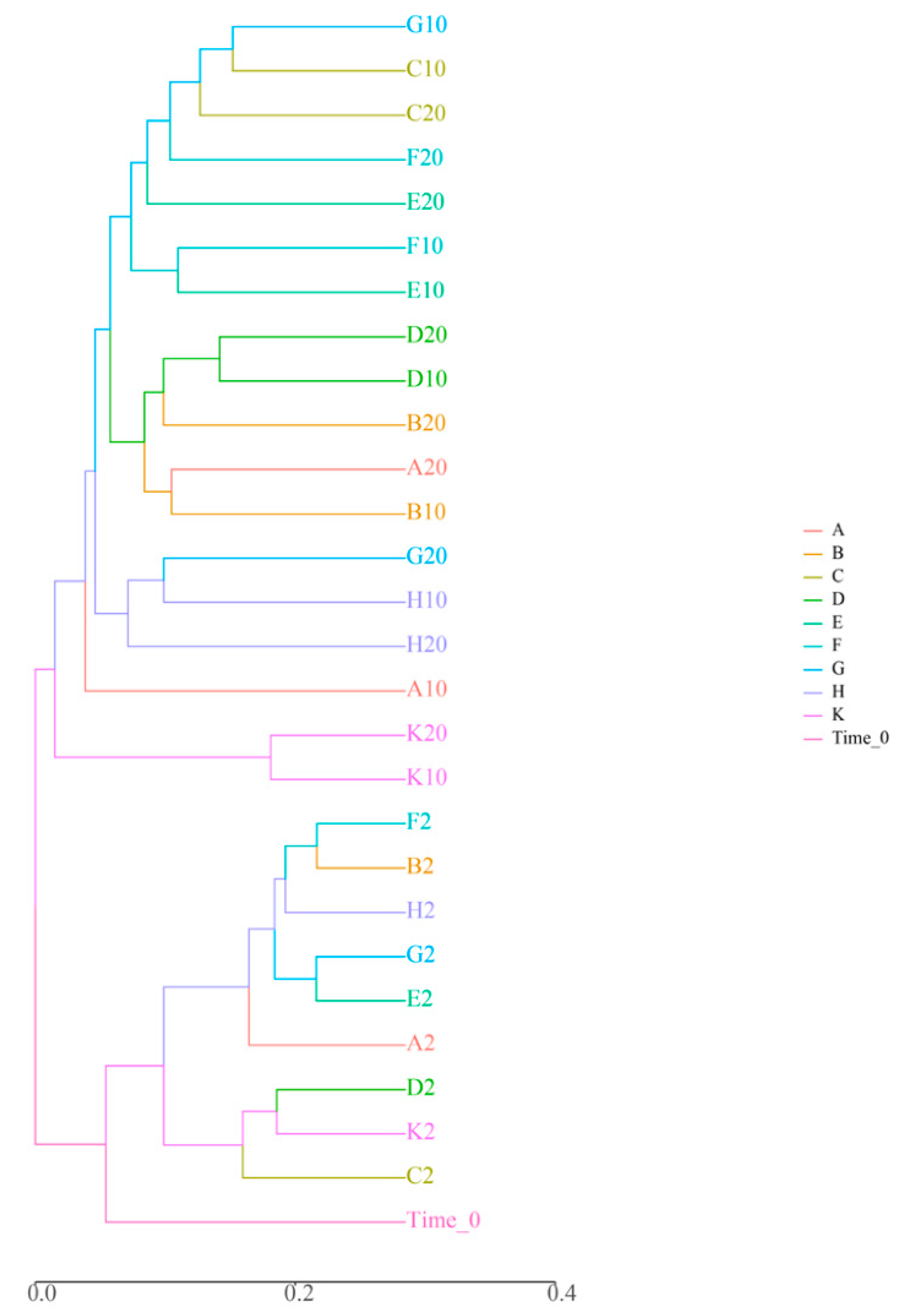
Compositional similarity between two or more sample bacterial colonies can be characterized using beta diversity. Two commonly-used methods to calculate beta diversity are cluster analysis (CA) and non-metric-multi-dimensional scaling (NMDS) (Figure 1). In the dendrogram, samples of high-density D. magna treatments (B, F, H), low-density D. magna treatments (A, E, G), and the two aquatic plant treatments (C, D) were clustered at similar coordinates at 2 d during the experiment, indicating that there was no difference in planktonic bacterial community species composition among treatment groups (Figure 1). On the 10th and 20th days of the experiment, the treatment groups A and B, E and F, G and H samples were gathered into one branch, and the samples of the control group K were gathered into another branch, far away from the samples of these groups. This indicates that, over time, both D. magna and submerged plants have an impact on planktonic bacteria community structure (Figure 1).
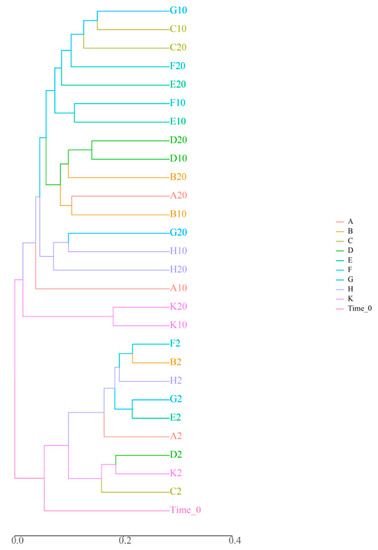
Figure 1.
Analysis results of the beta diversity of microbiota. Note: The axis represents the evolutionary distance among different microbials.
3.2. Influence of “Submerged Macrophytes—D. magna” Combination on Planktonic Bacterial Community Structure
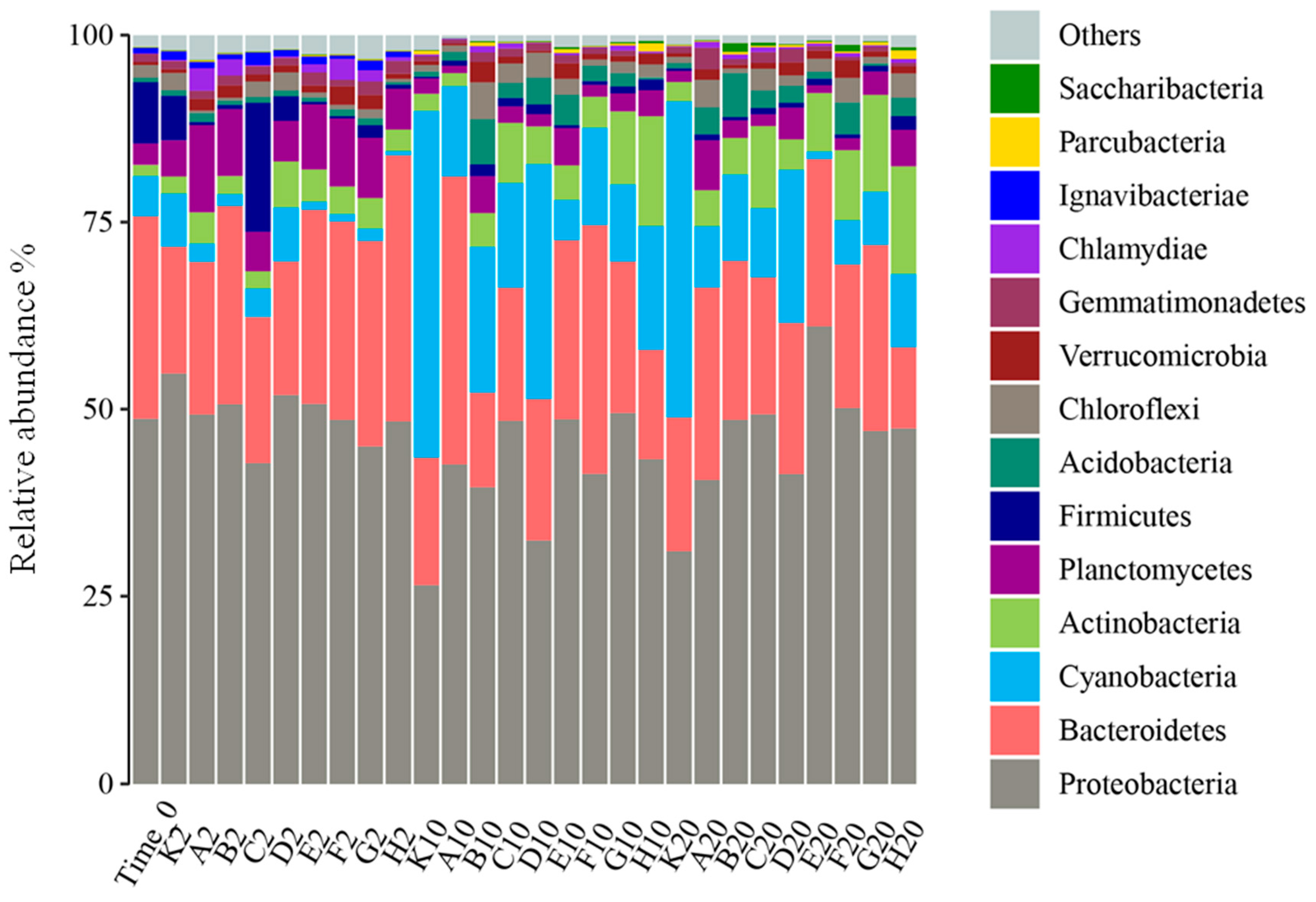
Bar graphs of the relative abundance of each sample at the phylum level during the experiment are shown in Figure 2. Overall, Proteobacteria, Bacteroidetes, Cyanobacteria, Actinobacteria, Planctomycetes, and Firmicutes were the predominant bacteriophyta in the samples, and their cumulative relative abundance accounted for more than 80% of each sample. Proteobacteria contained the most bacterial species, with ten genera among the top 20 in relative abundance. The bar graphs show that different samples had the same dominant species at the phylum level, but the relative abundance was different in each sample.
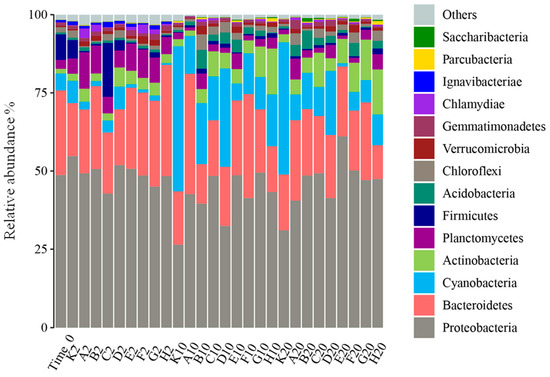
Figure 2.
Bacterial community composition at the phylum level.
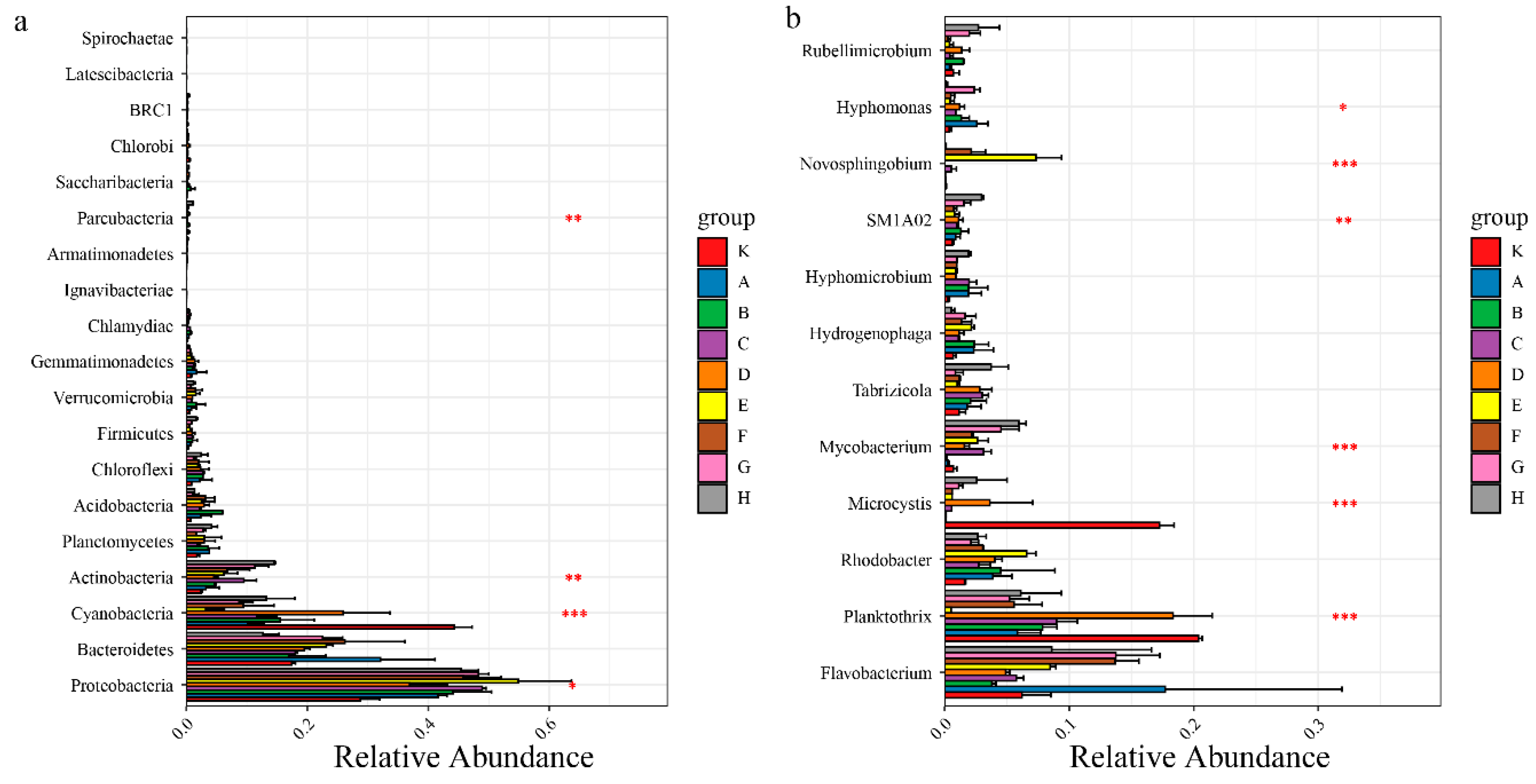
During the entire experiment, Flavobacterium relative abundance was continuously higher in each sample, while by the second day of the experiment, the dominant species in each group were Proteobacteria, with relative abundance of 42.83–54.81%. Among Proteobacteria, the proportions of Unclassified-f-Rhodobacteraceae, Hydrogenophaga, and Woodsholea were relatively higher in each group, and the composition at the genus level was similar in each group, which is consistent with the sample clustering results. At day 10 of the experiment, Planctomycetes appeared in all treatment groups, so the living space for other microorganism phyla was greatly compressed. Compared to the control (K10, K20), all the treatments better inhibited the growth of Microcystis, which is a Cyanobacteria (Figure 3). It is notable that Mycobacterium grew markedly from 10 to 20 days of the experiment in all treatments except K, A, and B. To further analyze the impacts of different treatments on planktonic bacterial community composition, differences in relative abundance among groups were analyzed for the top 20 species (Figure 3). At the phylum level, Cyanobacteria, Actinobacteria, and Proteobacteria significantly differed among all groups (p < 0.05), while at the genus level, Mycobacterium, Microcystis, and Planktothrix were the significantly dominant species (p < 0.05).
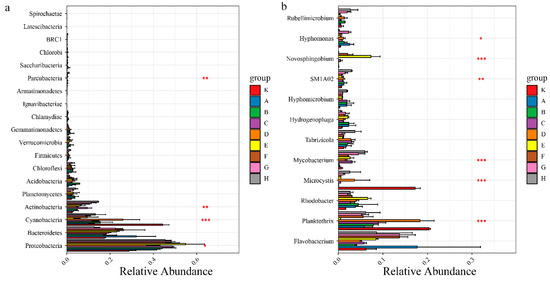
Figure 3.
Univariate ANOVA of intergroup differences in Microphylum (a) and genus (b) levels. Note: Asterisks indicates the significance among different microbials; * p < 0.05; ** p < 0.01; *** p < 0.001.
3.3. Relationships between Water Environmental Factors and the Planktonic Bacterial Community during “Submerged Macrophytes—D. magna” Combined Remediation
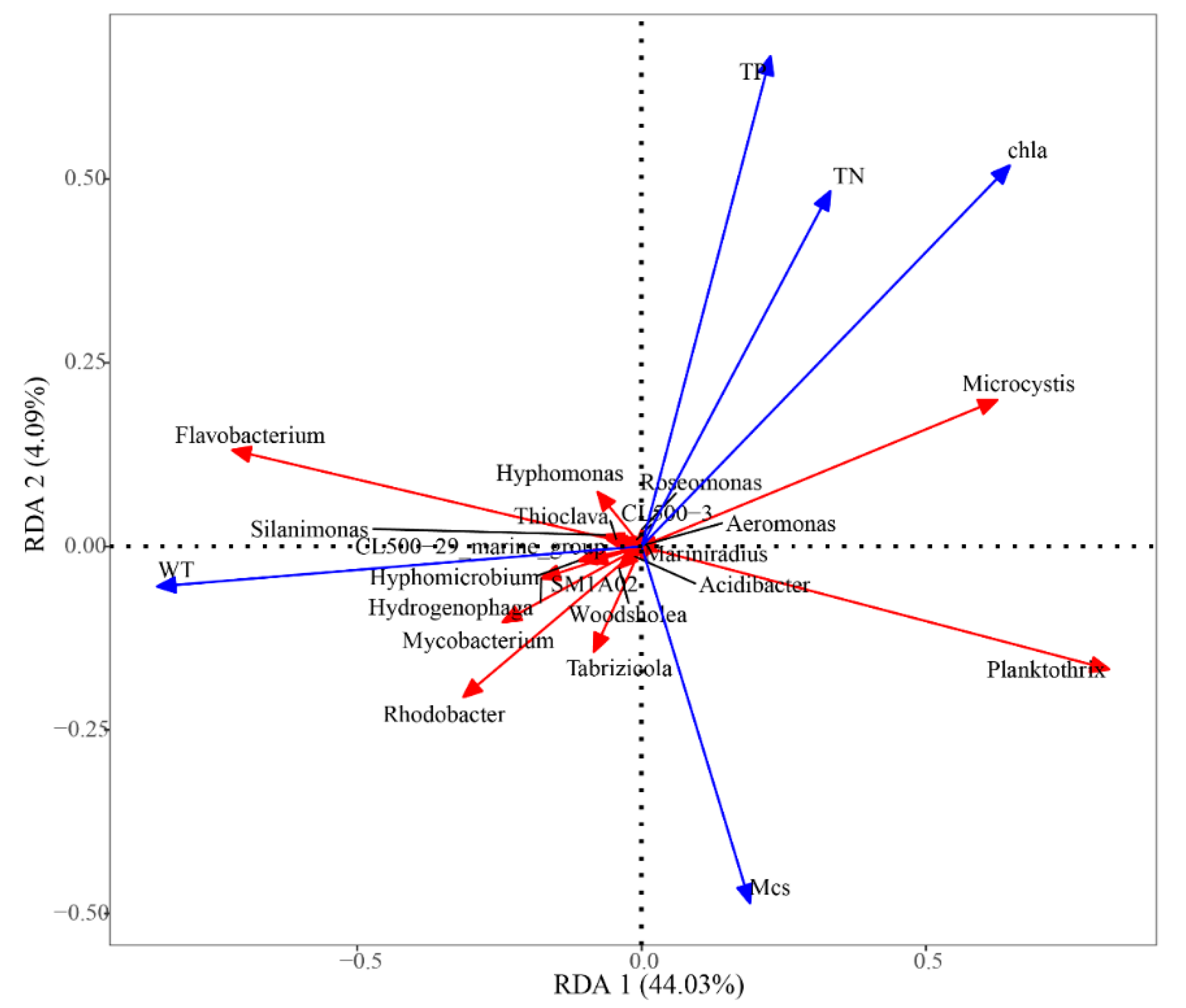
To further investigate interactions between water environmental factors and bacterial community composition during D. magna remediation, redundancy analysis (RDA) was performed. Water environmental factors including water TN, TP, and Chla concentrations, microcystins (MCs), and water transparency (WT) were used in a ranking analysis of relative abundance among groups of the top ten species at the genus level in all treatments (Figure 4). Chla concentration and water transparency were the key environmental factors that affected the bacterial community structure of the eutrophic water, and most bacterial species were positively correlated with WT.
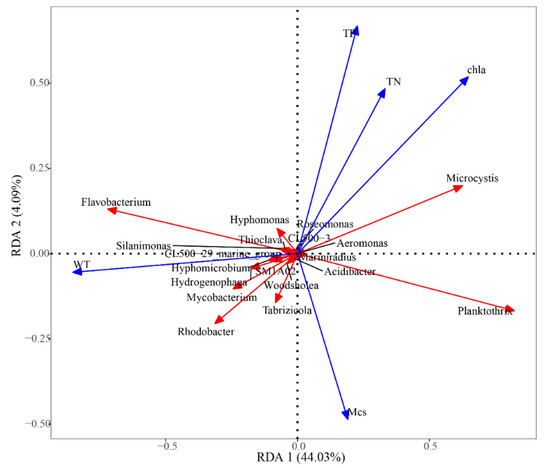
Figure 4.
Redundancy analysis of microbial community gate levels. The scale on the x- and y-axes represent the values generated by regression analysis with environmental factors for each sample or species.
4. Discussion
4.1. Effects of D. magna on Bacterioplankton in Eutrophic Water
D. magna exhibited different inhibitory effects due to Cyanobacteria of varying toxicity [25]. For Microcystis, the inhibitory effect of D. magna was visible; however, Planktothrix, a type of filamentous Cyanobacteria, can interfere with zooplankton abdominal movement in a winding manner, blocking their filter-feeding organs and causing them damage [26]. Therefore, D. magna has a weaker inhibitory effect on Planktothrix, and their feeding effects even increased the relative abundance of Cyanobacteria (Figure 2). It has been speculated that strong D. magna excretion accelerates nutrient recycling, facilitating Cyanobacteria growth [27]. Notably, high-density Daphnia has a stronger respiration that releases more CO2 into the water, due to the lower pH in B10 and B20 samplings, so the relative abundance of Acidobacteria (grows in acidic environment) was higher in the two samples.
4.2. Effects of Submerged Macrophytes on Bacterioplankton in Eutrophic Water
Our research results suggested that Myriophyllum spicatum and C. demersum have less influence on bacterial diversity. Liu et al. [28] found that bacterioplankton diversity responds slowly to environmental changes. Additionally, although our research period was short (20 d), the growth of submerged plants takes a certain amount of time and releasing allelochemicals into the water is a slow process; therefore, there were no significant changes in bacterial diversity. During the experiment, Bacteroidetes abundance was lower when Cyanobacteria abundance was high in the combined groups, indicating that these two bacteria have a competitive relationship. It has been confirmed that both C. demersum and M. spicatum have allelopathic inhibitory effects on Microcystis [29,30], but have large differences. In our study, this explains why M. spicatum had an obvious inhibitory effect on Microcystis and Planktothrix while C. demersum exhibited a strong inhibitory effect on Microcystis, but no significant inhibitory effect on Planktothrix.
4.3. Combined Effects of Submerged Macrophytes and D. magna on Bacterioplankton in Eutrophic Water
Notably, Mycobacterium grew better in the E, F, G, H treatments from 10 to 20 days, and this confirmed that the “phytoplankton + D. magna” treatment groups lowered eutrophication since Actinobacteria grow well in less eutrophic water [31]. While the inhibitory effect on Cyanobacteria in the combined group was better, the abundance of other taxa increased slightly, implying that the combination of “phytoplankton + D. magna” protected the stability of bacterioplankton composition due to submerged plant communities providing major habitats and refuges for D. magna, forming a benign water ecosystem [32]. The reduction of water transparency caused by algae proliferation is one of the biggest bottlenecks in the ecological restoration of eutrophic waters. The use of large fleas to quickly filter and eat algae and particles in eutrophic waters can quickly improve water transparency in the short-term. This makes it possible to transplant submerged plants, laying a foundation for further application of submerged vegetation for water purification [33]. This result is consistent with Kim et al. [34], who used an artificial food web composed of phytoplankton and Daphnia magna to remove nutrients from sewage.
4.4. Relationships between Environmental Factors and Bacterioplankton
TN concentration was negatively correlated with Mycobacterium, and it has been revealed that Mycobacterium possesses a denitrification function [35]. We also found that WT was negatively correlated with Planktothrix and Microcystis, indicating that their excessive growth may lower WT and inhibit the growth of other bacterioplankton. M. spicatum and C. demersum improved WT to some extent; however, since submerged plants need a certain time to grow, their effect on WT was not significant, implying that single submerged macrophytes only slowly improve water quality slightly [36]. It has been shown that Rhodobacter and Novosphingobium were negatively correlated with TN and TP, which is because Rhodobacter is an aerobic denitrifier that can degrade TN in water [37], while Novosphingobium can degrade TP in water and possesses an algicidal effect [38]. This indicates that the combination of submerged macrophytes and D. magna promotes the growth of functional bacteria and the removal of nitrogen and phosphorus.
4.5. Application Prospects
Some previous studies have tried to use biomanipulation in eutrophic water. In the case of typical South Asian eutrophication water bodies in Nanjing Yueya Lake, the application of ecological restoration combination techniques of submerged macrophytes and D. magna was conducted. The results showed that D. magna feeds on algae and organic suspended matter particles, and it achieved transformation of eutrophication material [39]. In addition to nitrification/denitrification, the oxidation sedimentation of phosphorus can also be achieved in submerged macrophytes. Nutrient salt changes caused by macrophytes and D. magna indirectly promoted bacterioplankton change. Eutrophication water quality could be improved, and therefore, it has practical significance and application prospects.
5. Conclusions
Compared with the control group, the low-density Daphnia magna and Myriophyllum verticillatum L. treatment had a greater effect on lowering phytoplankton density, Cyanophyta density, chla concentration, and microcystin toxin content by 91.58%, 92.05%, 92.35%, and 87.56%, respectively, in water. Additionally, the inhibitory effect of Ceratophyllum demersum L. on microcystin toxins and Planktothrix could be significantly improved by the grass-Daphnia combination treatment. Furthermore, the grass-Daphnia combination could also protect the stability of bacterial community structure, and significantly inhibit Cyanobacteria growth among each group. With the same density of Daphnia and plant species, the grass-Daphnia treatment better inhibited the explosive growth of Cyanobacteria than the Daphnia magna or submerged plant single treatments. Moreover, the grass-Daphnia combination had a significant inhibitory effect on Planktothrix and Microcystis growth, with the strongest inhibitory effect induced by the low-density Daphnia magna and Myriophyllum verticillatum L. combination. Furthermore, the grass-Daphnia combination could increase the relative abundance of the denitrification bacteria Mycobacterium in the water. Moreover, RDA analysis showed that the correlation coefficients of environmental factors with planktonic bacterial community ranked in decreasing order were WT, chla, TN, MCs, and TP. The relative abundance of Rhodobacter and Novosphingobium in the low-density Daphnia magna and Myriophyllum verticillatum L. combination was significantly higher than other groups.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/22/9548/s1. (Table S1 Next generation sequencing read statistics).
Author Contributions
B.Y., C.Z. and X.Z. designed the investigation. X.W. and H.W. conducted the experiment. B.Y., J.L. and B.L. interpreted the data. All authors were involved in writing the paper and approved the final manuscript.
Funding
This work was financially funded by the Nature Science Foundation of Tianjin (No.19JCQNJC13400) and the National Science Foundation of China (41807021).
Acknowledgments
We sincerely appreciate the anonymous reviewers and Editor Alison Liu for the critical and valuable comments to help improve this manuscript. This work was financially supported by grants from the Nature Science Foundation of Tianjin (No.19JCQNJC13400) and the National Science Foundation of China (41807021).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Hernández-Crespo, C.; Oliver, N.; Bixquert, J.; Gargallo, S.; Martin, M.A. Comparison of three plants in a surface flow constructed wetland treating eutrophic water in a Mediterranean climate. Hydrobiologia 2016, 774, 183–192. [Google Scholar] [CrossRef]
- Wojciech, K.; Joanna, K. Variations in zooplankton functional groups density in freshwater ecosystems exposed to cyanobacterial blooms. Sci. Total Environ. 2020, 730, 139044. [Google Scholar]
- Le, C.; Zha, Y.; Li, Y.; Sun, D.; Lu, H.; Yin, B. Eutrophication of Lake Waters in China: Cost, Causes, and Control. Environ. Manag. 2010, 45, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Bing, X.W.; Chen, J.Z. The control of eutrophic water in ponds byfloating-bed soilless culture of plants. J. Zhan-Jiang Ocean Univ. 2001, 21, 29–33. (In Chinese) [Google Scholar]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Gerke, M.; Hübner, D.; Schneider, J.; Winkelmann, C. Can top-down effects of cypriniform fish be used to mitigate eutrophication effects in medium-sized European rivers? Sci. Total Environ. 2021, 755, 142547. [Google Scholar] [CrossRef]
- Waajen, G.; Van Oosterhout, F.; Douglas, G.; Lürling, M. Management of eutrophication in lake de kuil (the Netherlands) using combined flocculant—Lanthanum modified bentonite treatment. Water Res. 2016, 97, 83–95. [Google Scholar] [CrossRef]
- Triest, L.; Stiers, I.; Van Onsem, S. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquat. Ecol. 2016, 50, 461–483. [Google Scholar] [CrossRef]
- Shen, R.; Gu, X.; Chen, H.; Mao, Z.; Zeng, Q.; Jeppesen, E. Combining bivalve (Corbicula fluminea) and filter-feeding fish (Aristichthys nobilis) enhances the bioremediation effect of algae: An outdoor mesocosm study. Sci. Total Environ. 2020, 727, 138692. [Google Scholar] [CrossRef]
- Søndergaard, M.; Liboriussen, L.; Pedersen, A.R.; Jeppesen, E. Lake Restoration by Fish Removal: Short- and Long-Term Effects in 36 Danish Lakes. Ecosystems 2008, 11, 1291–1305. [Google Scholar] [CrossRef]
- Bakker, E.S.; Sarneel, J.M.; Gulati, R.D.; Liu, Z.; Van Donk, E. Restoring macrophyte diversity in shallow temperate lakes: Biotic versus abiotic constraints. Hydrobiologia 2012, 710, 23–37. [Google Scholar] [CrossRef]
- Skov, C.; Nilsson, P.A. Evaluating stocking of YOY pike Esox lucius as a tool in the restoration of shallow lakes. Freshw. Biol. 2007, 52, 1834–1845. [Google Scholar] [CrossRef]
- Wojtal-Frankiewicz, A.; Frankiewicz, P. The impact of pelagic (Daphnia longispina) and benthic (Dreissena polymorpha) filter feeders on chlorophyll and nutrient concentration. Limnol.-Ecol. Manag. Inland Waters 2011, 41, 191–200. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Li, M.; Tu, W.; Luo, X.; Liu, X. A field study on the effects of combined biomanipulation on the water quality of a eutrophic lake. Environ. Pollut. 2020, 265, 115091. [Google Scholar] [CrossRef] [PubMed]
- Montemezzani, V.; Duggan, I.C.; Hogg, I.D.; Craggs, R.J. A review of potential methods for zooplankton control in wastewater treatment High Rate Algal Ponds and algal production raceways. Algal Res. 2015, 11, 211–226. [Google Scholar] [CrossRef]
- Van Gremberghe, I.; Vanormelingen, P.; Van der Gucht, K.; Mancheva, A.; D’hondt, S.; De Meester, L.; Vyverman, W. Influence of Daphniainfo chemicals on functional traits of Microcystisstrains (Cyanobacteria). Hydrobiologia 2009, 635, 147–155. [Google Scholar] [CrossRef]
- Chislock, M.F.; Sarnelle, O.; Jernigan, L.M.; Wilson, A.E. Do high concentrations of microcystin prevent Daphnia control of phytoplankton? Water Res. 2013, 47, 1961–1970. [Google Scholar] [CrossRef]
- Lyu, K.; Guan, H.; Wu, C.; Wang, X.; Wilson, A.E.; Yang, Z. Maternal consumption of non-toxic microcystis by daphnia magna induces tolerance to toxic microcystis in offspring. Freshw. Biol. 2016, 61, 219–228. [Google Scholar] [CrossRef]
- Zeng, L.; He, F.; Dai, Z.; Xu, D.; Liu, B.; Zhou, Q.; Wu, Z. Effect of submerged macrophyte restoration on improving aquatic ecosystem in a subtropical, shallow lake. Ecol. Eng. 2017, 106, 578–587. [Google Scholar] [CrossRef]
- Yasin, H.; Usman, M.; Rashid, H.; Nasir, A.; Randhawa, I.A. Alternative approaches for solid waste management a case study in faisalabad pakistan. Earth Sci. Pak. 2017, 1, 7. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, H.; Luo, X.; Zhang, J.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: Antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci. Total Environ. 2020, 699, 134325. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Hochmuth, J.D.; Schamphelaere, K.A.C.D. The effect of temperature on the sensitivity ofDaphnia magnato cyanobacteria is genus dependent. Environ. Toxicol. Chem. 2014, 33, 2333–2343. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Z.; Yu, G.; Zhu, M.; Yu, B.; Li, R. Genetic diversity and molecular phylogeny of Planktothrix (Oscillatoriales, cyanobacteria) strains from China. Harmful Algae 2010, 9, 87–97. [Google Scholar] [CrossRef]
- Asselman, J.; Hochmuth, J.; De Schamphelaere, K. A comparison of the sensitivities of Daphnia magna and Daphnia pulex to six different cyanobacteria. Harmful Algae 2014, 39, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Sun, G.; Xu, Z.; Xu, M. Phylogenetic diversity, composition and distribution of bacterioplankton community in the Dongjiang River, China. FEMS Microbiol. Ecol. 2012, 80, 30–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Mu, X.; Zhang, S.; Pang, S.; Ohore, O.E. Nitrate application decreased microbial biodiversity but stimulated denitrifiers in epiphytic biofilms on Ceratophyllum demersum. J. Environ. Manag. 2020, 269, 110814. [Google Scholar] [CrossRef]
- Nagengast, B.; Gąbka, M. Apparent niche partitioning of two congeneric submerged macrophytes in small water bodies: The case of Ceratophyllum demersum L. and C. submersum L. Aquat. Bot. 2017, 137, 1–8. [Google Scholar] [CrossRef]
- Haukka, K.; Heikkinen, E.; Kairesalo, T.; Karjalainen, H.; Sivonen, K. Effect of humic material on the bacterioplankton community composition in boreal lakes and mesocosms. Environ. Microbiol. 2005, 7, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Burks, R.L.; Lodge, D.M.; Jeppesen, E.; Lauridsen, T.L. Diel horizontal migration of zooplankton: Costs and benefits of inhabiting the littoral. Freshw. Biol. 2002, 47, 343–365. [Google Scholar] [CrossRef]
- Huo, Y.; He, W.-H.; Luo, K.; Wang, Y.-Y.; Zhang, Y.-J.; Tian, Q.-T.; He, P. Bioremediation efficiency of applying Daphnia magna and submerged plants: A case study in Dishui Lake of Shanghai, China. Chin. J. Appl. Ecol. 2010, 21, 495–499. [Google Scholar]
- Kim, S.-R.; Woo, S.-S.; Cheong, E.-H.; Ahn, T.-S. Nutrient removal from sewage by an artificial food web system composed of phytoplankton and Daphnia magna. Ecol. Eng. 2003, 21, 249–258. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Bonaunet, K. Biodegradation of Petroleum Hydrocarbons in Seawater at Low Temperatures (0–5 °C). Biodegradation 2006, 17, 71–82. [Google Scholar] [CrossRef]
- Zhu, T.; Cao, T.; Ni, L.; He, L.; Yi, C.; Yuan, C.; Xie, P. Improvement of water quality by sediment capping and re-vegetation with Vallisneria natans L.: A short-term investigation using an in situ enclosure experiment in Lake Erhai, China. Ecol. Eng. 2016, 86, 113–119. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, K.; Xiong, Z.J.; Miao, C.P.; Chen, Y.W.; Li-Hua, X.U.; Zhao, L.X. Effect of large-scale planting water hyacinth on cultivable bacterial community structure in the eutrophic lake. Microbiol. China 2015, 42, 42–53. (In Chinese) [Google Scholar]
- Bai, S.J.; Huang, L.P.; Su, J.Q.; Tian, Y.; Zheng, T.L. Algicidal Effects of a Novel Marine Actinomycete on the Toxic Dinoflagellate Alexandrium tamarense. Curr. Microbiol. 2011, 62, 1774–1781. [Google Scholar] [CrossRef]
- Yuan, P.; Xu, L.K.; Ke, B.L.; Sun, F.; Gao, H.J. Treatment and ecological restoration of black and odorous water body in Yueya Lake in Nanjing City. J. Environ. Eng. Technol. 2020, 10, 696–701. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).