Dehumidification Effect of Polymeric Superabsorbent SAP-LiCl Composite Desiccant-Coated Heat Exchanger with Different Cyclic Switching Time
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Desiccant Coating Preparation and Static Performance Measurement
2.2. Dynamic Performance Measurement
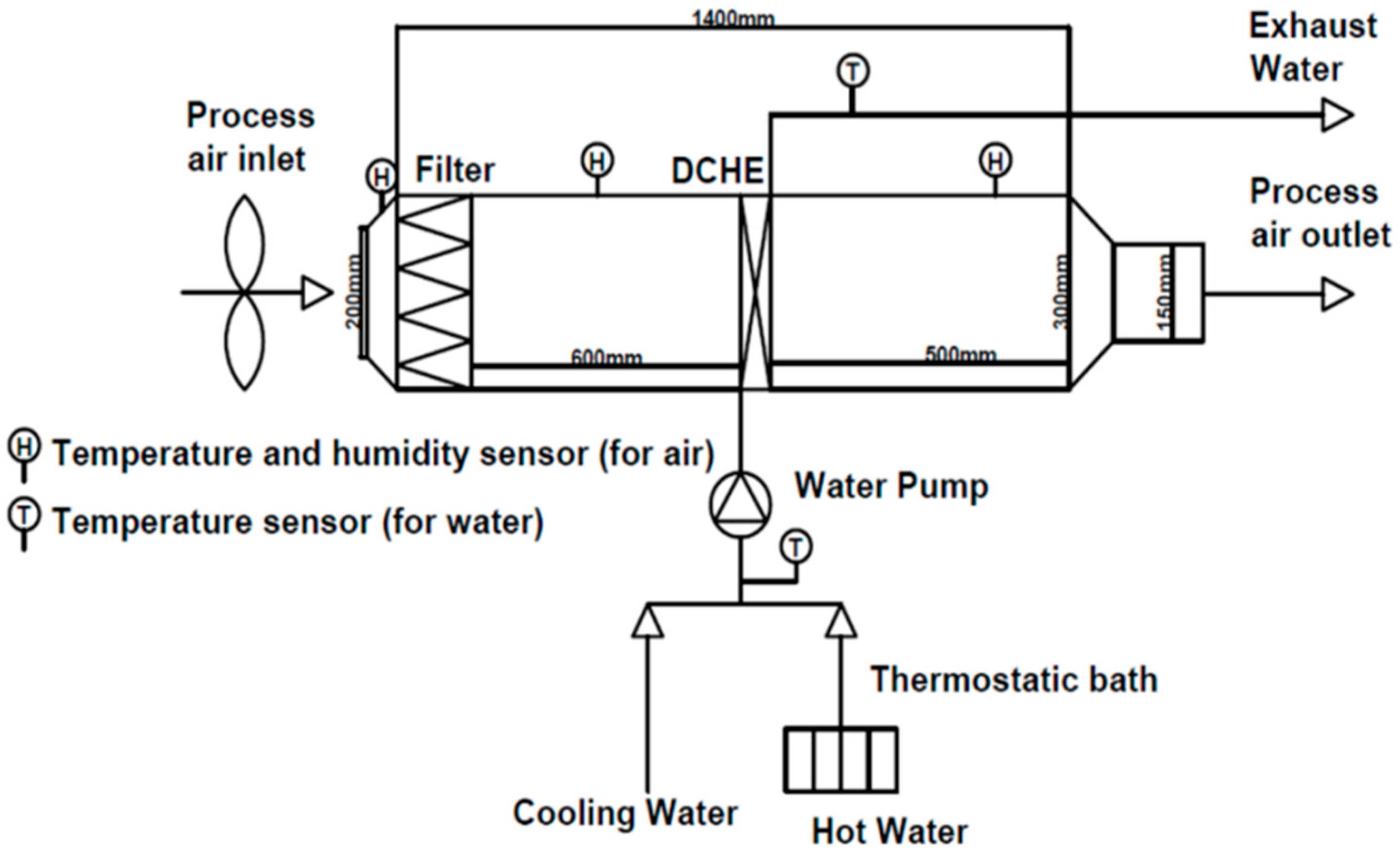
2.2.1. Immersion Coating on FTHE
2.2.2. Experimental Setup
2.2.3. Theoretical Performance Analysis
2.2.4. Uncertainty Analysis
3. Results and Discussions
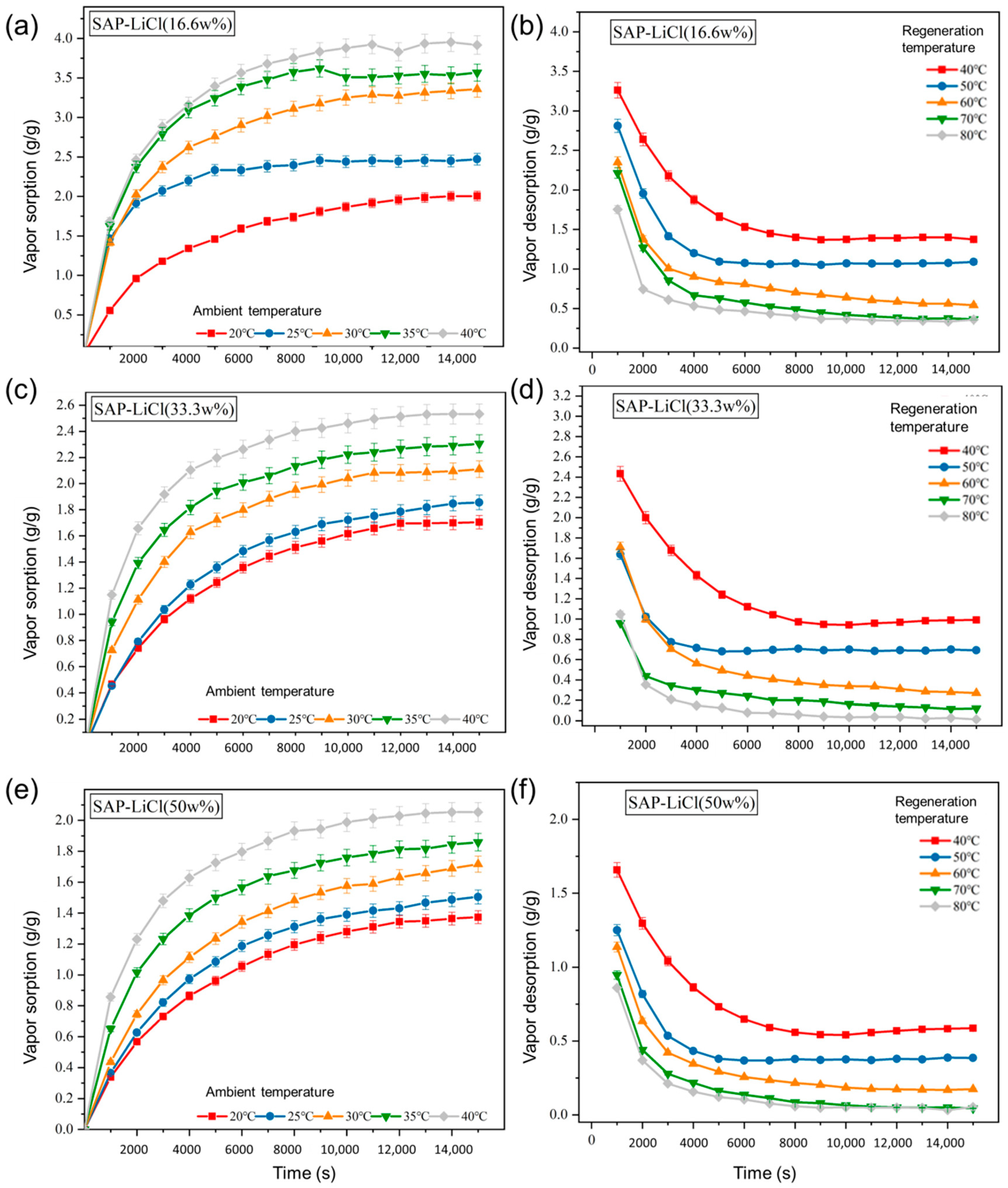
3.1. Performance Analysis of Desiccant in a Static Test Environment
3.2. Performance Analysis of Composite Desiccant-Coated FTHEs in a Dynamic Test Condition
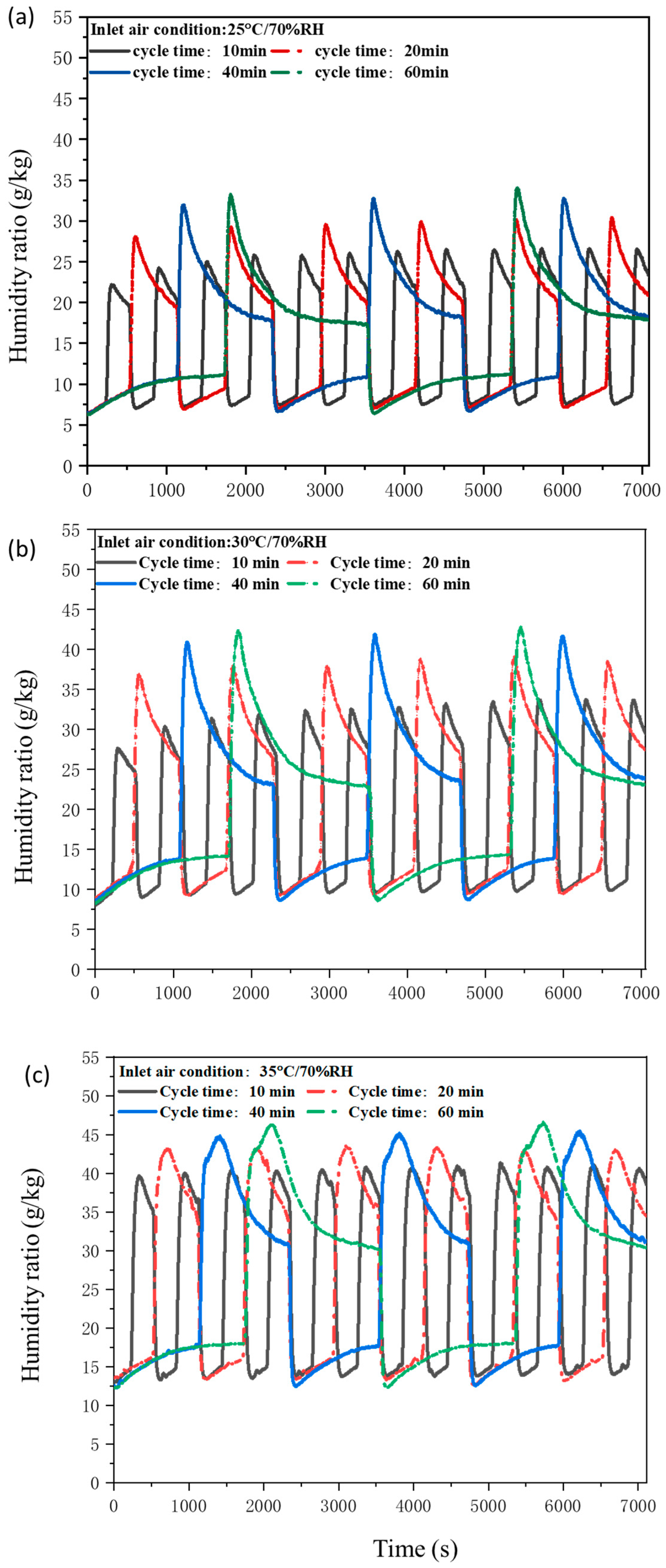
3.2.1. Moisture Removal Capability of Desiccant-Coated FTHEs at Different Humidity Conditions
3.2.2. Vapor Sorption or Desorption Capability of Desiccant-Coated FTHEs at Different Operating Conditions
3.2.3. Thermal Coefficient of Performance (COPth)
3.2.4. Moisture Removal Capability of Desiccant-Coated FTHE with Different Cyclic Switching Time
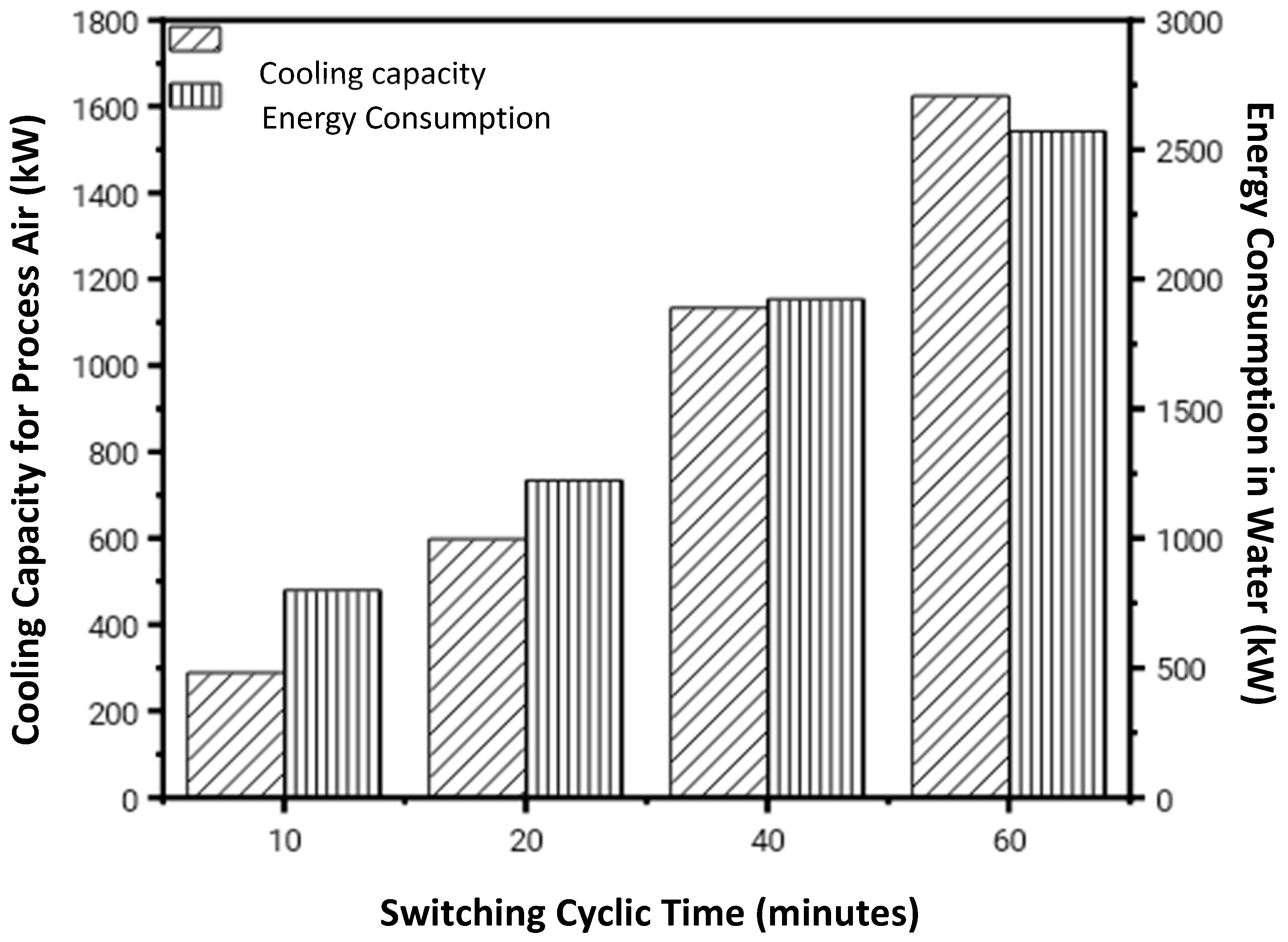
3.2.5. The Overall and Instantaneous COPth of Desiccant-Coated FTHE Corresponding to Different Cyclic Switching Times
4. Conclusions
- (1)
- The static experiment was carried out with a desiccant-coated Al sheet in a programmable constant temperature and humidity system. For superior sorption as well as desorption functionality, the final weight concentration (wt %) of SAP in the polymer composite desiccant solution (SAP-LiCl) was optimized as 33 wt %.
- (2)
- Under high humidity conditions (70% RH and 80% RH), a significant change in the humidity ratio curve was noticed during both the dehumidification as well as the regeneration process, suggesting the efficacy of the proposed desiccant-coated FTHE in high humidity conditions.
- (3)
- The sorption and desorption amount of SAP-LiCl-coated FTHE was found to be superior compared to silica gel-coated FTHE. For instance, at a processing inlet airflow temperature setting of 30 °C with humidity ratio of 70% RH, the sorption amount (Gvap) of the SAP-LiCl-coated FTHE was accounted as 1115.1 g compared to a value of 86.75 g of silica gel-coated FTHE.
- (4)
- The sorption amount and COPth of DCHE were found to be better with processed air inflow with a temperature setting of 30 °C compared to 25 °C. With processed air inflow temperature, relative humidity and regeneration temperature setting of 30 °C, 80% RH and 70 °C, DCHE’s COPth is recorded as high as 0.39.
- (5)
- The dehumidification ability of SAP-LiCl-coated FTHE was analyzed under different switching time cycles, and it was observed that with the increase in the switching time cycle, the overall thermal coefficient of performance increases corresponding to the decrease in the overall sorption and desorption ability of the DCHEs. Energy analysis suggests that a longer switching cycle decreases energy loss during each cycle, resulting in a net improvement in the thermal coefficient of performance (COPth) of DCHE.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| Moisture removal rate | |
| Mass flow rate of air | |
| Humidity ratio of process air inlet | |
| Humidity ratio of process air outlet | |
| Gvap | Vapor sorption/desorption amount |
| Τ | Period for vapor sorption or desorption |
| Vapor sorption/desorption amounts per unit area of desiccant | |
| Total area of the fins | |
| Gm | Sorption or desorption ability per unit mass of the desiccant |
| COPth | Coefficient of thermal performance |
| Cp | Specific heat |
| Qreg | Average heat exchange of water in an effective regeneration process |
| Qcool | The ratio of the total energy exchange of the processed air in an effective dehumidification process |
| RH% | Relative humidity in percentage |
| Temperatures of water supply to DCHE | |
| Enthalpies of the process air after of DCHE | |
| Enthalpies of the process before of DCHE | |
| Mass flow rate of water | |
| Temperatures of water exhaust from DCHE |
References
- Vivekh, P.; Kumja, M.; Bui, D.; Chua, K. Recent developments in solid desiccant coated heat exchangers—A review. Appl. Energy 2018, 229, 778–803. [Google Scholar] [CrossRef]
- Ma, Z.; Song, J.; Zhang, J. Energy consumption prediction of air-conditioning systems in buildings by selecting similar days based on combined weights. Energy Build. 2017, 151, 157–166. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Ge, T.; Zheng, X. Comfortable, high-efficiency heat pump with desiccant-coated, water-sorbing heat exchangers. Sci. Rep. 2017, 7, 40437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.; Al-Alili, A. A review on desiccant coated heat exchangers. Sci. Technol. Built Environ. 2017, 23, 136–150. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.; Ma, W. Dehumidification assessment for desiccant coated heat exchanger systems in different buildings and climates: Fast choice of desiccants. Energy Build. 2020, 110083. [Google Scholar] [CrossRef]
- Wu, X.; Ge, T.; Dai, Y.; Wang, R. Review on substrate of solid desiccant dehumidification system. Renew. Sustain. Energy Rev. 2018, 82, 3236–3249. [Google Scholar] [CrossRef]
- Hu, L.; Ge, T.; Jiang, Y.; Wang, R. Performance study on composite desiccant material coated fin-tube heat exchangers. Int. J. Heat Mass Transf. 2015, 90, 109–120. [Google Scholar] [CrossRef]
- Kummer, H.; Jeremias, F.; Warlo, A.; Füldner, G.; Fröhlich, D.; Janiak, C.; Gläser, R.; Henninger, S.K. A functional full-scale heat exchanger coated with aluminum fumarate metal–organic framework for adsorption heat transformation. Ind. Eng. Chem. Res. 2017, 56, 8393–8398. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivekh, P.; Islam, M.; Chua, K. Experimental performance evaluation of a composite superabsorbent polymer coated heat exchanger based air dehumidification system. Appl. Energy 2020, 260, 114256. [Google Scholar] [CrossRef]
- Leiming, H.; Tianshu, G.; Yu, J.; Ruzhu, W. Hygroscopic property of metal matrix composite desiccant. J. Refrig. 2014, 2, 12. [Google Scholar]
- Ge, T.; Zhang, J.; Dai, Y.; Wang, R. Experimental study on performance of silica gel and potassium formate composite desiccant coated heat exchanger. Energy 2017, 141, 149–158. [Google Scholar] [CrossRef]
- Cui, S.; Qin, M.; Marandi, A.; Steggles, V.; Wang, S.; Feng, X.; Nouar, F.; Serre, C. Metal-Organic Frameworks as advanced moisture sorbents for energy-efficient high temperature cooling. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Wang, R.; Ge, T. Experimental study and performance predication of carbon based composite desiccants for desiccant coated heat exchangers. Int. J. Refrig. 2016, 72, 124–131. [Google Scholar] [CrossRef]
- Chang, C.-C.; Luo, W.-J.; Lu, C.-W.; Cheng, Y.-S.; Tsai, B.-Y.; Lin, Z.-H. Effects of process air conditions and switching cycle period on dehumidification performance of desiccant-coated heat exchangers. Sci. Technol. Built Environ. 2017, 23, 81–90. [Google Scholar] [CrossRef]
- Yang, Y.; Rana, D.; Lan, C.Q. Development of solid super desiccants based on a polymeric superabsorbent hydrogel composite. RSC Adv. 2015, 5, 59583–59590. [Google Scholar] [CrossRef]
- Luo, W.-J.; Faridah, D.; Fasya, F.R.; Chen, Y.-S.; Mulki, F.H.; Adilah, U.N. Performance Enhancement of Hybrid Solid Desiccant Cooling Systems by Integrating Solar Water Collectors in Taiwan. Energies 2019, 12, 3470. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, D.-Y. Sorption characteristics of a novel polymeric desiccant. Int. J. Refrig. 2012, 35, 1940–1949. [Google Scholar] [CrossRef]
- Kline, S.J. Describing uncertainty in single sample experiments. Mech. Eng. 1953, 75, 3–8. [Google Scholar]
- Li, K.-Y.; Luo, W.-J.; Tsai, B.-Y.; Kuan, Y.-D. Performance Analysis of Two-Stage Solid Desiccant Densely Coated Heat Exchangers. Sustainability 2020, 12, 7357. [Google Scholar] [CrossRef]


| Vapor Sorption and Desorption Amount of SAP-LiCl-Coated FTHE | |||||||
|---|---|---|---|---|---|---|---|
| 60% RH | 70% RH | 80% RH | |||||
| Regeneration Temperature | Sorption Amount | Desorption Amount | Sorption Amount | Desorption Amount | Sorption Amount | Desorption Amount | |
| Case-I—Processed air inflow temperature of 25 °C | |||||||
| Gvap (g) | 50 °C | 252.3 | 361.8 | 289.6 | 424.9 | 348.9 | 457.8 |
| 60 °C | 287.7 | 526.6 | 346.7 | 597.4 | 402.2 | 709.1 | |
| 70 °C | 315.0 | 667.6 | 380.3 | 777.6 | 442.5 | 945.1 | |
| Ga (g/cm2) | 50 °C | 0.052 | 0.075 | 0.060 | 0.088 | 0.072 | 0.095 |
| 60 °C | 0.060 | 0.109 | 0.072 | 0.124 | 0.083 | 0.147 | |
| 70 °C | 0.065 | 0.138 | 0.079 | 0.161 | 0.092 | 0.196 | |
| Gm (g/g) | 50 °C | 0.661 | 0.948 | 0.759 | 1.114 | 0.914 | 1.200 |
| 60 °C | 0.754 | 1.380 | 0.909 | 1.566 | 1.054 | 1.859 | |
| 70 °C | 0.826 | 1.750 | 0.997 | 2.038 | 1.160 | 2.478 | |
| Case-II—Processed air inflow temperature of 30 °C | |||||||
| Gvap (g) | 50 °C | 282.9 | 420.5 | 309.0 | 504.0 | 342.0 | 584.4 |
| 60 °C | 303.7 | 641.3 | 396.0 | 732.9 | 418.0 | 1006.1 | |
| 70 °C | 336.8 | 800.9 | 432.9 | 969.7 | 486.4 | 1115.1 | |
| Ga (g/cm2) | 50 °C | 0.059 | 0.087 | 0.064 | 0.104 | 0.071 | 0.121 |
| 60 °C | 0.063 | 0.133 | 0.082 | 0.152 | 0.087 | 0.208 | |
| 70 °C | 0.070 | 0.166 | 0.090 | 0.201 | 0.101 | 0.231 | |
| Gm (g/g) | 50 °C | 0.742 | 1.102 | 0.810 | 1.321 | 0.879 | 1.502 |
| 60 °C | 0.796 | 1.681 | 1.038 | 1.921 | 1.075 | 2.586 | |
| 70 °C | 0.883 | 2.099 | 1.135 | 2.542 | 1.250 | 2.867 | |
| Dehumidification and Regeneration Amount () for an Individual First Cycle and Overall Cycle Time. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case-I: 25 °C/70% RH | Case-II: °C/70% RH | Case-III: 35 °C/70% RH | ||||||||||
| Sorption Amount (g) | Desorption Amount (g) | Sorption Amount (g) | Desorption Amount (g) | Sorption Amount (g) | Desorption Amount (g) | |||||||
| Cycle Time (min) | First Cycle | Overall | First Cycle | Overall | First Cycle | Overall | First Cycle | Overall | First Cycle | Overall | First Cycle | Overall |
| 10 | 67 | 801 | 86 | 1026 | 85 | 1018 | 101 | 1203 | 100 | 128 | 1204 | 1536 |
| 20 | 127 | 764 | 186 | 1113 | 161 | 964 | 243 | 1456 | 198 | 280 | 1192 | 1680 |
| 40 | 222 | 666 | 311 | 933 | 287 | 861 | 401 | 1203 | 361 | 495 | 1084 | 1486 |
| 60 | 308 | 616 | 398 | 796 | 386 | 772 | 538 | 1076 | 504 | 683 | 1009 | 1366 |
| COPth of DCHE under Different Switching Times | ||||||
|---|---|---|---|---|---|---|
| Case-I: 25 °C/70% RH | Case-II: 30 °C/70% RH | Case-III: 35 °C/70% RH | ||||
| Cycle Time (min) | First Cycle | Overall | First Cycle | Overall | First Cycle | Overall |
| 10 | 0.1890 | 0.1940 | 0.2799 | 0.2885 | 0.3612 | 0.3693 |
| 20 | 0.2649 | 0.2673 | 0.3838 | 0.3837 | 0.4887 | 0.4939 |
| 40 | 0.3033 | 0.3084 | 0.4539 | 0.4659 | 0.5895 | 0.5913 |
| 60 | 0.3213 | 0.3254 | 0.4561 | 0.4557 | 0.6318 | 0.6207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panigrahi, B.; Chen, Y.S.; Luo, W.J.; Wang, H.W. Dehumidification Effect of Polymeric Superabsorbent SAP-LiCl Composite Desiccant-Coated Heat Exchanger with Different Cyclic Switching Time. Sustainability 2020, 12, 9673. https://doi.org/10.3390/su12229673
Panigrahi B, Chen YS, Luo WJ, Wang HW. Dehumidification Effect of Polymeric Superabsorbent SAP-LiCl Composite Desiccant-Coated Heat Exchanger with Different Cyclic Switching Time. Sustainability. 2020; 12(22):9673. https://doi.org/10.3390/su12229673
Chicago/Turabian StylePanigrahi, Bivas, Yu Sheng Chen, Win Jet Luo, and Hung Wei Wang. 2020. "Dehumidification Effect of Polymeric Superabsorbent SAP-LiCl Composite Desiccant-Coated Heat Exchanger with Different Cyclic Switching Time" Sustainability 12, no. 22: 9673. https://doi.org/10.3390/su12229673





