Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition
Abstract
1. Introduction
2. Materials and Methods
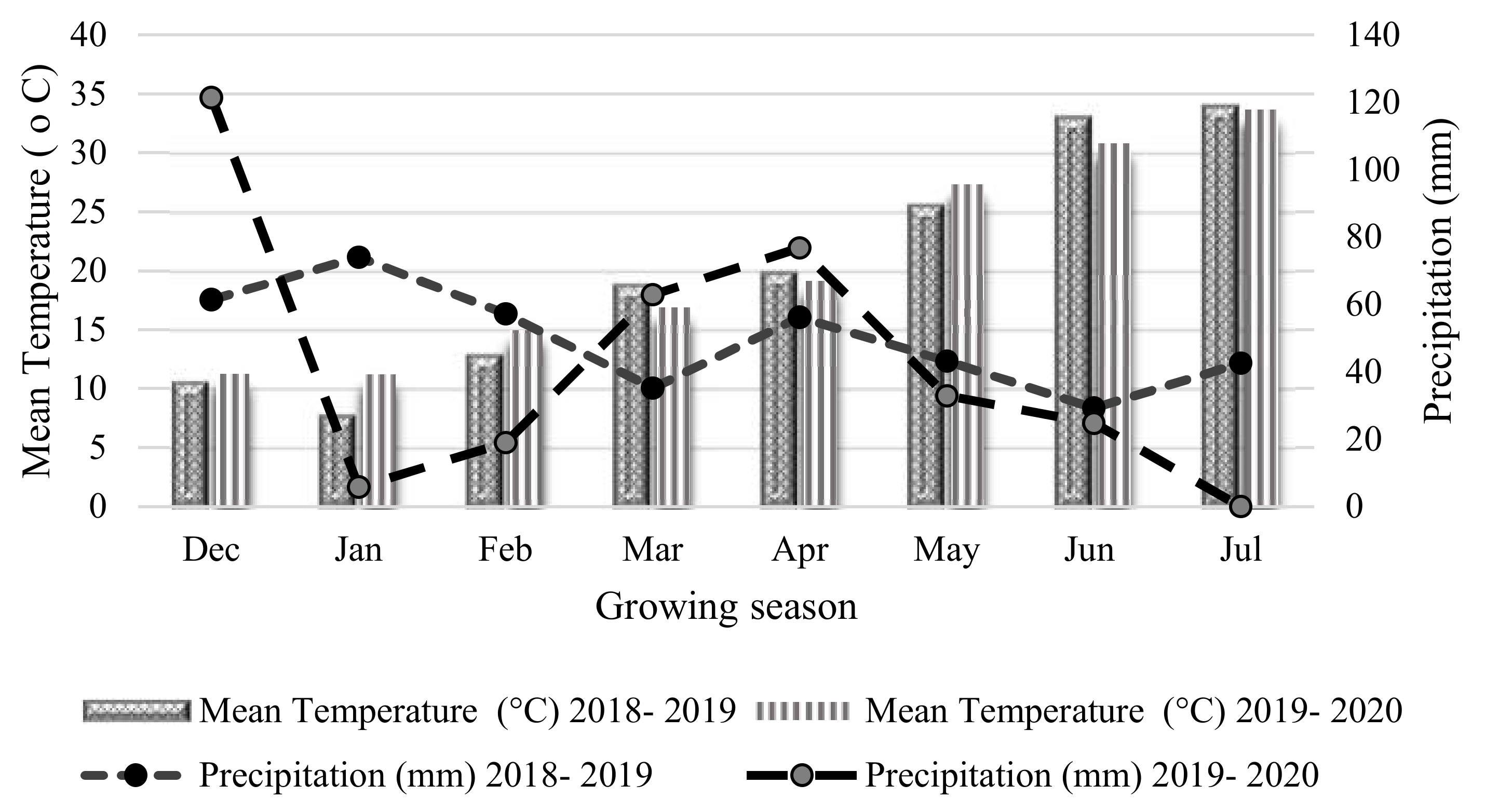
2.1. Experimental Design
2.2. Plant Materials
2.3. Sampling and Analytical Methods
2.4. Calculations and Statistics
3. Results
3.1. Agronomic Characteristics
3.2. Quality Characteristics
3.3. Tillering, Fungi Infection, Vigor, and Relative Production Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RCBD | Randomized Complete Block Design |
| C | Clay |
| DAS | Days After Sowing |
| GDDs | Growing Degree Days |
| RPE | Relative Production Efficiency |
References
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Agraib, L.M.; Al-Awwad, N.J.; Heath, D.D.; Bani-Hani, K.E. Consumption of whole grains, refined cereals, and legumes and its association with colorectal cancer among jordanians. Integr. Cancer Ther. 2016, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Reyneri, A.; Locatelli, M.; Coïsson, J.D.; Blandino, M. Distribution of bioactive compounds in pearled fractions of tritordeum. Food Chem. 2019, 301, 125228. [Google Scholar] [CrossRef] [PubMed]
- Bothmer, R.; Jacobsen, N.; Baden, C.; Jorgensen, R.B.; Linde-Laursen, I. An Ecogeographical Study of the Genus Hordeum, 2nd ed.; International Board for Plant Genetic Resources: Svalov, Sweden, 1995; p. 129. ISBN 978-92-9043-229-6. [Google Scholar]
- Erlandsson, A. Tritordeum Evaluation of a New Food Cereal. Master’s Thesis, Agricultural Program Food Science, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2010. [Google Scholar]
- Martin, A.; Cabrera, A.; Hernández, P.; Ramirez, M.C.; Rubiales, D.; Ballesteros, J. Prospect for the use of Hordeum chilense in durum wheat breeding. In Durum Wheat Improvement in the Mediterranean Region: New Challenges; CIHEAM: Zaragoza, Spain, 2000; pp. 111–115. [Google Scholar]
- Ballesteros, J.; Ramirez, M.C.; Martinez, C.; Atienza, S.G.; Martıin, A. Registration of HT621, a high carotenoid content Tritordeum germplasm line. Crop Sci. 2005, 45, 2662–2663. [Google Scholar] [CrossRef]
- Villegas, D.; Casadesús, J.; Atienza, S.G.; Martos, V.; Maalouf, F.; Karam, F.; Aranjuelo, I.; Nogués, S. Tritordeum, wheat and triticale yield components under multi-local mediterranean drought conditions. Field Crops Res. 2010, 116, 68–74. [Google Scholar] [CrossRef]
- Millan, T.; Martin, A.; de Haro, A. Field trial of Tritordeum. Cereal Res. Commun. 1988, 16, 31–38. [Google Scholar]
- Atienza, S.G.; Ballesteros, J.; Martín, A.; Hornero-Méndez, D. Genetic variability of carotenoid concentration and degree of esterification among tritordeum (Tritordeum Ascherson et Graebner) and durum wheat accessions. J. Agric. Food Chem. 2007, 55, 4244–4251. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Hornero-Méndez, D. Carotenoid profiling of Hordeum chilense grains: The parental proof for the origin of the high carotenoid content and esterification pattern of tritordeum. J. Cereal Sci. 2015, 62, 15–21. [Google Scholar] [CrossRef]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2017, 98, 2201–2209. [Google Scholar] [CrossRef]
- IPCC. Climate change: The physical science basis. In Contribution of Working Group I to the Fourth Annual Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 996. [Google Scholar]
- Kotschi, J. Agricultural biodiversity is essential for adapting to climate change. GAIA Ecol. Perspect. Sci. Soc. 2007, 16, 98–101. [Google Scholar] [CrossRef]
- Mannion, A.M. Biotechnology and environmental quality. Prog. Phys. Geogr. 1995, 19, 192–215. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and water relations in C3 cereals: What to breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, F.; Isidro, J.; Villegas, D.; Garcia del Moral, F.L.; Royo, C. Breeding effect on grain filling, biomass partitioning, and remobilization in Mediterranean durum wheat. Agron. J. 2008, 100, 361–370. [Google Scholar] [CrossRef]
- Gallardo, M.; Fereres, E. Resistencia a la sequia del triticale. tritordeo (Hordeum chilense × Triticum aestivum en relacion a la del trigo, cebada y triticale. Investig. Agrar. Prod. Prot. Veg. 1989, 4, 361–375. [Google Scholar]
- Barro, F.; Gonzalez-Fontes, A.; Maldonado, J.M. Relation between photosynthesis and dark respiration in cereal leaves. J. Plant Physiol. 1996, 149, 64–68. [Google Scholar] [CrossRef]
- Anandhi, A. Growing degree days—Ecosystem indicator for changing diurnal temperatures and their impact on corn growth stages in Kansas. Ecol. Indic. 2016, 61, 149–158. [Google Scholar] [CrossRef]
- Miller, P.; Lanier, W.; Brandt, S. Using Growing Degree Days to Predict Plant Stages. Ag/Extension Communications Coordinator, Communications Services; Montana State University-Bozeman: Bozeman, MO, USA, 2001; pp. 1–7. [Google Scholar]
- Ram, H.; Gupta, N.; Saini, J.S. Growing degree day requirements and yield ability of irrigated durum wheat as influenced by sowing time. Agric. Res. J. 2016, 53, 303–306. [Google Scholar] [CrossRef]
- Liu, Y.; Su, L.; Wang, Q.; Zhang, J.; Shan, Y.; Deng, M. Comprehensive and quantitative analysis of growth characteristics of winter wheat in China based on growing degree days. Adv. Agron. 2020, 237–273. [Google Scholar] [CrossRef]
- Li, Q.; Yin, J.; Liu, W.; Zhou, S.; Li, L.; Niu, J.; Niu, H.; Ma, Y. Determination of Optimum Growing Degree-Days (GDD) Range before Winter for Wheat Cultivars with Different Growth Characteristics in North China Plain. J. Integr. Agric. 2012, 11, 405–415. [Google Scholar] [CrossRef]
- Bonhomme, R. Bases and limits to using “degree.day” units. Eur. J. Agron. 2000, 13, 1–10. [Google Scholar] [CrossRef]
- Haggard, G.B.; Weindorf, D.; Cacovean, H.; Rusu, T.; Lofton, J. Analysis of Growing Degree Days in the transylvanian plain, Romania. Geographia 2010, 2, 13–20. [Google Scholar]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress-opportunities, challenges and future directions. Field Crop. Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Sadras, V.O.; Monzon, J.P. Modelled wheat phenology captures rising temperature trends: Shortened time to flowering and maturity in Australia and Argentina. Field Crop. Res. 2006, 99, 136–146. [Google Scholar] [CrossRef]
- Bilalis, D.; Papastylianou, P.; Travlos, H. Agronomy; Pedio: Athens, Greece, 2019; p. 227. ISBN 978-960-546-039-6. [Google Scholar]
- Gavian, S.; Ehui, S. Measuring the production efficiency of alternative land tenure contracts in a mixed crop–livestock system in Ethiopia. Agric. Econ. 1999, 20, 37–49. [Google Scholar]
- Peterson, R. Wheat Crop Series; Polunin, N., Ed.; Inter Science Publication Inc.: New York, NY, USA, 1965; p. 422. [Google Scholar]
- Kimball, B.A.; White, J.W.; Wall, G.W.; Ottman, M.J. Infrared-Warmed and Unwarmed Wheat Vegetation Indices Coalesce Using Canopy-Temperature–Based Growing Degree Days. Agron. J. 2012, 104, 114–118. [Google Scholar] [CrossRef]
- Stewart, D.W.; Dwyer, L.M. Analysis of phenological observations on barley (Hordeum vulgare) using the feekes scale. Agric. For. Meteorol. 1987, 39, 37–48. [Google Scholar] [CrossRef]
- Karagoz, A.; Zencirci, N. Variation in wheat (Triticum spp.) landraces from different altitudes of three regions of Turkey. Gen. Resour. Crop Evol. 2004, 52, 775–785. [Google Scholar] [CrossRef]
- Preece, C.; Livarda, A.; Christin, P.A.; Wallace, M.; Martin, G.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 2017, 31, 387–397. [Google Scholar] [CrossRef]
- Hoyle, A.; Brennan, M.; Jackson, G.E.; Hoad, S. 2019 Increased grain density of spring barley (Hordeum vulgare L.) is associated with an increase in grain nitrogen. J. Cereal Sci. 2019, 89. [Google Scholar] [CrossRef]
- Jenner, C.F. Starch synthesis in the kernel of wheat under high temperature conditions. Aust. J. Plant Physiol. 1994, 21, 791–806. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.; Fitzgerald, G.; Seneweera, S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012, 133, 1307–1311. [Google Scholar] [CrossRef]
- Jolankai, M.; Kassai, K.M.; Tarnawa, A.; Posa, B.; Birkas, M. Impact of precipitation and temperature on the grain and protein yield of wheat (Triticum aestivum L) varieties. Quart. J. Hung. Meteorol. Serv. 2018, 122, 31–40. [Google Scholar] [CrossRef]
- Prats, E.; Fondevilla, S.; Rubiales, D.; Carver, T.L. Cellular basis of resistance to different formae speciales of Blumeria graminis in Hordeum chilense, wheat, and tritordeum and agroticum amphiloids. Can. J. Plant Pathol. 2006, 28, 577–587. [Google Scholar] [CrossRef]
- Botwright, T.L.; Condon, A.G.; Rebetzke, G.J.; Richards, R.A. Field evaluation of early vigour for genetic improvement of grain yield in wheat. Aust. J. Agric. Res. 2002, 53, 1137–1145. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, S. Soil Quality Indices and Relative Production Efficiency for Maize and Wheat Crops in Agroclimates of Northwest India. Soil Sci. 2010, 175, 44–49. [Google Scholar] [CrossRef]
| Physical Composition (%) | Exchangeable Bases (cmol kg−1) | ||
|---|---|---|---|
| sand | 23 | Na | 0.37 |
| Silt | 24 | K | 1 |
| Clay | 53 | Ca | 28 |
| Textural class | Clay | ||
| Chemical characteristics | |||
| pH (H2O 1:1) (25 °C) | 7.4 | Total N (Kjeldahl) (g/100g) | 0.11 |
| Organic Matter (%) | 1.4 | CaCO3 | 1 |
| (POlsen) (mg/kg) | 15 | EC | 524 |
| Lines/Variety | DAS |
|---|---|
| HT-460 | 143 |
| HT-1704 | 134 |
| HT-1707 | 146 |
| HT-15-54-32 | 144 |
| AUCAN | 145 |
| BULEL | 140 |
| GENESIS | 139 |
| FALADO | 136 |
| Varieties/Lines | Head Emergence (GDDs) | Period from Head Emergence to Harvest (GDDs) | Plant Height (cm) | 1000 Grain Weight (g) | Yield (kg ha−1) | Protein Yield (kg ha−1) | Grain Specific Weight (kg hl−1) |
|---|---|---|---|---|---|---|---|
| 2019 | |||||||
| HT-460 | 497.5 a | 1288.3 a | 69.3 a | 28.3 a | 4695 a,b | 1.7 ns | 66.4 a |
| HT-1704 | 402.7 b | 1383.1 b | 60 b,e | 28.2 a | 4055 b | 2 ns | 70.9 b |
| HT-1707 | 532.7 b | 1253.1 a | 65 c | 31.9 b | 3806 c | 2 ns | 67.8 c |
| HT-15-54-32 | 514.8 c | 1270.9 a | 71.8 c | 29.8 c | 4754 a | 1.9 ns | 67.9 c |
| Aucan | 520.2 b | 1265.6 a | 68.5 d,f | 31.4 b | 3666 c,d | 2 ns | 67.6 c |
| Bulel | 473.6 d | 1312.1 b | 68 b,f | 28.5 a | 3211 d | 1.7 ns | 71.4 b |
| Genesis (bread wheat) | 461.3 c | 1324.5 b | 67.3 e | 33.4 d | 6706 e | 1.8 ns | 75.3 d |
| Falado (bread wheat) | 430.1 a | 1355.6 b | 76.3 a,d | 23.7 d | 7966 f | 1.5ns | 75.3 d |
| 2020 | |||||||
| HT-460 | 572.4 a | 1068.9 a | 69.3 a | 28.4 a | 3813 a | 1.7 ns | 61.8 a |
| HT-1704 | 496.3 b | 1145 b | 65.3 b | 28.4 a | 3173 b | 1.9 ns | 66.6 b |
| HT-1707 | 610.7 a | 1030.5 a | 64 b | 32.2 b | 3029 b | 1.8 ns | 69.2 c |
| HT-15-54-32 | 601.4 a | 1039.9 a | 70 c | 30.1 c | 3905 a | 1.7 ns | 66.6 b |
| Aucan | 622.4 c | 1019 a | 69.8 a | 31.3 c | 3189 b | 1.9 ns | 62.2 a |
| Bulel | 546.6 a | 1094.7 a | 69.8 a | 28.7 d | 3196 b | 1.7 ns | 70 d |
| Genesis (bread wheat) | 560.8 c | 1080.4 a | 69.8 a | 33.3 b | 4315 d | 1.8 ns | 69.8 c |
| Falado (bread wheat) | 538.1 c | 1103.2 b | 77 d | 25.6 e | 3799 a | 1.8 ns | 70.4 d |
| FLines/Varieties | ns | 8.9 ** | 10.2 *** | 49 *** | 39.7 *** | ns | 30.8 *** |
| Fyear | 9.9 ** | 420.8 *** | ns | ns | 133.2 *** | ns | 62.8 *** |
| FLines/Varieties x year | ns | ns | ns | ns | ns | ns | ns |
| Varieties/Lines | Moisture Content (%) | Gluten (%) | Protein Content (%) | Gluten/Protein | Threshing Efficiency (%) |
|---|---|---|---|---|---|
| 2019 | |||||
| HT-460 | 10.8 a | 26.2 a | 13 a | 2 a | 89.5 a |
| HT-1704 | 11 b | 37.1 b | 15.5 b | 2.4 b | 84.3 b |
| HT-1707 | 11 b | 33.5 c | 15.1 b | 2.2 c | 83.3 c |
| HT-15-54-32 | 11 b | 31.7 d | 14.3 c | 2.2 c | 92.8 d |
| Aucan | 10.7 a | 33.2 c | 15.2 b | 2.2 c | 84.3 b |
| Bulel | 11 b | 27.6 e | 13 a | 2.1 a | 83.8 c |
| Genesis (bread wheat) | 9.7 c | 34.2 d | 14.1 c | 2.4 b | 94.3 d |
| Falado (bread wheat) | 10 c | 27.2e | 11.7 d | 2.3 b | 93 d |
| 2020 | |||||
| HT-460 | 10.6 a | 26.7 a | 13.3 a | 2 a | 90.5 a |
| HT-1704 | 10.9 a | 36.4 b | 14.7 b | 2.5 b | 84 b |
| HT-1707 | 11 b | 32.1 c | 14 b | 2.3 c | 81.5 c |
| HT-15-54-32 | 11.1 b | 32.4 c | 13.4 a | 2.4 c | 93.3 d |
| Aucan | 10.7 a | 33.9 d | 14.5 b | 2.3 c | 84.3 b |
| Bulel | 10.9 a | 29 a | 13.3 a | 2.2 a | 83.8 b |
| Genesis (bread wheat) | 12.1 c | 33.3 d | 14.1 b | 2.4 b | 93.3 d |
| Falado (bread wheat) | 12.4 c | 32.3 c | 13.7 a | 2.4 b | 92.3 d |
| FLines/Varieties | ns | 16.9 *** | 12.9 *** | 4.4 * | 19.9 *** |
| Fyear | 21.7 *** | ns | ns | ns | ns |
| FLines/Varieties x year | ns | ns | ns | ns | ns |
| Varieties/Lines | Tillering (1 = Low, 5 = High) | Fungi Infection (1 = Low, 5 = High) | Vigour (1 = Low, 5 = High) | Relative Production Efficiency (%) |
|---|---|---|---|---|
| 2019 | ||||
| HT-460 | 3 a | 2.5 a | 3 a | −36 a |
| HT-1704 | 4.5 b | 2 a | 3.5 a | −44.7 b |
| HT-1707 | 4 c | 1.3 b | 4 b | −48.1 b |
| HT-15-54-32 | 4.8 d | 1 b | 4.8 c | −35.2 a |
| Aucan | 4 c | 1.3 b | 4 b | −50 b |
| Bulel | 4.8 d | 1.3 b | 4.5 c | −56.2 b |
| Genesis (bread wheat) | 4.3 b | 1.8 c | 3.5 a | |
| Falado (bread wheat) | 4 c | 2 a | 4 b | |
| 2020 | ||||
| HT-460 | 3 a | 2.3 a | 3.3 a | -6 a |
| HT-1704 | 4 b | 1.5 b | 3.8 b | −21.8 b |
| HT-1707 | 3.8 b | 1.3 b | 4.3 c | −25.3 b |
| HT-15-54-32 | 4.3 c | 1 c | 4.8 c | -3.8 a |
| Aucan | 4.3 c | 1.3 b | 4.3 c | −21.4 b |
| Bulel | 4.5 d | 1 c | 4.3 c | −21.2 b |
| Genesis (bread wheat) | 4.5 d | 1.3 b | 4.5 c | |
| Falado (bread wheat) | 2.5 a | 1.3 b | 3 a | |
| FLines/Varieties | 9.2 *** | 5.1 *** | 7.7 *** | 6.8 * |
| Fyear | 5.2 * | 4.2 * | ns | 15.8 ** |
| FLines/Varieties x year | ns | ns | ns | ns |
| Crop Properties | Height (cm) | 1000 Grain Weight (g) | Yield (kg ha−1) | Protein Content (%) | Moisture Content (%) | Grain Specific Weight (kg hl−1) | Gluten (%) | Threshing Efficiency (%) | Tillering (1 = Low. 5 = High) | Fungi Infection (1 = Low. 5 = High) | Vigour (1 = Low. 5 = High) | Head Emergence (GDDs) | Period from Head Emergence to Harvest (GDDs) | Protein Yield (kg ha−1) | Gluten/Protein | Relative Production Efficiency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height (cm) | 1.0 | 0.51 *** | 0.0 | 0.62 *** | 0.63 *** | 0.51 *** | 0.52 *** | 0.53 *** | 0.60 *** | 0.61 *** | 0.61 *** | −0.0.40 ** | −0.0.20 ns | −0.0.30 * | −0.0.20 ns | 0.21 ns |
| 1000 grain weight (g) | 0.51 *** | 1.0 | −0.0.41 *** | 0.10 ** | 0.10 ** | 0.72 *** | 0.91 ** | 0.32 * | 1.0 ** | 1.0 ** | 1.0 ** | −0.52 *** | −0.0.30 ns | 0.20 ns | 0.10 ns | −0.0.10 ns |
| Yield (kg ha−1) | 0.0 | −0.0.41 *** | 1.0 | −0.0.41 ** | −0.0.44 ** | 0.10 ns | −0.0.41 ** | 0.47 *** | −0.0.30 ** | −0.0.30 ** | −0.0.42 ** | −0.0.21 ns | 0.41 ** | 0.10ns | −0.0.22 ns | 0.12 ns |
| Protein content (%) | 0.62 *** | 1.0 ** | −0.41 ** | 1.0 | 1.0 ** | 0.72 *** | 0.97 ** | 0.35 * | 1.0 ** | 1.0 ** | 1.0 ** | −0.0.65 *** | 0.35 ns | 1.0 | 0.46 * | −0.0.28 ns |
| Moisture Content (%) | 0.63 *** | 1.0 ** | −0.44 ** | 1.0 ** | 1.0 | 0.74 *** | 0.91 ** | 0.33 * | 1.0 ** | 1.0 ** | 1.0 ** | −0.0.64 *** | 0.12 ns | −0.0.11 ns | 0.21 ns | 0.02 ns |
| Grain specific weight (kg hl−1) | 0.51 *** | 0.72 *** | 0.10 ns | 0.72 *** | 0.74 *** | 1.0 | 0.61 *** | 0.35 * | 0.78 *** | 0.79 *** | 0.74 *** | −0.0.88 *** | 0.55 *** | −0.0.18ns | 0.19ns | −0.0.65 *** |
| Gluten (%) | 0.52 *** | 0.91 ** | −0.41 ** | 0.97 ** | 0.91 ** | 0.61 *** | 1.0 | 0.21 ns | 0.92 ** | 0.94 ** | 0.97 ** | −0.0.61 *** | 0.12 ns | 0.80 *** | 0.81 *** | 0.01 ns |
| Threshing efficiency (%) | 0.53 *** | 0.32 * | 0.47 *** | 0.35 * | 0.33 * | 0.35 * | 0.21 ns | 1.0 | 0.31 * | 0.33 * | 0.37 * | −0.0.22 * | −0.0.11 ns | −0.22 ns | −0.28 ns | 0.45 ns |
| Tillering (1 = low.5 = high) | 0.60 *** | 1.0 ** | −0.30** | 1.0 ** | 1.0 ** | 0.78 *** | 0.92 ** | 0.31 * | 1.0 | 1.0 ** | 1.0 ** | −0.0.61 *** | 0.24 ns | 0.12 ns | 0.35 * | −0.0.28 ns |
| Fungi Infection (1 = low. 5 = high) | 0.61 *** | 1.0 ** | −0.30** | 1.0 ** | 1.0 ** | 0.79 *** | 0.94 ** | 0.33 * | 1.0 ** | 1.0 | 1.0 ** | −0.77 *** | 0.22 ns | −0.0.25 ns | −0.0.38 * | 0.01 ns |
| Vigour (1 = low. 5 = high) | 0.61 *** | 1.0 ** | −0.42 ** | 1.0 ** | 1.0 ** | 0.74 *** | 0.97 ** | 0.37 * | 1.0 ** | 1.0 ** | 1.0 | −0.65 *** | −0.0.27 ns | 0.02 ns | 0.14 ns | 0.02 ns |
| Head emergence (GDDs) | −0.40 ** | −0.0.52 ** | −0.21 ns | −0.65 *** | −0.0.63 *** | −0.0.88 *** | −0.0.61 *** | −0.0.22* | −0.0.61 *** | −0.0.77 *** | −0.65 *** | 1.0 | −0.0.97 *** | −0.0.34 * | −0.0.21 ns | 0.65 *** |
| Period from head emergence to harvest (GDDs) | −0.0.20 ns | −0.0.30 ns | 0.41 ** | 0.35 ns | 0.12 ns | 0.55 *** | 0.18 ns | −0.0.11 ns | 0.24 ns | 0.22 ns | −0.0.27 ns | −0.0.97 *** | 1.0 | 0.37 ns | −0.0.26 ns | −0.0.87 *** |
| Protein yield(kg ha−1) | −0.0.30 * | 0.20 ns | 0.10 ns | 1.0 | −0.0.11 ns | −0.0.19 ns | 0.80 *** | −0.0.22 ns | 0.12 ns | −0.0.25 ns | 0.02 ns | −0.0.34 * | 0.37 ns | 1.0 | 0.45 * | −0.0.27 ns |
| Gluten/Protein | −0.0.21 ns | 0.10 ns | −0.0.22 ns | 0.46 * | 0.21 ns | 0.19 ns | 0.81 *** | −0.0.28 ns | 0.35 * | −0.0.38 * | 0.14 ns | −0.0.21 ns | −0.0.26 ns | 0.45 * | 1.0 | 0.23 ns |
| Relative ProductionEfficiency (%) | 0.21 ns | −0.0.10 ns | 0.12 ns | −0.28 ns | 0.02 ns | −0.0.65 *** | 0.01 ns | 0.45 ** | −0.0.28 ns | 0.01 ns | 0.02 ns | 0.65 *** | −0.0.87 *** | −0.0.27 ns | 0.23 ns | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakabouki, I.; Beslemes, D.F.; Tigka, E.L.; Folina, A.; Karydogianni, S.; Zisi, C.; Papastylianou, P. Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability 2020, 12, 9700. https://doi.org/10.3390/su12229700
Kakabouki I, Beslemes DF, Tigka EL, Folina A, Karydogianni S, Zisi C, Papastylianou P. Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability. 2020; 12(22):9700. https://doi.org/10.3390/su12229700
Chicago/Turabian StyleKakabouki, Ioanna, Dimitrios F. Beslemes, Evangelia L. Tigka, Antigolena Folina, Stella Karydogianni, Charikleia Zisi, and Panagiota Papastylianou. 2020. "Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition" Sustainability 12, no. 22: 9700. https://doi.org/10.3390/su12229700
APA StyleKakabouki, I., Beslemes, D. F., Tigka, E. L., Folina, A., Karydogianni, S., Zisi, C., & Papastylianou, P. (2020). Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability, 12(22), 9700. https://doi.org/10.3390/su12229700






